Abstract
There is interest in understanding post-translational modifications of proteins in inflammatory disease. Neddylation is the conjugation of the molecule neural precursor cell expressed, developmentally down-regulated 8 (NEDD8) to promote protein stabilization. Cullins are a family of NEDD8 targets important in the stabilization and degradation of proteins, such as hypoxia-inducible factor (HIF; via Cullin-2). Here, we elucidate the role of human deneddylase-1 (DEN-1, also called SENP8) in inflammatory responses in vitro and in vivo and define conditions for targeting neddylation in models of mucosal inflammation. HIF provides protection in inflammatory models, so we examined the contribution of DEN-1 to HIF stabilization. Pharmacologic targeting of neddylation activity with MLN4924 (IC50, 4.7 nM) stabilized HIF-1α, activated HIF promoter activity by 2.5-fold, and induced HIF-target genes in human epithelial cells up to 5-fold. Knockdown of DEN-1 in human intestinal epithelial cells resulted in increased kinetics in barrier formation, decreased permeability, and enhanced barrier restitution by 2 ± 0.5-fold. Parallel studies in vivo revealed that MLN4924 abrogated disease severity in murine dextran sulfate sodium colitis, including weight loss, colon length, and histologic severity. We conclude that DEN-1 is a regulator of cullin neddylation and fine-tunes the inflammatory response in vitro and in vivo. Pharmacologic inhibition of cullin neddylation may provide a therapeutic opportunity in mucosal inflammatory disease.—Curtis, V. F., Ehrentraut, S. F., Campbell, E. L., Glover, L. E., Bayless, A., Kelly, C. J., Kominsky, D. J., Colgan, S. P. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses.
Keywords: hypoxia, intestinal epithelia, inflammation
Inflammatory bowel disease (IBD) is composed of chronic mucosal inflammatory disorders, including ulcerative colitis (UC) and Crohn’s disease (CD). IBD is thought to be caused by a dysregulation of the mucosal immune response (1). There is significant interest in understanding the mechanisms of resolution of this immune response in IBD. Of particular interest are post-translational modifications of proteins, which are central to nearly all biologic processes because they are important in the establishment and progression of inflammatory diseases. The role of particular modifications, such as phosphorylation in the NF-κB pathway and hydroxylation in the HIF (hypoxia-inducible factor) pathway, and their role in the mucosal immune response have been extensively studied (2). However, upstream of these signaling cascades is the ubiquitin proteasome pathway that is regulated by a post-translational protein modification known as neddylation.
Neddylation involves the conjugation of the ubiquitin-like molecule neural precursor cell expressed, developmentally down-regulated 8 (NEDD8) to specific targets to promote protein stabilization or degradation (3). Cullins are a major family of NEDD8 targets and are subunits of the E3 ligase machinery, which bring together E2 ubiquitin-conjugating enzymes and substrates that have been targeted for degradation. Previous work has demonstrated the major role that neddylation plays in modulating pathways important in the inflammatory response, such as the NF-κB pathway [via Cullin-1 (Cul-1)] (4, 5) and the HIF pathway [via Cullin-2 (Cul-2)] (6, 7). Cul-1 mutants that cannot be neddylated are unable to stimulate ubiquitination and processing of NF-κB components (8). Other work has demonstrated the role of enteric commensal bacteria in modulating Cul-1 neddylation and the consequent effects on NF-κB and β-catenin signaling (9, 10). Additionally, we have previously shown that the hypoxic microenvironment in tissues due to inflammation induces an extracellular accumulation of adenosine resulting in the deneddylation of Cul-1 and suppression of proinflammatory NF-κB activity (11).
Cul-1 is a subunit of a ubiquitin E3 ligase that ubiquitinates IκB. After treatment with the purine nucleoside adenosine, this ubiquitin activity is abrogated, blocking IκB degradation, even in the presence of TNF, therefore sequestering NF-κB in the cytoplasm and reducing the transcription of NF-κB target genes (11). An analogous complex exists upstream of the HIF-1α protein, where Cul-2 is part of the E3 ligase along with the von Hippel-Lindau protein (pVHL), which ubiquitinates HIF, leading to its degradation and blocking its signaling pathway (6, 7, 12). Cul-2 neddylation is required for activation of the Elongin B/C, Cul-2, pVHL E3 ubiquitin ligase complex once hydroxylated HIF-1α binds to pVHL (13). Because previous studies have demonstrated the protective effect of hypoxia and the HIF pathway (14, 15), modulation of the HIF target gene response via the neddylation pathway has become a subject of great interest in the treatment of inflammatory diseases, such as IBD.
Upstream of the E3 ligase activity is the protein human deneddylase-1 (DEN-1), a dual NEDD-specific protease (16, 17). DEN-1 cleaves the C terminus of a proform of NEDD8 to its mature form, allowing NEDD8 to be conjugated it to substrates, such as cullins (18). DEN-1 also deconjugates these neddylated cullin proteins. Previous work has highlighted the importance of DEN-1 in fine-tuning the vascular response to inflammatory stimuli via NF-κB and HIF-1α (19).
Given our observation that adenosine deneddylates Cul-1 (11), we were particularly interested in the identification of MLN4924, a molecule structurally related to AMP that acts by inhibiting cullin neddylation and down-regulating the ubiquitin pathway (20). MLN4924 inhibits the neddylation of multiple cullins (21) and acts by binding to the NEDD8-activating enzyme (NAE) protein complex, a heterodimer of NAE1 and UBA3 subunits, and forming an adduct with NEDD8, preventing the activation of NEDD8 and its subsequent conjugation to target proteins (22). Treatment of cancer cells with MLN4924 in both in vivo and in vitro models has demonstrated its viability as a therapeutic. By disrupting ubiquitin ligase activity and subsequent protein turnover, apoptosis was induced via S phase defects (20). Previous investigations into the vascular inflammatory response revealed the importance of neddylation and the ability of MLN4924 to abrogate proinflammatory responses and promote anti-inflammatory responses (19).
There is significant interest in modifying neddylation pathways for therapeutic purposes beyond cancer. MLN4924’s action as a neddylation inhibitor and its similar structure to AMP, a precursor to adenosine, made it an intriguing small molecule to test in our inflammatory models where we are interested in activating the HIF pathway to enhance the expression of proresolving genes. Here, we demonstrate the role of neddylation in mucosal inflammation and further define the mechanisms that control the inflammatory response and identify a potential therapeutic option.
MATERIALS AND METHODS
Cell culture
Human T84 intestinal epithelial cells and HeLa cells were cultured in 95% air with 5% CO2 at 37°C. Lentiviral particles encoding short hairpin RNA (shRNA) against DEN-1 (MISSION TRC shRNA; Sigma-Aldrich, St. Louis, MO, USA) were transduced into T84 intestinal epithelial cells using established protocols.
Transcriptional analysis
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to isolate RNA from HeLa or T84 cells. cDNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). PCR analysis was performed using SYBR Green (Applied Biosystems, Carlsbad, CA, USA) and the following primer sequences: BNIP3L, forward, 5′-GCACAACAACAACAACAACTG-3′, reverse, 5′-CATTGCCATTATCATTGCCATTG-3′; α-enolase (Eno1), forward, 5′-ATCGCCAAGGCCGTGAA-3′, reverse, 5′-ACGGAGCCAATCTGGTTGAC-3′; glucose transporter 1 (Glut1), forward 5′- GGGTCAGGCTCCATTAGGATT-3′, reverse, 5′- CCCAACTGGTCTCAGGAAAGAA-3′; DEN-1, forward, 5′-CAACACAGAGGTGCCTGAAG-3′, reverse, 5′-CGGGGTCCATCTTGTACTGA-3′; and β-actin, forward, 5′-GGAGAAAATCTGGCACCACA-3′, reverse, 5′-AGAGGCGTACAGGGATAGCA-3′.
Generation of anti-Den-1 polyclonal antibody
Anti-Den-1 antibody was generated by immunizing rabbits with the following peptide coupled to keyhole limpet hemocyanin: Ac-CYITKKRGEWKDLI-amide (synthesized by New England Peptide, Gardner, MA, USA). This peptide antigen represents amino acids 194–206 of full-length Den-1. To purify the antibody, the rabbit serum was passed over a column of cyanogen bromide-activated Sepharose 4B coupled to the peptide immunogen. The column was washed repeatedly with wash buffer [50 mM Tris (pH 8.0), 150 mM NaCl]. The bound antibodies were eluted in 1 ml fractions with 100 mM glycine and neutralized immediately with1 M Tris (pH 8.0).
Western blot analysis
The NE-PER extraction kit was used to prepare nuclear and cytoplasmic lysates from HeLa cells and confluent T84 monolayers per the manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA). Western blotting of these lysates was performed using Cul-2 Rb pAb (Invitrogen), HIF-1α Ms mAb (BD Biosciences, San Jose, CA, USA), HIF-1α-OH Rb pAb (Novus Biologicals, Littleton, CO, USA), NEDD8 Rb pAb (Cell Signaling, Danvers, MA, USA), TATA-binding protein, Ms mAb (Abcam, Cambridge, United Kingdom), and human β-actin Rb pAb (Abcam).
Luciferase assays
A hypoxia response element (HRE)-containing luciferase reporter plasmid (100 ng) (23) and a Renilla reporter plasmid (1 ng) were transfected into subconfluent HeLa cells using PolyFect Reagent (Invitrogen). Luciferase activity was determined at 24 h using Promega Dual-Luciferase reagents (Promega, Madison, WI, USA), and luminescence was determined using the GloMax Multi plate reader (Promega).
Proliferation assay
To measure the proliferation rate of the DEN-1 knockdown (KD) cells and short hairpin nontargeting control (shNTC) cells, a cell-counting assay was performed over 6 d. In brief, 2 × 105 cells were plated in each well of 6-well plates. The cells were trypsinized, stained with trypan blue 0.4% (Life Technologies, Carlsbad, CA, USA) to identify live cells, and counted daily using a hemacytometer (Hausser Scientific, Horsham, PA, USA). The concentration of cells was plotted, and the linear portion of the graph was used to calculate average doubling time using the following formula: doubling time = (t2 − t1)/3.32 × (log(n2) − log(n1)), where t indicates time and n indicates the concentration of cells.
Barrier formation, Ca2+ switch, and permeability assays
T84 cells (shNTC and DEN-1 KD) were plated on 0.33 cm2, 0.4 µm permeable polyester inserts (Corning, Corning, NY, USA). Transepithelial resistance (TER) was measured daily using the EVOM2 voltohmmeter (World Precision Instruments, Sarasota, FL, USA) to monitor barrier formation. For the Ca2+ switch assays, T84 cells (wild-type or shNTC and DEN-1 KD) were grown to confluence on 0.33 cm2, 0.4 µm permeable polyester inserts. Calcium switch was performed by equilibrating cells in HBSS with Ca2+ (HBSS+; Sigma-Aldrich) for 20 min and then by incubating the cells in Ca2+-free HBSS (HBSS−) with 2 mM EDTA for 5 min. The cells were then returned to HBSS+ alone for experiments with shNTC and DEN-1KD cells or containing MLN4924 (330 nM) or an equivalent dilution of DMSO as a control for experiments with wild-type cells. TER was measured using the EVOM2. Rates of restitution were calculated from resultant tracings by plotting the TER recovery phase (135 min following repletion of Ca2+) as a best-fit exponential function (all R2 > 0.95).
Paracellular permeability was assayed using FITC-dextran flux assay described previously (24). In brief, confluent T84 monolayers on 0.33 cm2, 0.4 µm permeable polyester inserts were washed and equilibrated in HBSS+. In total, 1 mg/ml FITC-dextran (molecular size of 3 kDa; Sigma-Aldrich) was added to the apical compartment, and samples were taken from the basolateral compartment every 30 min for 2 h. Fluorescence was determined using a GloMax Multi fluorescent plate reader (Promega) and represented as the change in fluorescence over time.
Animals
Wild-type C57BL/6 mice (Mus musculus) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Colorado. To induce colitis, the drinking water of 8-wk-old mice was supplemented with 2.5% dextran sulfate sodium (DSS; MP Biomedicals, Solon, OH, USA), ad libitum. Animals were administered either MLN4924 (0.1 mg/kg) or cyclodextrin (Sigma-Aldrich) via intraperitoneal injection at day −1 of DSS exposure and continued daily. The DSS was withdrawn from the drinking water on day 8, and mice were killed on day 12, 4 d after the removal of DSS exposure.
Histology
Histologic examination was performed on 5 samples of the distal colon per group; samples were fixed in 10% formalin before staining with hematoxylin and eosin (H&E). All histologic quantitation was performed blinded by the same individual using a scoring system previously described (25). The 3 independent parameters measured were as follows: severity of inflammation (0–3: none, slight, moderate, or severe); extent of injury (0–3: none, mucosal, mucosal and submucosal, or transmural); and crypt damage (0–4: none, basal one-third damaged, basal two-third damaged, only surface epithelium intact, or entire crypt and epithelium lost). The score of each parameter was multiplied by a factor reflecting the percentage of tissue involvement (×1: 0–25%; ×2: 26–50%; ×3: 51–75%; and ×4: 76–100%), and all numbers were summed. Maximum possible score was 40.
Statistical analysis
Data are expressed as mean values ± sem. Data were analyzed with Student’s t test between 2 groups or ANOVA coupled with post hoc Bonferroni test for multiple pairwise comparisons. P values <0.05 were considered to be statistically significant.
RESULTS
Pharmacologic inhibition of neddylation influences the Cul-2/HIF pathway
Our previous studies revealed that deneddylation of Cul-1 inhibited NF-κB activation (19). Because a parallel pathway exists for Cul-2 and HIF (6, 7, 12), we initially defined these principles using the neddylation inhibitor MLN4924. As shown in Fig. 1, the exposure of HeLa cells to MLN4924 (330 nM for 1 h based on pilot studies) resulted in prominent deneddylation of Cul-2 by Western blot (0.31 ± 0.05 vs. 0.86 ± 0.10 ratio of neddylated:unneddylated Cul-2 with and without MLN4924, respectively) and a robust stabilization of HIF-1α (Fig. 1A–C). We next performed a time course treatment of MLN4924 at 330 nM in HeLa cells. The conjugation of NEDD8 to cullin proteins is shown using a NEDD8 antibody at the approximate size of neddylated cullin proteins (90–95 kDa), and this conjugation was lost as early as 5 min, and a stabilization of HIF-1α in the nuclear fraction was observed as early as 15 min, which increased up to 2 h. By use of an antibody specific for the hydroxylated form of HIF-1α (HIF-1α-OH), we confirmed that HIF-1α-OH was present at increasing amounts over the time course in the nuclear fraction (Fig. 1D).
Figure 1.
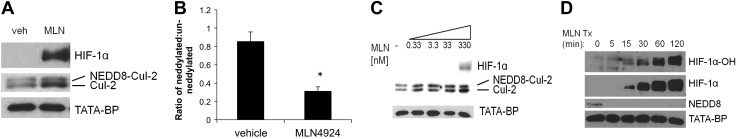
MLN4924 deneddylates Cul-2 and stabilizes HIF-1α in HeLa cells. A) Western blotting of HeLa cells treated with vehicle control (veh) or 330 nM MLN4924 for 1 h or increasing doses of MLN4924 indicates deneddylation of Cul-2 and stabilization of HIF-1α in the nuclear fraction. B) Densitometry of the ratio of neddylated:unneddylated Cul-2 protein in HeLa cells treated with vehicle control or 330 nM MLN4924 for 1 h (n = 3 independent experiments). *P < 0.01 C) Western blotting of HeLa cells treated with vehicle control (veh) or increasing doses of MLN4924 for 1 h indicates deneddylation of Cul-2 and stabilization of HIF-1α in the nuclear fraction. D) Western blotting of HeLa cells with 330 nM MLN4924 over a time course demonstrates a loss of NEDD8 conjugated to cullin proteins at T = 5, and a stabilization of HIF-1α and HIF-1α-OH at T = 15 in the nuclear fraction. In all cases, n = 3 independent experiments.
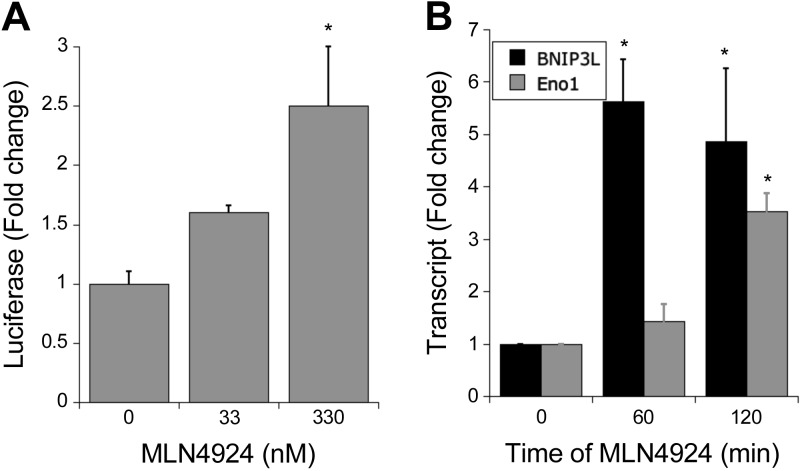
To determine whether HIF-1α stabilized by MLN4924 was functional, we performed 2 assays: an HIF luciferase reporter assay and PCR on HIF target genes. HeLa cells were transfected with a plasmid containing 4 HREs upstream of a firefly luciferase gene. The results from the luciferase reporter assay showed that exposure to MLN4924 (330 nM for 6 h) increased luciferase activity, which titrated out with a 10-fold lower concentration of MLN4924 (Fig. 2A). We also tested the influence of HIF-1α stabilization on HIF target genes. After treatment of HeLa cells with MLN4924 (330 nM, 1 and 2 h), mRNA was extracted, and cDNA was generated for PCR analysis. We analyzed the levels of the HIF-1 target genes BNIP3L and Eno1 and revealed a significant increase at both 1 and 2 h compared with vehicle control (Fig. 2B), suggesting that the HIF-1α protein stabilized by MLN4924 treatment is transcriptionally active.
Figure 2.
HIF-1α stabilization via MLN4924 can promote HIF target gene transcription. A) Treatment of HeLa cells transfected with an HRE-containing luciferase reporter plasmid with MLN4924 increased luciferase expression at 330 nM concentration. B) RT-PCR analysis of HeLa cells treated with 330 nM MLN4924 reveals significant up-regulation of BNIP3L and Eno1 mRNA at T = 60 and T = 120 compared to T = 0. In all cases, n = 3 independent experiments. *P < 0.05, as determined by Student’s t test. Error bars represent sem.
shRNA KD of DEN-1/Cul-2/HIF pathway enhances barrier function
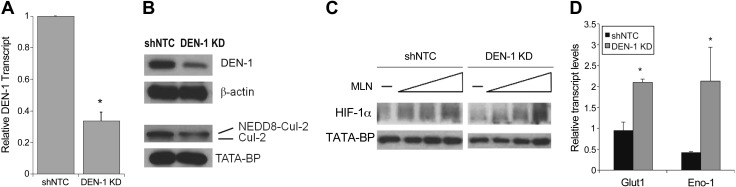
Our next approach in analyzing the role of neddylation in the mucosal inflammatory response involved investigating the contribution of DEN-1 to the neddylation pathway in human intestinal epithelial cells. We used a lentiviral shRNA construct to KD DEN-1 expression in T84 epithelial cells. As shown in Fig. 3A, PCR analysis of DEN-1 following selection revealed a 70 ± 0.05% KD (P < 0.001) compared with cells infected with a nontargeting control construct (shNTC). We confirmed KD of the DEN-1 protein by Western blot, which showed a 71% KD (Fig. 3B). Parallel analysis of Cul-2 revealed a prominent decrease in the neddylated fraction of Cul-2 in DEN-1 KD cells (43% decrease by Western blot; P < 0.001) compared with the control cells. Treatment with increasing doses of MLN4924 revealed a slightly more robust stabilization of HIF-1α in the DEN-1 KD compared with the shNTC cells, whereas a 2 h treatment with the prolyl hydroxylase domain (PHD) inhibitor AKB4924 led to an increased transcription of HIF-1α target genes Glut1 and Eno1 in the DEN-1 KD cells that was not observed in the shNTC cells (Fig. 3C and D). A cell-counting proliferation assay revealed no significant difference in the doubling time of the shNTC cells (1.15 ± 0.01 d) compared with the DEN-1 KD cells (1.15 ± 0.03 d).
Figure 3.
Lentiviral KD of DEN-1 in T84 cells. A) Lentiviral shRNA-mediated KD of DEN-1 in T84 intestinal epithelial cells results in diminished DEN-1 mRNA relative to shNTC. B) DEN-1 KD results in loss of DEN-1 protein in cytoplasmic fraction and Cul-2 neddylation in nuclear fraction (n = 3 independent experiments). C) Western blotting of T84 shNTC and DEN-1 KD cells after increasing doses of MLN4924 for 1 h demonstrating increased HIF-1α stabilization in nuclear fraction (n = 3). D) Relative mRNA expression of HIF targets Glut and Eno-1 in shNTC and DEN-1 KD cells after 2 h treatment with 10 μM AKB4924 (n = 2). *P < 0.05, as determined by Student’s t test. Error bars represent sem.
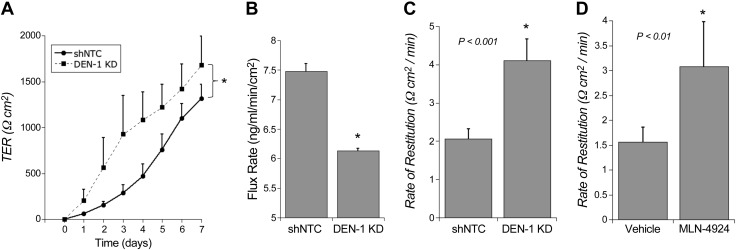
We next characterized the functional consequences of DEN-1 KD in intestinal epithelial cells. Given the role of HIF in the regulation of epithelial barrier development and maintenance (26), we examined barrier function in DEN-1 KD cells. Initially, we examined barrier development. After plating both shNTC and DEN-1 KD cells onto permeable supports, we observed that the DEN-1 KD cells showed increased kinetics of barrier formation over time compared to controls as measured by TER (Fig. 4A). When these cells were tested at various time points over the course of a week, we consistently observed a lower flux rate for the DEN-1 KD cells compared to the shNTC cells, as measured by a FITC-dextran flux assay. As shown in Fig. 4B, significant differences in paracellular permeability were observed using 3 kDa molecular size (Stokes radius 17 Å; P < 0.05) FITC-dextran. These results indicate that the lack of DEN-1 in the cells is attributed to enhanced barrier development.
Figure 4.
Inhibition of the neddylation pathway enhances barrier function of intestinal epithelial cells. A) TER measurements reveal a significant increase in transcellular barrier kinetics over time in DEN-1 KD T84 cells compared to shNTC. B) FITC-dextran flux assay of DEN-1 KD T84 cells reveals significant decrease in paracellular permeability compared to shNTC on day 3 of barrier formation. C) shNTC and DEN-1 KD T84 cells were subjected to Ca2+ switch, and TER was monitored over time. D) T84 cells were subjected to Ca2+ switch, recovered in vehicle- or 330 nM MLN4924-containing buffers, and TER was monitored over time. In all cases, n = 3 independent experiments. *P < 0.05, unless otherwise indicated, as determined by Student’s t test. Error bars represent sem.
We next used the Ca2+switch assay to assess the ability of the DEN-1 KD cells to recover from barrier disruption. The DEN-1 KD and shNTC cells were grown to confluence on transwells, equilibrated in HBSS+, and then placed into HBSS− containing 2 mM EDTA to disrupt the barrier by depleting extracellular Ca2+. TER was then monitored following Ca2+ repletion. As shown in Fig. 4C, these studies revealed that the rate of epithelial restitution was 2.0 ± 0.4-fold increased in DEN-1 KD cells compared with shNTC (P < 0.001), suggesting that inhibition of the neddylation pathway in intestinal epithelial cells promotes restitution. As a corollary, we also demonstrated that pharmacologic inhibition of neddylation using MLN4924 resulted in a nearly identical increase in barrier restitution (1.9 ± 0.6-fold increase over vehicle controls; P < 0.01; Fig. 4D). Taken together, these results indicate that cullin neddylation contributes to barrier formation in intestinal epithelial cells.
MLN4924 treatment abrogates mucosal inflammation
We next turned our attention to cullin neddylation in vivo. Reagents to define specific aspects of NEDD8/cullin conjugation pathways in vivo have been limiting due to the embryonic lethality of many of the knockout mouse lines. Thus, we used a pharmacologic approach by examining the influence of MLN4924 on disease activity in murine DSS-colitis. Mice were pretreated on day −1 with MLN4924 at a dose of 0.1 mg/kg in 15% cyclodextrin in water, or vehicle consisting of 15% cyclodextrin alone in water (n = 10 mice per group), and this treatment continued daily for the duration of the experiment. On day 0, the MLN4924 and vehicle groups were subdivided, and 5 mice from each group received either tap water or 2.5% DSS in tap water. Weights were monitored daily, and DSS was withdrawn on day 8. Mice were allowed to recover and were sacrificed on day 12. As shown in Fig. 5A, mice receiving water and MLN4924 or vehicle maintained their weights, and there was no significant difference in weight percentage between these 2 groups. However, in the DSS-treated mice, the mice receiving MLN4924 did not lose as great a percentage of weight compared with the vehicle-treated mice. The MLN4924-treated DSS mice also started to recover significantly faster and to a greater extent compared to the vehicle-treated DSS mice.
Figure 5.
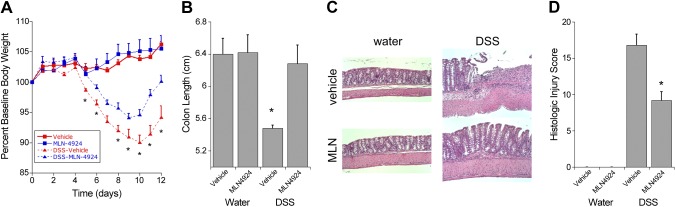
MLN4924 attenuates inflammation in a mouse model of colitis. A) Weight loss curve for C57BL/6 mice receiving water or 2.5% DSS. Results indicate that pretreatment with 0.1 MLN4924 mg/kg per day significantly decreases weight loss and increases weight recovery (n = 5 mice per group). B) After euthanasia, colon length measurements indicate that MLN4924 treatment in mice receiving DSS significantly prevented colon shortening compared to vehicle-treated DSS mice. C) Representative H&E colon sections from each of the 4 treatment groups. D) MLN4924 treatment in mice receiving DSS significantly decreased the histologic injury score compared to vehicle-treated DSS mice. Results are presented as the mean ± sem. *P < 0.05.
These results corresponded with the observed colon lengths of the mice after being killed because the colon length shortening was prevented in MLN4924-treated DSS mice compared with vehicle-treated DSS mice, which had significantly shorter colons compared to all the other groups (Fig. 5B). Although both groups of DSS-treated mice displayed the typical crypt lengthening observed in a colitis model, the MLN4924-treated mice maintained more crypt architecture compared to the vehicle-treated mice (Fig. 5C). These results were reflected in the histologic injury score because the MLN4924-treated DSS mice had a significantly lower injury score compared to the vehicle-treated DSS mice (Fig. 5D). These findings suggest that inhibition of neddylation in a mouse model of colitis can protect the mucosal epithelium from inflammation-induced damage. Further studies are needed to fully explore the mechanism of this protection. From such results, we propose that decreased Cul-2 neddylation, and resultant HIF-1α stabilization, promotes inflammatory resolution during ongoing mucosal inflammation.
DISCUSSION
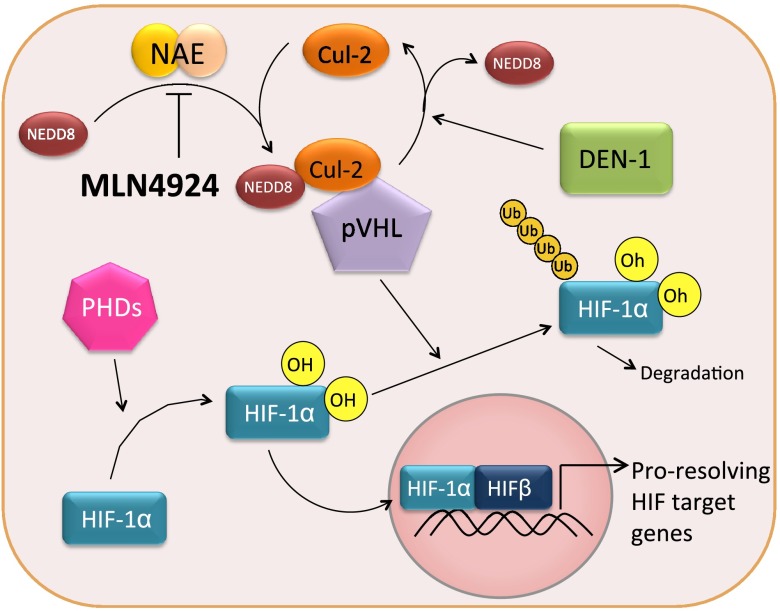
Inflammatory responses in diseases such as IBD are regulated by transcription factors (e.g., NF-κB and HIF) that are post-translationally modified to control the kinetics of their expression, primarily through proteasomal degradation pathways (2). Cullin proteins, as components of ubiquitin E3 ligases, are neddylated for the polyubiquitination of effectors (e.g., IκB and HIF-α) (Fig. 6). This neddylation reaction is regulated, in part, by the deneddylase DEN-1, as a mechanism to control E3 ligase activity.
Figure 6.
Neddylation and the HIF pathway. Under normoxic conditions, HIF-1α is hydroxylated by PHDs. In its hydroxylated form, HIF-1α is recognized by the Cul-2-Nedd8-pVHL complex, leading to polyubiquitination and degradation by the proteasome. Pharmacologic inhibition of Cul-2 neddylation using MLN4924 stabilizes cellular HIF-1α levels, leading to increased transcription of proresolving HIF target genes and increased barrier function. Loss of DEN-1 also positively influences barrier function of intestinal epithelial cells.
We have only recently begun to understand the role of neddylation and cullin regulation in the inflammatory response. Previous work has focused on the polyubiquitylation activity of E3 ligases in processing the p105 precursor of the p50 subunit of NF-κB and how inhibiting this activity limits inflammatory responses (8). Recent work has demonstrated that pharmacologically targeting neddylation via MLN4924 significantly abrogated NF-κB responses and reduced secretion of TNF-α-elicited proinflammatory cytokines in human microvascular endothelial cells (19). Mice treated with MLN4924 for 6 h prior to treatment with the proinflammatory stimulator, LPS, did not exhibit an increase in proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, whereas increased levels of the anti-inflammatory cytokine, IL-10, were maintained.
Environmental signals can also influence cullin neddylation. For example, commensal bacteria can alter the status of Cul-1 neddylation through soluble bacterial fermentation products, such as butyrate (10). Viable bacteria and physiologic concentrations of butyrate induce reactive oxygen species that alter the redox balance and inactivate the NEDD8-conjugating enzyme Ubc12. This inactivation leads to deneddylation of Cul-1, stabilizing IκB-α, and suppression of NF-κB activity. Recent work has shown that supplementation with tributyrin, a modified form of butyrate, in animal models of colitis is protective against intestinal injury and inflammation, further supporting the notion that inhibition of the neddylation pathway may be beneficial in mucosal models of inflammation (27, 28).
Far less is known about neddylation targets other than NF-κB. Because a parallel pathway involving Cul-2 and HIF exists (15, 29), in the present work, we focused on cullin regulation of HIF. A notably strong association has been established between tissue hypoxia and HIF stabilization in mucosal inflammation (30, 31). Multiple studies have shown that pathways driven by HIF protect epithelial barrier and that pharmacologic stabilization of HIF is protective (30, 31). Our studies here demonstrate that MLN4924 is a particularly potent HIF stabilizer. Indeed, whereas micromolar concentrations of direct PHD inhibitors are required for in vitro HIF stabilization (32), nanomolar concentrations of MLN4924 were shown to rapidly stabilize HIF in epithelial cells. A role of DEN-1 was revealed through stable KD approaches. Indeed, KD of DEN-1 expression likely decreases the amount of NEDD8 precursor cleavage into mature forms, thereby inhibiting its conjugation to target cullin proteins and leading to the functional phenotypes observed here. A prominent functional endpoint was epithelial barrier function. Both barrier formation and the rate of epithelial restitution were significantly enhanced by DEN-1 loss of function and MLN4924 exposure. Given previous observations that adenosine actively promotes epithelial restitution (33) and in parallel deneddylates cullin proteins (11), it is likely that adenosine functions on epithelial barrier are, at least in part, mediated by regulation of the activation state of DEN-1.
The therapeutic potential of low-dose MLN4924 was prominently highlighted in a mouse model of colitis. Multiple studies have shown that agents that stabilize HIF (especially PHD inhibitors) hold great promise in promoting the resolution of ongoing mucosal inflammation (30). Our results here support these studies and indicate that pretreatment of mice with low-dose MLN4924 (0.1 mg/kg per day) significantly decreases disease severity resulting from DSS colitis in wild-type C57/BL6 mice. Whether DEN-1 represents a viable therapeutic target for intestinal inflammation is not known at the present time. It is notable that the HIF-1 neddylation partner Cul-2 represents a rare coding variant in genome-wide association studies of patients with both CD (34) and UC (35). This observation has been more recently validated in an independent cohort of 350 patients with CD (36). Further studies with this variant will be necessary to determine disease relevance.
In conclusion, we demonstrate a relative importance for DEN-1 at multiple functional levels within the mucosa. In particular, these studies identify MLN4924 as a potent inhibitor of Cul-2 neddylation and a prominent stabilizer of HIF-1α in epithelial cells. Loss-of-function analysis supports a role for DEN-1 in enhanced epithelial barrier function in vitro, and pharmacologic inhibition of neddylation in vivo decreases inflammation and mucosal disease severity.
Acknowledgments
This work was supported by U.S. National Institutes of Health Grants DK50189, HL60569, and DK95491 and by grants from the Crohn’s and Colitis Foundation of America. The authors declare no conflicts of interest.
Glossary
- CD
Crohn’s disease
- DEN-1
human deneddylase-1
- Cul-1
Cullin-1
- Cul-2
Cullin-2
- DSS
dextran sulfate sodium
- Eno1
α-enolase
- Glut1
glucose transporter 1
- H&E
hematoxylin and eosin
- HIF
hypoxia-inducible factor
- HRE
hypoxia response element
- IBD
inflammatory bowel disease
- KD
knockdown
- NAE
NEDD8 activating enzyme
- NEDD8
neural precursor cell expressed, developmentally down-regulated 8
- PHD
prolyl hydroxylase domain
- pVHL
von Hippel-Lindau protein
- shNTC
short hairpin nontargeting control
- shRNA
short hairpin RNA
- TER
transepithelial resistance
- UC
ulcerative colitis
REFERENCES
- 1.Xavier R. J., Podolsky D. K. (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 [DOI] [PubMed] [Google Scholar]
- 2.Ehrentraut S. F., Colgan S. P. (2012) Implications of protein post-translational modifications in IBD. Inflamm. Bowel Dis. 18, 1378–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xirodimas D. P. (2008) Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem. Soc. Trans. 36, 802–806 [DOI] [PubMed] [Google Scholar]
- 4.Mikus P., Zundel W. (2005) COPing with hypoxia. Semin. Cell Dev. Biol. 16, 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read M. A., Brownell J. E., Gladysheva T. B., Hottelet M., Parent L. A., Coggins M. B., Pierce J. W., Podust V. N., Luo R. S., Chau V., Palombella V. J. (2000) Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol. Cell. Biol. 20, 2326–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liakopoulos D., Büsgen T., Brychzy A., Jentsch S., Pause A. (1999) Conjugation of the ubiquitin-like protein NEDD8 to cullin-2 is linked to von Hippel-Lindau tumor suppressor function. Proc. Natl. Acad. Sci. USA 96, 5510–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada H., Yeh E. T., Kamitani T. (1999) Identification of NEDD8-conjugation site in human cullin-2. Biochem. Biophys. Res. Commun. 257, 100–105 [DOI] [PubMed] [Google Scholar]
- 8.Amir R. E., Iwai K., Ciechanover A. (2002) The NEDD8 pathway is essential for SCF(beta -TrCP)-mediated ubiquitination and processing of the NF-kappa B precursor p105. J. Biol. Chem. 277, 23253–23259 [DOI] [PubMed] [Google Scholar]
- 9.Collier-Hyams L. S., Sloane V., Batten B. C., Neish A. S. (2005) Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J. Immunol. 175, 4194–4198 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Wu H., Collier-Hyams L. S., Kwon Y. M., Hanson J. M., Neish A. S. (2009) The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J. Immunol. 182, 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury J., Ibla J. C., Neish A. S., Colgan S. P. (2007) Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J. Clin. Invest. 117, 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 13.Sufan R. I., Ohh M. (2006) Role of the NEDD8 modification of Cul2 in the sequential activation of ECV complex. Neoplasia 8, 956–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgan S. P., Taylor C. T. (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacManus C. F., Campbell E. L., Keely S., Burgess A., Kominsky D. J., Colgan S. P. (2011) Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J. 25, 1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza H. M., Shen L. N., Botting C., Lewis A., Chen J., Ink B., Hay R. T. (2003) NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J. Biol. Chem. 278, 25637–25643 [DOI] [PubMed] [Google Scholar]
- 17.Gan-Erdene T., Nagamalleswari K., Yin L., Wu K., Pan Z. Q., Wilkinson K. D. (2003) Identification and characterization of DEN1, a deneddylase of the ULP family. J. Biol. Chem. 278, 28892–28900 [DOI] [PubMed] [Google Scholar]
- 18.Wu K., Yamoah K., Dolios G., Gan-Erdene T., Tan P., Chen A., Lee C. G., Wei N., Wilkinson K. D., Wang R., Pan Z. Q. (2003) DEN1 is a dual function protease capable of processing the C terminus of Nedd8 and deconjugating hyper-neddylated CUL1. J. Biol. Chem. 278, 28882–28891 [DOI] [PubMed] [Google Scholar]
- 19.Ehrentraut S. F., Kominsky D. J., Glover L. E., Campbell E. L., Kelly C. J., Bowers B. E., Bayless A. J., Colgan S. P. (2013) Central role for endothelial human deneddylase-1/SENP8 in fine-tuning the vascular inflammatory response. J. Immunol. 190, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soucy T. A., Smith P. G., Milhollen M. A., Berger A. J., Gavin J. M., Adhikari S., Brownell J. E., Burke K. E., Cardin D. P., Critchley S., Cullis C. A., Doucette A., Garnsey J. J., Gaulin J. L., Gershman R. E., Lublinsky A. R., McDonald A., Mizutani H., Narayanan U., Olhava E. J., Peluso S., Rezaei M., Sintchak M. D., Talreja T., Thomas M. P., Traore T., Vyskocil S., Weatherhead G. S., Yu J., Zhang J., Dick L. R., Claiborne C. F., Rolfe M., Bolen J. B., Langston S. P. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 [DOI] [PubMed] [Google Scholar]
- 21.Boh B. K., Smith P. G., Hagen T. (2011) Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J. Mol. Biol. 409, 136–145 [DOI] [PubMed] [Google Scholar]
- 22.Brownell J. E., Sintchak M. D., Gavin J. M., Liao H., Bruzzese F. J., Bump N. J., Soucy T. A., Milhollen M. A., Yang X., Burkhardt A. L., Ma J., Loke H. K., Lingaraj T., Wu D., Hamman K. B., Spelman J. J., Cullins C. A., Langston S. P., Vyskocil S., Sells T. B., Mallender W. D., Visiers I., Li P., Claiborne C. F., Rolfe M., Bolen J. B., Dick L. R. (2010) Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol. Cell 37, 102–111 [DOI] [PubMed] [Google Scholar]
- 23.Sheta E. A., Trout H., Gildea J. J., Harding M. A., Theodorescu D. (2001) Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene 20, 7624–7634 [DOI] [PubMed] [Google Scholar]
- 24.Furuta G. T., Turner J. R., Taylor C. T., Hershberg R. M., Comerford K., Narravula S., Podolsky D. K., Colgan S. P. (2001) Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieleman L. A., Palmen M. J., Akol H., Bloemena E., Peña A. S., Meuwissen S. G., Van Rees E. P. (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover L. E., Bowers B. E., Saeedi B., Ehrentraut S. F., Campbell E. L., Bayless A. J., Dobrinskikh E., Kendrick A. A., Kelly C. J., Burgess A., Miller L., Kominsky D. J., Jedlicka P., Colgan S. P. (2013) Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl. Acad. Sci. USA 110, 19820–19825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou Y., Wang L., Yi D., Ding B., Chen X., Wang Q., Zhu H., Liu Y., Yin Y., Gong J., Wu G. (2014) Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br. J. Nutr. 111, 1748–1758 [DOI] [PubMed] [Google Scholar]
- 28.Leonel A. J., Teixeira L. G., Oliveira R. P., Santiago A. F., Batista N. V., Ferreira T. R., Santos R. C., Cardoso V. N., Cara D. C., Faria A. M., Alvarez-Leite J. (2013) Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br. J. Nutr. 109, 1396–1407 [DOI] [PubMed] [Google Scholar]
- 29.Kamura T., Maenaka K., Kotoshiba S., Matsumoto M., Kohda D., Conaway R. C., Conaway J. W., Nakayama K. I. (2004) VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 18, 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colgan S. P., Taylor C. T. (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover L. E., Colgan S. P. (2011) Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology 140, 1748–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keely S., Campbell E. L., Baird A. W., Hansbro P. M., Shalwitz R. A., Kotsakis A., McNamee E. N., Eltzschig H. K., Kominsky D. J., Colgan S. P. (2014) Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 7, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colgan S. P., Eltzschig H. K. (2012) Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 74, 153–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franke A., McGovern D. P., Barrett J. C., Wang K., Radford-Smith G. L., Ahmad T., Lees C. W., Balschun T., Lee J., Roberts R., Anderson C. A., Bis J. C., Bumpstead S., Ellinghaus D., Festen E. M., Georges M., Green T., Haritunians T., Jostins L., Latiano A., Matthew C. G., Montgomery G. W., Prescott N. J., Raychaudhuri S., Rotter J. I., Schumm P., Sharma Y., Simms L. A., Taylor K. D., Whiteman D., Wijmenga C., Baldassano R. N., Barclay M., Bayless T. M., Brand S., Brüning C., Cohen A., Colombel J. F., Cottone M., Stronati L., Denson T., De Vos M., D’Inca R., Dubinsky M., Edwards C., Florin T., Franchimont D., Gearry R., Glas J., Van Gossum A., Guthery S. L., Halfvarson J., Verspaget H. W., Hugot J. P., Karban A., Laukens D., Lawrance I., Lemann M., Levine A., Libioulle C., Louis E., Mowat C., Newman W., Panés J., Phillips A., Proctor D. D., Regueiro M., Russell R., Rutgeerts P., Sanderson J., Sans M., Seibold F., Steinhart A. H., Stokkers P. C., Torkvist L., Kullak-Ublick G., Wilson D., Walters T., Targan S. R., Brant S. R., Rioux J. D., D’Amato M., Weersma R. K., Kugathasan S., Griffiths A. M., Mansfield J. C., Vermeire S., Dueer R. H., Silverberg M. S., Satsangi J., Schreiber S., Cho J. H., Annese V., Hakonarson H., Daly M. J., Parkes M. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGovern D. P., Gardet A., Törkvist L., Goyette P., Essers J., Taylor K. D., Neale B. M., Ong R. T., Lagacé C., Li C.,Green T., Stevens C. R., Beauchamp C., Fleshner P. R., Carlson M., D’Amato M., Halfvarson J., Hibberd M. L., Lordal M., Padyukov L., Andriulli A., Colombo E., Latiano A., Palmieri O., Bernard E. J., Deslandres C., Hommes D. W., de Jong D. J., Stokkers P. C., Weersma R. K., Sharma Y., Silverberg M. S., Cho J. H., Wu J., Roeder K., Brant S. R., Schumm L. P., Duerr R. H., Dubinsky M. C., Glazer N. L., Haritunians T., Ippoliti A., Melmed G. Y., Siscovick D. S., Vasiliauskas E. A., Targan S. R., Annese V., Wijmenga C., Pettersson S., Rotter J. I., Xavier R. J., Daly M. J., Rioux J. D., Seielstad M. (2010) Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 42, 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas M. A., Beaudoin M., Gardet A., Stevens C., Sharma Y., Zhang C. K., Boucher G., Ripke S., Ellinghaus D., Burtt N., Fennell T., Kirby A., Latiano A., Goyette P., Green T., Halfvarson J., Haritunians T., Korn J. M., Kuruvilla F., Lagace C., Neale B., Lo K. S., Schumm P., Torkvist L., Dubinsky M. C., Brant S. R., Silverberg M. S., Duerr R. H., Altshuler D., Gabriel S., Lettre G., Franke A., D’Amato M., McGovern D. P., Cho J. H., Rioux J. D., Xavier R. J., Daly M. J. (2011) Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 43, 1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]