Abstract
A cycle of cis-to-trans isomerization of the chromophore is intrinsic to vertebrate vision where rod and cone photoreceptors mediate dim- and bright-light vision, respectively. Daylight illumination can greatly exceed the rate at which the photoproduct can be recycled back to the chromophore by the canonical visual cycle. Thus, an additional supply pathway(s) must exist to sustain cone-dependent vision. Two-photon microscopy revealed that the eyes of the zebrafish (Danio rerio) contain high levels of 11-cis-retinyl esters (11-REs) within the retinal pigment epithelium. HPLC analyses demonstrate that 11-REs are bleached by bright light and regenerated in the dark. Pharmacologic treatment with all-trans-retinylamine (Ret-NH2), a potent and specific inhibitor of the trans-to-cis reisomerization reaction of the canonical visual cycle, impeded the regeneration of 11-REs. Intervention with 11-cis-retinol restored the regeneration of 11-REs in the presence of all-trans-Ret-NH2. We used the XOPS:mCFP transgenic zebrafish line with a functional cone-only retina to directly demonstrate that this 11-RE cycle is critical to maintain vision under bright-light conditions. Thus, our analyses reveal that a dark-generated pool of 11-REs helps to supply photoreceptors with the chromophore under the varying light conditions present in natural environments.—Babino, D., Perkins, B. D., Kindermann, A., Oberhauser, V., von Lintig, J. The role of 11-cis-retinyl esters in vertebrate cone vision.
Keywords: visual cycle, retinosomes
The vertebrate retina accommodates 2 different types of photoreceptors with rod and cone-like morphology. The more sensitive rods offer visual perception at dim-light (scotopic) conditions, whereas cones mediate high-resolution color vision under bright-light (photopic) conditions (1). Despite this functional difference, both photoreceptors’ visual pigments use the same vitamin A-derived chromophore, 11-cis-retinal (11-RAL), to mediate phototransduction (2). This process begins when light absorption causes an 11-cis to all-trans isomerization of the protein-bound retinylidene chromophore. This conformational change triggers a G-protein-mediated signaling cascade that eventually leads to a neuronal response (3). The photoproduct all-trans-RAL (at-RAL) is then released from the protein moiety of visual pigments by hydrolysis, converted to all-trans-retinol (at-ROL), and reisomerized into 11-RAL through a multistep biochemical pathway (4, 5).
The regeneration pathway for the chromophore is termed the visual or retinoid cycle (6) and has been extensively studied in rod-dominated bovine and mice eyes (7). This canonical visual cycle involves 2 cellular compartments: the photoreceptor outer segments (POSs) and the closely associated retinal pigment epithelium (RPE). In the RPE, the light-independent reisomerization reaction of the chromophore is achieved by a 2-step enzymatic reaction (8, 9). at-ROL is converted into all-trans-retinyl esters (at-REs) by the action of lecithin retinol acyltransferase (LRAT) (10). at-REs are then processed into 11-cis-retinol (11-ROL) by a retinoid isomerase encoded by the RPE-specific 65 kDa gene (Rpe65) (11–13). This isomerohydrolase reaction is regarded as the rate-limiting step in the visual cycle (14, 15).
The response kinetic of cones significantly differs from rods. Following light flashes that generate similar membrane currents, cones recover sensitivity approximately 10-fold faster than rods (16). Moreover, the rod photoresponse is saturated at photoisomerization rates above 500 per second (17), but cones remain responsive to light at photoisomerization rates up to 1 million per second (18). This may necessitate a specific mechanism(s) that accommodates the different regeneration rates of the 2 types of photoreceptors.
A hallmark of eyes with high-resolution color vision, including human eyes, is the existence of 11-cis-retinyl esters (11-REs). Biochemical studies in chicken and ground squirrel suggest that these vitamin A derivatives are critical for cone visual pigment regeneration (19, 20). However, the role of 11-REs for cone vision is not well defined (19, 20) and lacks description in live animals with cone-rich retinas.
The eyes of the zebrafish (Danio rerio) larva are amenable for genetic and pharmacologic manipulations and have been successfully used to study various aspects of photoreceptor development (21). Similar to tetrapods, teleosts display cone and rods with different adaptive properties to varying degrees of illumination (22, 23). Previously (24, 25), we characterized key components of the canonical visual cycle of the fish, including LRAT, RPE65, and the cellular retinal binding protein (CRALBP) (26–29). We now took advantage of the fish to clarify the role of 11-REs for visual pigment regeneration under photopic conditions.
MATERIALS AND METHODS
Fish maintenance and strains
Rearing, breeding, and staging of zebrafish (Danio rerio) were performed and maintained under standard conditions at 28°C (30). The XOPS:mCFP (XM) transgenic line has been described previously (31). Wild-type zebrafish were composed of AB/TL and TU strains. The stages of the embryos in days postfertilization (dpf) were logged from time of fertilization, and larvae were used around the same time of day when experiments permitted. Embryos used for 2-photon microscopy and in situ hybridization experiments were raised in the presence of 200 μm 1-phenyl-2-thiourea (Sigma-Aldrich, St. Louis, MO, USA) in order to inhibit pigmentation. Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Trusted platform module imaging
Trusted platform module (TPM) images were obtained with a Leica TCS SP5 confocal MP system (Wetzlar, Germany) equipped with an upright DM6000 CFS stand and a tunable laser Vision S (Coherent, Santa Clara, CA, USA), Ti-Sapphire femtosecond laser. Laser light at 730 nm was focused on the sample with a 20 × 1.0 NA water-immersion Leica objective. Two-photon excited fluorescence was collected by the same lens, and after filtering excitation light by a Chroma ET680sp filter (Chroma Technology Corporation, Bellows Falls, VT, USA), the beam was directed to HyD detector (Leica) in a nondescanned manner. Emission spectra were obtained with a TCS SP5 spectrally sensitive HyD detector in a descanned configuration. For imaging structures in the intact eye in living zebrafish, laser light penetrated through the front of the eye. TPM 3-dimensional reconstructions were analyzed off-line with a Leica LAS AF 3.0.0.
Light treatments of zebrafish larvae
For all light-treatment experiments, zebrafish larvae were placed into 60 × 15-mm sized Petri dishes that were wrapped on the bottom and sides with reflective aluminum foil. Controlled illumination was achieved with a Cold-Light Haloid Lamp Unit HL-150A (AmScope, Irvine, CA, USA), and luminous emittance was measured using a Lux Light Meter 401025 (Extech Instruments, Nashua, NH, USA). Temperature readings of the water in which fish were placed were taken before, during, and after light treatments to protect against overheating. Upon the different illumination regimens, larvae were immediately collected, sacrificed on ice, and stored at −80°C prior to HPLC analysis devoid of any water. Unless otherwise noted, 100 larvae per experiment were used, and at least 3 trials were performed per experiment. Total amounts of retinoids were then averaged per total numbers of larvae used. In some experiments, larval heads were severed from the rest of the bodies, whereas others used whole bodies for retinoid analysis.
Retinylamine synthesis and treatments
Retinylamine (Ret-NH2) was synthesized as described previously (32). Prior to the experiments, Ret-NH2 was dissolved in ethanol and added to the egg water of 5 dpf larvae to obtain a final concentration of 5 μM. The incubation times of treatment with Ret-NH2 depended on the experiment and are described under Results accordingly.
HPLC analysis of retinoids of zebrafish larvae
For extraction of retinoids, zebrafish larvae were thawed on ice and subsequently transferred to a glass homogenizer in 200 μl 2 M NH2OH, 400 μl methanol was added, and the larvae were homogenized for 2 min. This mixture was left at room temperature for 10 min before being transferred to a 2 ml Eppendorf microcentrifuge tube (Hamburg, Germany). A total of 800 μl acetone was added to the homogenized solution. Then, 500 μl hexane was added and vigorously vortexed. After centrifugation at 5000 × g, the upper phase was collected. The extraction was repeated once more, and the collected organic phases were dried using a rotary SpeedVac system (Vacufuge Plus, Eppendorf, Hamburg, Germany). The dried pellet was dissolved in HPLC solvent. Retinoid analyses were performed with a normal phase Zorbax Sil (5 μm, 4.6 × 150 mm) column (Agilent Technologies, Santa Clara, CA, USA). For retinyl ester separation, a linear gradient of 0.5% ethyl acetate in hexane over 15 min followed by 20 min of 10% ethyl acetate in hexane was used with a continuous flow rate of 1.4 ml/min with detection at 325 nm. After each run, a 10 min equilibration period of 0.5% ethyl acetate in hexane was added. For molar quantification of retinoids, the HPLC was previously scaled with the pattern compounds ROL, RE (Sigma-Aldrich), and BC (Calbiochem, Darmstadt, Germany).
Immunohistochemistry
Immunostaining was performed as described previously (33).Whole larvae were submerged in paraformaldehyde [4% in PBS (pH 7)] overnight at 4°C. After rehydration into 80% HBSS, samples were cryoprotected in 20% sucrose at 4°C until larvae became completely submerged and then in 30% sucrose overnight at 4°C. Whole samples were mounted in OCT medium (Miles Scientific, Elkhart, IN, USA) and frozen on a methanol, dry ice liquid bath. Then, 10 µm sections were cut on a cryostat, mounted on gelatin-coated glass slides, and allowed to air-dry at room temperature for 2 h. After 2 washes in PBS and 2 washes in PBS with Tween 20 (PBST; 0.05%), slides were blocked in PBST containing 1% bovine serum albumin for 1 h at room temperature. Slides were then incubated in primary antibody for 1 h at room temperature in a humidified chamber. After 2 washes in PBST, slides were then incubated in the appropriate fluorescent dye-conjugated secondary antibody for 1 h at room temperature in the dark. Slides were washed 2 times in PBST, 2 times in PBS, counterstained with DAPI (Sigma-Aldrich), and mounted with Prolong Gold antifade reagent (Invitrogen, Eugene, OR, USA). Sections were examined under a Zeiss LSM 510 UVMETA confocal microscope (Jena, Germany) with an HCX Plan ×40 numerical aperture 1.4 oil-immersion objective lens. Images were acquired with Zeiss confocal software version 2.0 and later processed with Adobe Photoshop CS5.1 (Adobe Systems, San Jose, CA, USA).
The following primary antibodies and dilutions were used: RPE65 (1:100 dilution), a monoclonal antibody that recognizes the proteins RPE65a and RPE65b; Zpr1 (1:20 dilution), a monoclonal antibody that recognizes red and green cones (Zebrafish International Resource Center); and anti-rhodopsin (Rho) (1:100 dilution), a monoclonal antibody that recognizes rod POSs. Alexa Fluor 488 goat anti-mouse and 594 goat anti-mouse (Jackson ImmunoResearch, West Grove, PA, USA) secondary antibodies were all used at a 1:100 dilution.
Optokinetic response assays
Visual behavior was assayed as described in Rinner et al. (34).
RESULTS
Chemical identification and localization of 11-REs
Dark-adapted eyes of zebrafish larvae and adults contain high levels of retinyl esters (27, 35). To assess the incidence and distribution of different RE stereoisomers in 5 dpf zebrafish larvae, we performed HPLC analyses and multiphoton excitation fluorescence microscopy (MPM). Using an adjusted linear gradient (36), we achieved detection and separation of 11-REs and at-REs (Fig. 1A). The stereoisomers existed in approximately equal molar amounts in the dark-adapted larval eyes. The other major ocular retinoid was 11-RAL. Additionally, we detected small amounts of at-RAL and at-ROL. Previously (27), our laboratory established the identification of these retinoids, extracted from zebrafish larvae, by using their spectral characteristics as compared to authentic standards (Fig. 1B).
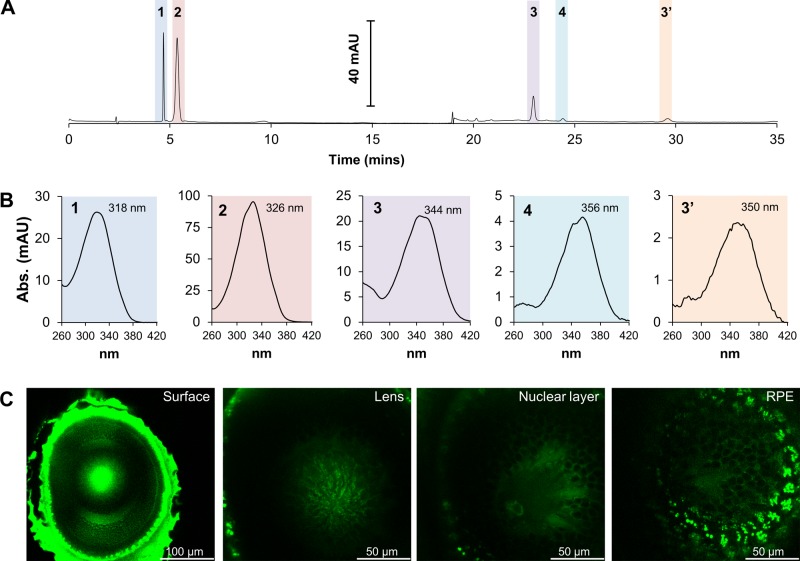
Figure 1.
11-REs localize to the RPE in zebrafish larvae. A) Representative HPLC chromatogram at 325 nm of lipophilic extracts of 5 dpf zebrafish larvae. B) Spectral characteristics of extracted retinoids from zebrafish larvae. C) In vivo 2-photon microscopy images of different layers of the zebrafish larval eye. Retinosomes were exclusively detected in the RPE (far-right image). 1, 11-REs; 2, at-REs; 3, syn 11-RAL oxime (syn 11-RAL); 3′, anti 11-RAL oxime (anti 11-RAL); 4, syn all-trans-retinal (all-RAL); Abs., absorbance; AU, absorbance units.
Once having established the presence of 11-REs within the larval eyes, we proceeded to identify the ocular cell layer to which these compounds localize. Given the minute size of the zebrafish larva, physical dissection of hundreds of eyes to separate the RPE from the retina would be difficult. This procedure, even in larger specimens, is vulnerable to a high occurrence of cross-contamination. Previously, retinosomes, clusters of REs with phospholipids and helper proteins, were visualized in live mice by using a 730 nm-femtosecond laser (37). We followed suit by subjecting zebrafish larvae eyes, in situ, to the same in vivo 2-photon fluorescence microscopy technique. Scanning across the zebrafish larval eye, from the surface of the eye to the RPE, demonstrated that retinosomes were exclusively detected in the RPE (Fig. 1C). Intense fluorescence across the surface of the eye could not be attributed to any particular chemical or anatomic feature and is most likely the result of an intrinsic autofluorescence in this region. Across the lens, some fluorescent particles are clearly visible in the images, but spectral analysis of these regions revealed that they did not concur with characteristic spectra reported earlier for retinosomes (37). Similarly, small, faint fluorescent particles in the inner nuclear layer could not be attributed to retinosomes by spectral analysis. It was only in the region of the RPE (rightmost TPM image in Fig. 1C) where subcellular structures are visible with the bright fluorescent spots indicating retinosomes. Normalized fluorescence spectrum obtained as a function of wavelength of these bright fluorescent regions (encircled in red in Fig. 2A and B) parallels spectra shown in (37) that were identified as at-REs. The slight discrepancy in the emission spectrum may be due to the mixture of 11-REs and at-REs found in zebrafish as opposed to the exclusively detectable amounts of at-REs in mice, which was the model used in the cited literature.
Figure 2.
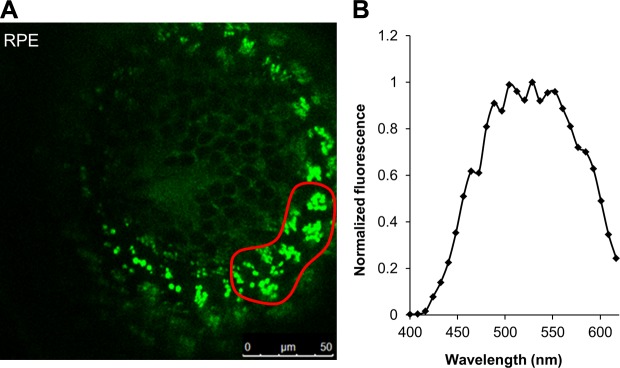
Multiphoton excitation of a 5 dpf zebrafish larval eye at 730 nm produced emission spectra indicating presence of retinosomes. A) TPM image of an intact zebrafish larva RPE at 730 nm. The fluorescent green structures are retinosomes containing 11-REs and at-REs. B) Fluorescence emission spectra from the region in the RPE encircled in red in (A), left.
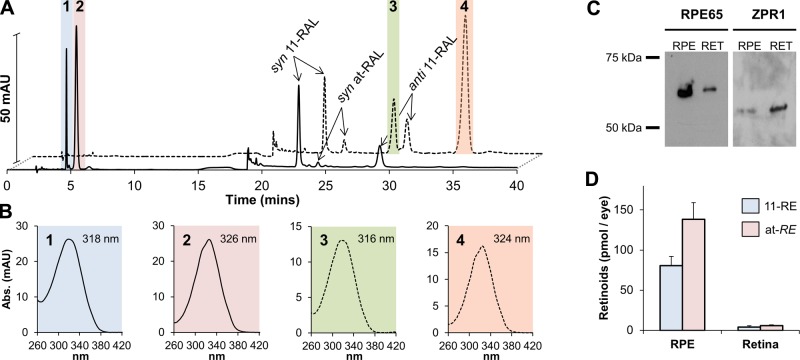
HPLC analysis of extracted retinoids from adult zebrafish eyes revealed a consistent accumulation of 11-REs and at-REs similar to larvae (Fig. 3A). A saponification of ocular retinoid extracts provided further identification of 11-REs and at-REs with their respective conversion to 11-ROL and at-ROL (Fig. 3A) as identified by their spectral characteristics (Fig. 3B). To distinguish between 11-RE and at-RE and to confirm their localization to the RPE, we dissected the eyes of adult fish into retina and RPE. Because this procedure is vulnerable to a high occurrence of cross-contamination, we performed immunoblot analysis for RPE65 and Zrp1, respectively, RPE and retina marker proteins. This staining revealed the strong enrichment of either marker protein in respective fractions (Fig. 3C). HPLC analysis with lipid extracts from isolated retina and RPE showed that 11-RE and at-RE mainly existed in the RPE fraction, thus corroborating the conclusions from the microscopic analysis of the larval eyes (Fig. 3D).
Figure 3.
11-REs localize to the RPE in adult zebrafish eyes. A) Representative HPLC chromatogram at 325 nm of control (solid line) and saponified (dashed line) lipophilic extracts of adult zebrafish eyes. B) Spectral characteristics of extracted retinoids, identified in (A), from zebrafish eyes. C) Immunoblot analysis of RPE65 and ZPR1 levels in isolated RPE and retina (RET) from adult zebrafish eyes. Contamination of each laminar marker in the noncorresponding layer indicates difficulty in separating the RPE and retinal layers. D) 11-RE and at-RE levels (mean ± sd; n ≥ 3 eyes). 11-RE levels were predominantly recorded in the RPE with minimal levels found in the retina most likely from cross-contamination from dissections. 1, 11-REs; 2, at-REs, 3, 11-ROL, 4, at-ROL.
Time- and light intensity-dependent variation of ocular 11-RE levels
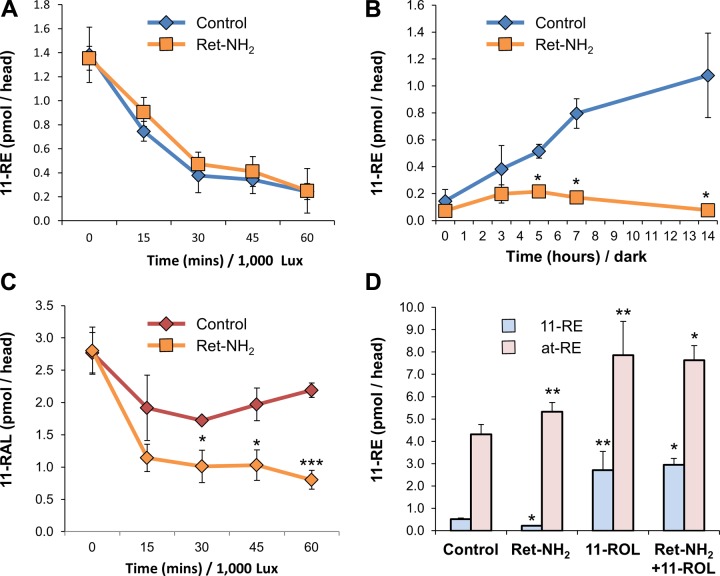
Having identified and localized 11-REs to the RPE, we set out to address the possible role that they play in larval zebrafish vision. We subjected 5 dpf fish larvae to varying light treatments in order to test 11-RE’s dependency on time and luminous emittance. Upon exposing dark-adapted animals (12 h) to a continuous illuminance of 1000 lux in time steps up to 1 h and subsequently performing HPLC retinoid analysis, we observed that the concentration of 11-REs steadily decreased over time from about 1.4 pmol per head to about 0.24 pmol per head (Fig. 4A). In comparison, average levels of 11-RAL continuously decreased for about 30 min until a steady state was achieved, at which point levels of 11-RAL were maintained above 1.7 pmol per head, and on average, levels even began to slightly increase after 30 min (Fig. 4A). In testing illuminance dependency, larvae were exposed to varying degrees of light intensity, dark adapted, 1000 lux, or 20,000 lux for a set amount of time of 2 min. For the period tested, as was observed in time-dependent experiments, levels of 11-RAL decreased as a result of photopigment bleaching (Fig. 4B). As expected, an increase in production of all-trans-retinoid isomers was correlated with light intensity (Fig. 4B). The levels of 11-REs were inversely correlated with light intensity, and an exposure to highly intense light significantly bleached the 11-RE pool.
Figure 4.
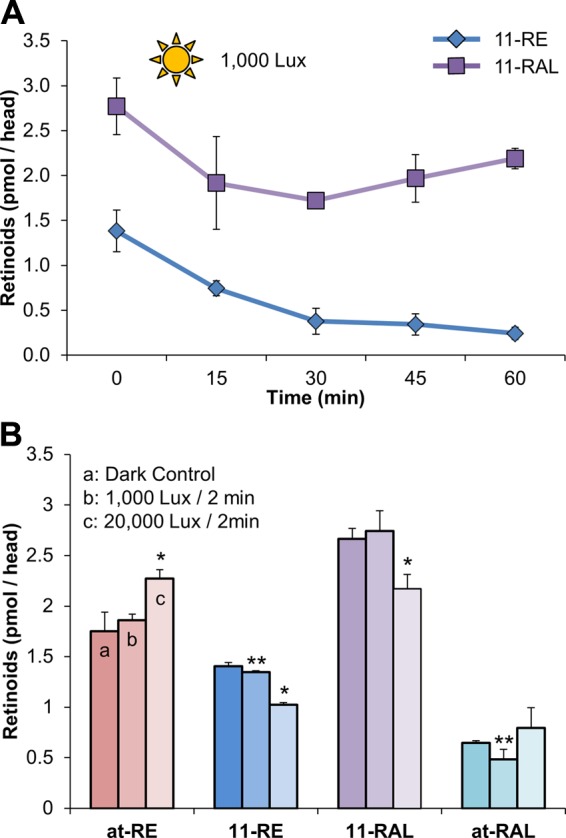
The consumption of 11-cis-RE, in wild-type zebrafish larvae, is time and light intensity dependent. A) Quantification, by HPLC, of 11-RE and 11-RAL levels of overnight dark-adapted 5 dpf zebrafish larvae when placed under light treatment (1000 lux) for 1 h. B) Retinoid profile of overnight dark-adapted, illuminated with 1000 lux for 2 min and illuminated at 20,000 lux for 2 min, 5 dpf larvae. All values given are an average (mean ± sd; n ≥ 3 experiments) of at least 3 independent experiments for each condition with 100 larvae per experiment. Statistical significance was tested by applying a 2-tailed Student’s t test to each illuminance treatment when compared to the dark control. *P < 0.003; **P < 0.03.
11-REs regenerate in the dark and are dependent on RPE65 enzyme function
Previously (27), our laboratory used Ret-NH2, a potent inhibitor of RPE65 (32), to determine the role of this enzyme in vision of 5 dpf zebrafish larvae. One particular detail that was not scrutinized during that study was the effect that RPE65 inhibition had on 11-RE levels. To determine this effect, if any, larvae were subjected to varying light treatments in the absence (control) or presence of Ret-NH2. When larvae were treated with a constant illuminance of 1000 lux in time intervals up to 1 h, in the presence of Ret-NH2, the bleaching process of 11-REs was not altered compared to control groups (Fig. 5A). To determine if RPE65 inhibition had an effect on 11-RE synthesis during dark adaptation, bleached larvae (1000 lux for 1 h) were placed in darkness for a period of 14 h, in the absence or presence of Ret-NH2, and 11-RE levels were recorded throughout (Fig. 5B). Here, we discovered that 11-RE regeneration during dark adaptation took place in control larvae but was absent in larvae treated with Ret-NH2. This finding indicated that RPE65 is required for the regeneration of 11-REs during dark adaptation. To confirm this assumption, we performed HPLC analysis on 11-RAL levels under the same conditions. Bleaching experiments demonstrated that when treated with Ret-NH2, larvae were unable to reach steady-state levels of the chromophore under constant illumination as opposed to control groups (Fig. 5C).
Figure 5.
11-RE regeneration in wild-type zebrafish occurs in the dark and is prevented by inhibition of RPE65. A) Quantification, by HPLC, of 11-RE levels of overnight dark-adapted 5 dpf wild-type zebrafish larvae when placed under light treatment (1000 lux) for 1 h in the presence and absence (control) of Ret-NH2. B) Quantification, by HPLC, of 11-RE levels of light-adapted (1000 Lux) wild-type zebrafish larvae, subsequently set to dark adapt in the presence and absence (control) of Ret-NH2. Levels were recorded throughout a 14 h period. 11-REs only regenerated in the absence of RetNH2. C) Quantification, by HPLC, of 11-RAL levels of overnight dark-adapted 5 dpf wild-type zebrafish larvae when placed under light treatment (1000 lux) for 1 h in the presence and absence (control) of RetNH2. D) Levels of 11-RE and at-RE of larvae that were light adapted for 12 h (1000 Lux) and then dark adapted for 5 h under various conditions. Control, the presence of Ret-NH2 (5 µM), 11-ROL (2 µM), and both Ret-NH2 and 11-ROL. All values given are an average (mean ± sd; n ≥ 3 experiments) of at least 3 independent experiments for each condition with 100 larvae per experiment. Statistical significance was tested by applying a 2-tailed Student’s t test to each illuminance treatment when compared to the dark control. *P < 0.008; **P < 0.05; ***P < 0.0005.
Seemingly, the data indicated that 11-RE production was achieved, at least in part, by the same molecular players of the canonical visual cycle responsible for at-RE and 11-RAL production. In order to delineate this process further, we supplemented live, Ret-NH2-treated larvae with 11-ROL, the immediate product of RPE65’s enzymatic activity. For this purpose, larvae were bleached for a period of 12 h under an illumination of 1000 lux and were then dark adapted for 5 h under 3 different conditions: in the presence of Ret-NH2, 2 µM 11-ROL, or both Ret-NH2 and 11-ROL (Fig. 5D). Similar to treatments described previously, 11-ROL was dissolved in DMSO and pipetted into Petri dishes holding the live larvae. As observed previously, larvae treated with Ret-NH2 were unable to regenerate 11-RE to control levels. Treatment with 11-ROL caused a surge in the production of both at-REs and 11-REs, with a >5-time-fold increase in 11-RE production over control larvae. The concomitant increase of both 11-RE and at-RE is likely explained by some thermal isomerization of 11-ROL in the fish water. When Ret-NH2-inhibited larvae were treated with 11-ROL, production of 11-REs achieved the same levels as with treatment with 11-ROL alone. This finding demonstrated that RPE65’s catalysis product 11-ROL is readily converted into 11-RE in the larval eyes.
The XM zebrafish transgenic line displays a functional cone-only retina
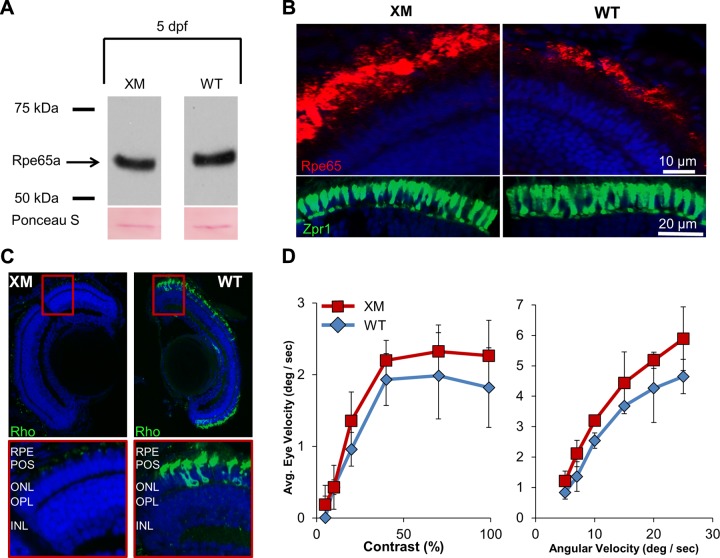
Some electroretinogram and optomotor response studies suggest that zebrafish rod photoreceptors are not functional in 5 dpf larvae (23, 38, 39). However, other studies report that cone and rod functional circuitry and photoreceptor synaptic terminals are distinguishable in the 5 dpf fish eyes (25, 40). We showed that 11-REs were synthesized and recycled in the larva eyes. To provide definite evidence that 11-RE and RPE65 are required for cone visual pigment regeneration, we used the XM transgenic zebrafish line (31). In the XM line, the cytotoxic effect of a rod-targeted fluorescent reporter gene causes degeneration of rods. To evaluate the effect of rod degeneration on general eye morphology and cone function, we examined standard histologic sections and performed optokinetic response (OKR) tests. First, we established that protein levels of RPE65 were not altered in the XM line as compared to wild-type larvae by immunoblot analysis (Fig. 6A). Immunohistochemistry for RPE65 determined an intact RPE and retinal lamination in the XM line similar to wild-type zebrafish (Fig. 6B, top panels). We then performed immunostainings against double cones (Zpr1 staining in Fig. 6B, bottom panels) and rods (Rho staining in Fig. 6C) to determine whether the distribution and/or morphology of the photoreceptors was affected in the XM line. Double-cone morphology and distribution were identical in the wild-type and XM zebrafish larvae. As expected and previously reported (31), rod photoreceptor cells were virtually undetectable at 5 dpf in the XM line (Fig. 6C). Considering that an intact morphology, despite rod degeneration, may not necessarily ensure visual function, we performed OKR tests on the XM line and wild-type to compare visual performance. Visual response of the larvae was measured in 2 ways: as eye velocity vs. angular velocity of a moving grating, and vs. the change in contrast of that grating at a set velocity (Fig. 6D). A significant difference between the XM and wild-type larvae in their OKR was not found, indicating that cone visual function was not altered due to rod photoreceptor degeneration.
Figure 6.
The XM zebrafish line is a cone-only model. A) Immunoblot analysis for RPE65 in protein extracts derived from 10 heads of 5 dpf wild-type and XM transgenic zebrafish larvae. B) Retinal cross-sections from 5 dpf larvae, XM and wild-type (WT) labeled with Rpe65 (labeling RPE65 in the RPE) in the upper panels, and Zpr1 antibodies (labeling double cones) in the lower panels. C) Immunostaining for Rho (green). D) Eye movements triggered by a wide range of stimulus speeds (left panel) and varying contrast (right panel) showed no significant differences between WT (blue) and XM (red) zebrafish.
Retinoid analysis of XM mutants
Having established that the XM zebrafish line was a functional cone-only model, we set out to determine if 11-REs are mandatory for cone photoreceptor functioning. Overnight dark-adapted 5 dpf XM larvae that were placed in time steps up to 1 h under an illumination of 2000 lux showed similar ocular retinoid dynamics as wild-type larvae. 11-RAL levels achieved a steady state after 30 min, whereas 11-RE levels continually decreased during illumination (compare Figs. 4A and 7A). Experiments with various light intensity also proved analogous because 11-RE levels decreased with increasing luminance without reaching a steady state within the period tested (Fig. 7B). Hence, the bleaching of 11-REs in the wild-type and XM models was time and intensity dependent and attributable to cone photoreceptors.
Figure 7.
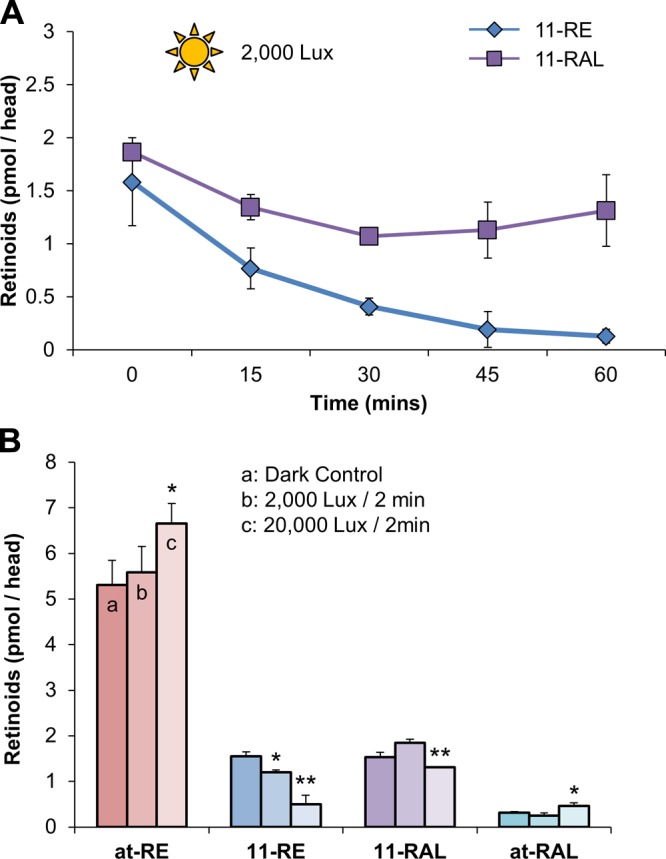
The consumption of 11-cis-RE, in the XM transgenic zebrafish larvae, is time and light intensity dependent. A) Quantification, by HPLC, of 11-cis-RE and 11-cis-RAL levels of overnight dark-adapted 5 dpf zebrafish larvae when placed under light treatment (2000 lux) for 1 h. B) Retinoid profile of overnight dark-adapted, illuminated with 2000 lux for 2 min and illuminated at 20,000 lux for 2 min, 5 dpf larvae. All values given are an average (mean ± sd; n ≥ 3 experiments) of at least 3 independent experiments for each condition with 100 larvae per experiment. Statistical significance was tested by applying a 2-tailed Student’s t test to each illuminance treatment when compared to the dark control. *P < 0.003; **P < 0.001.
Analyses of RPE65-inhibited XM eyes/Ret-NH2
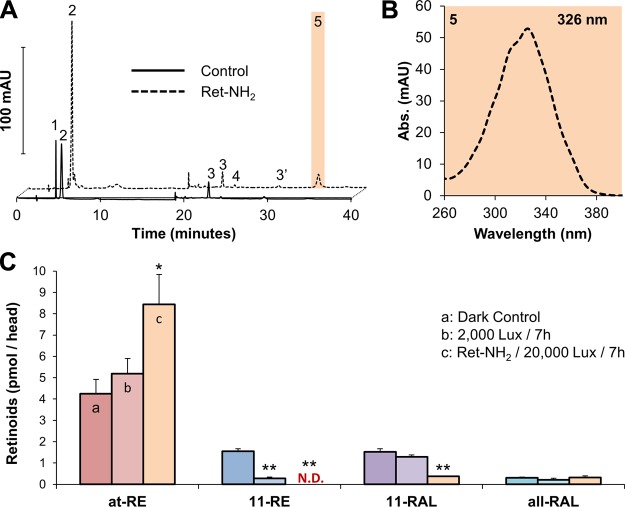
Having established that 11-REs support cone photoreceptor function, we proceeded to analyze the effect of Ret-NH2 inhibition on this process. The production of the retinylamide demonstrated that Ret-NH2 was readily absorbed by the larval eyes of XM mutants (Fig. 8A). In HPLC analyses, retinoids were then once again identified by elution time and spectral characteristics (Fig. 8A and 8B). From the previous experiment with wild-type larvae (Fig. 8B), it was surprising to find that even when exposed for an extended time period to bright light, Ret-NH2-treated larvae still maintained low levels of 11-RE and 11-RAL. This finding might be explained by several scenarios: one being that even high amounts of Ret-NH2 (5 μM) did not completely inhibit RPE65 activity. Another was that the period of light exposure for the set intensity was not sufficient to completely bleach out 11-REs and 11-RAL. Finally, the existence of an alternate, RPE65-independent pathway may account for the lack of a complete bleach of 11-REs and 11-RAL. To distinguish between these possibilities, dark-adapted larvae (12 h) were subjected to 2000 lux illuminance for 7 h in the presence or absence of Ret-NH2. In these experiments, larvae were pre-exposed to Ret-NH2 in the dark for 1 h to allow efficient binding of the inhibitor to its target. Immediately after bright-light treatments, larvae were subjected to HPLC analysis for retinoids. Under these conditions, 11-RE levels were absent in illuminated larvae treated with Ret-NH2 (Fig. 8C). A total of 2000 lux illuminance was used here, as opposed to 1000 lux used earlier, in order to increase the likelihood of bleaching out visual pigments. In comparison, 11-RE levels were significantly decreased in Ret-NH2,-untreated larvae when compared to dark-adapted control animals but were still detectable (Fig. 8C). Accordingly, 11-RAL levels also were highly reduced in RetNH2-treated larvae, whereas steady-state levels were maintained in controls (Fig. 8C). Thus, a complete bleaching of 11-RE and a nearly one of 11-RAL were achieved by inhibiting RPE65 in the cone-only retina under constant bright-light illumination.
Figure 8.
11-RE regeneration in the XM zebrafish line is prevented by inhibition of RPE65 and can be completely bleached out with continuous light treatment. A) Representative HPLC chromatograms at 325 nm of lipophilic extracts of 5 dpf zebrafish larvae in the presence and absence (control) of Ret-NH2. B) Spectral characteristic of retinylamide, metabolite of Ret-NH2. C) Retinoid profile of XM zebrafish after dark adaption for 12 h, placed in the presence or absence of Ret-NH2 under illumination (2000 Lux) for 7 h. After light treatment, 11-RE levels, in zebrafish exposed to Ret-NH2, were not detectable via HPLC analysis. All values given are an average (mean ± sd; n ≥ 3 experiments) of at least 3 independent experiments for each condition with 100 larvae per experiment. Statistical significance was tested by applying a 2-tailed Student’s t test to each illuminance and pharmacologic treatment when compared to the dark control. 1, 11-REs; 2, at-REs; 3, syn 11-RAL oxime (syn 11-RAL); 3′, anti 11-RAL oxime (anti 11-RAL); 4, syn all-trans-retinal (all-RAL); 5, retinylamide; N.D., not detected. *P < 0.013; **P < 0.0001.
DISCUSSION
Continuous vision requires an incessant regeneration of the chromophore. This is achieved through a stepwise enzyme-catalyzed chemical transformation cascade of the photoproduct, at-RAL back to 11-RAL, in a pathway referred to as the visual cycle. The canonical visual cycle has been resolved in exquisite molecular detail in rod-dominant animals such as mice and bovine (41) but lacks major inquiry in species with cone-rich retinas. Cones operate under light conditions that saturate rods, but rods still consume 11-RAL. This scenario may require a specific mechanism to avoid competition for 11-RAL between different photoreceptor types (21).
A hallmark of eyes with high-resolution color vision, including the human eyes, is the existence of relatively high levels of 11-RE, a vitamin A derivative whose role had yet to be defined in vision. How this vitamin A metabolite is synthesized and whether it can support cone vision were the questions of this study. Our analyses revealed that 11-REs existed in the RPE of dark-adapted eyes of both 5 dpf larvae and adult zebrafish. This conclusion was drawn based on the chemical and physical properties of retinoids as compared to standards as well as on mechanical and microscopic dissection of retina and RPE. The optic approach used MPM, a noninvasive imaging modality that has previously been established to monitor REs and retinoid condensation products in live mouse eye (37). In the 5 dpf larval eyes, the levels of 11-REs were comparable to that of at-REs, also known to be present in the RPE as storage pools for chromophore production. Previous studies have reported that hydrolysis of 11-RE occurs in both homogenates of human retinal epithelial cells (42) and bovine RPE (43). In the former report, the hydrolysis of the 11-cis isomer was 20 times greater than that of the all-trans isomer as measured in vitro. Because it has been reported that cones recover sensitivity approximately 10-fold faster than rods, a quicker turnover of 11-RAL would be necessary to maintain uninterrupted vision (16, 44). Thus, if the hydrolysis kinetics of 11-REs were to hold true in vivo, the faster hydrolysis of 11-REs would provide a source of the chromophore for cones under immediate and continuous bright-light conditions. Our time-dependent experiments under constant, bright illumination provided evidence of this assumption (Figs. 4A and 7A). In both wild-type and XM larvae, there was an initial bleaching of 11-RAL for a period of 30 min with an ∼1.7-fold decrease in levels. However, after this initial drop, levels of 11-RAL achieved a steady state, whereas levels of 11-REs continued to diminish to nearly undetectable levels. Seemingly, the steady-state levels of 11-RAL were achieved both by continuous recycling of the photoproduct through the canonical visual cycle and the hydrolysis of 11-REs, as seen by the inhibition by Ret-NH2 (Figs. 5C and 8C). Additionally, this process was shown to be light intensity dependent with increased illuminance (10-fold), for a constant time period, causing a bleaching of 11-REs while maintaining 11-RAL levels similar to dark control (Figs. 4B and 7B).
To begin elucidating the process by which 11-REs were generated in the zebrafish eyes, we looked to evaluate the role that molecular players within the canonical visual cycle played on 11-RE levels. In a previous report, we used gene knockdown techniques and pharmacologic means to study the role of RPE65 in zebrafish vision and found that disruption and inhibition of RPE65 led to reduced levels of 11-RAL, but we did not examine the role that RPE65 played on 11-REs (27). Here, we scrutinized this role and found that inhibition of RPE65 in 5 dpf zebrafish larvae, by Ret-NH2, prohibited regeneration of 11-REs in the dark, in both wild-type and XM zebrafish larvae that had been bleached of 11-REs (Fig. 5B). When treated in conjunction with 11-ROL, zebrafish exposed to Ret-NH2 were able to generate levels of 11-RE exceeding control amounts by almost 6-fold (Fig. 5D). The supplied 11-ROL is most likely acted upon by LRAT to produce the pools of 11-REs found in the RPE (29). 11-RE levels were only exhausted when Ret-NH2-treated XM fish were kept under constant bright light for a period of 7 h (Fig. 8C). This gives further credence that in zebrafish larvae, the enzymes of the canonical visual cycle play a major role in sustaining cone vision. This finding revises the interpretation of the data of our previous study in zebrafish (27). In larvae with partial knockdown of RPE65 and treated with Ret-NH2, some 11-RAL persisted after 20 min illumination. In light of our current study, this 11-RAL more likely stemmed from the 11-RE pool synthesized by the residual RPE65 than from an RPE65-independent pathway. Thus, like in mice (45, 46), RPE65 is indispensable for cone vision. It remains to be investigated whether RPE65-dependent synthesis of the 11-RE pool also contributes to sustained cone vision in eyes of diurnal mammals including humans.
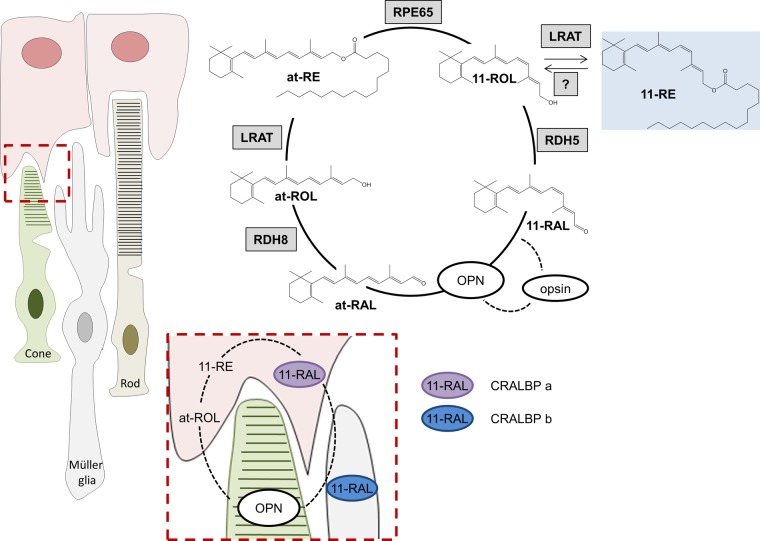
The hydrophobic nature of retinoids limits their solubility and diffusion. Thus, animals have evolved specific binding proteins for these compounds. These binding proteins protect retinoids from chemical modification and, vice versa, their surrounding from the chemical reactivity of these compounds. The role of CRALBPs as a substrate carrier that chaperones 11-RAL in the visual cycle is imperative to proper functioning of the visual cycle (47, 48). CRALBP knockout mice display a reduction in 11-RAL production and dark adaptation along with an increased accumulation of at-REs (49). In zebrafish, 2 paralogs of the cralbp gene are encoded, cralbp a and b, each expressed differentially in the RPE and to Müller glia cells, respectively (26, 50). Knockdown of either, with antisense morpholinos, produced larvae whose 11-RAL regeneration ability was diminished, and larvae targeted to reduce levels of CRALBP in Müller glia cells (Cralbp b) showed significant visual impairment. The existence of separate CRALBPs lends itself to our proposed “11-cis-retinyl ester cycle” in which 11-REs, whose production is dependent on RPE65, serve as precursors of 11-RAL for cones. This pathway would rely on all presently known molecular players of the canonical visual cycle and also rely on an unidentified 11-RE-specific light-dependent hydrolase (Fig. 9). The production of 11-RE provides a pool of chromophore precursors that can be utilized under bright-light luminance when the relatively slow RPE65-catalyzed isomerization reaction becomes rate limiting (14, 15). Under this condition, 11-REs would be hydrolyzed to produce 11-ROL, which would subsequently be oxidized by cis-stereoisomer-specific retinal dehydrogenases into 11-RAL. This newly produced 11-RAL would be protected from isomerization and helped be shuttled to cones by binding to CRALBPs (Fig. 9).
Figure 9.
Proposed 11-RE cycle in the cone-rich zebrafish retina. Within the RPE, under dark conditions, RPE65 would form 11-ROL in a canonical fashion, and subsequently, this 11-ROL would be esterified by LRAT for storage in retinosomes in the form of 11-RE. An unidentified light-dependent hydrolase would hydrolyze 11-RE into 11-RAL when demand for the chromophore would arise. The newly formed 11-RAL would be shuttled from the RPE to Müller glia cells via a CRALBP a-dependent manner and then to the cone photoreceptors via CRALBP b. In the cones, 11-RAL would bind to cone opsins to form the cone opsin pigment molecules for use in phototransduction. Recycling of the released chromophore, at-RAL, would proceed in a canonical fashion. OPN, cone opsin.
Notably, RDH5 knockout mice, which lack most of the 11-cis-RDH activity in the RPE, undergo abnormal accumulation of 11-REs and 13-cis-retinyl esters (51). Excessive amounts of 11-ROL, due to a lack of RDH5 activity, would be most likely esterified by LRAT, explaining the increased levels of 11-REs in the RDH5−/− mice despite the fact that 11-REs normally do not accumulate in this nocturnal animal. In humans, several mutations in the RDH5 gene cause a rare form of human night blindness, autosomal recessive fundus albipunctatus (52), and some patients with fundus albipunctatus develop progressive cone dystrophy (53, 54).
In summary, our finding of continued recycling of 11-REs by components of canonical visual cycle in the RPE well into adulthood clearly indicates the importance of this pathway in zebrafish vision. We showed that their bleaching process under bright-light illumination parallels a continued supply of 11-RAL in order to maintain cone vision in wild-type and a cone-only model. This mechanism elegantly and efficiently supplies cones with the chromophore under the varying light conditions present in natural environments.
Acknowledgments
The authors thank Marcin Golczak for providing Ret-NH2, Grazyna Palczewska for expert help in obtaining the TPM images presented, and Maryanne Pendergast and the Neurosciences Imaging Center for assistance with confocal microscopy. This work was supported by U.S. National Institutes of Health Grants EY019641 (to J.v.L.) and EY017037 (to B.D.P.) and a Doris and Jules Stein Professorship from the Research to Prevent Blindness (to B.D.P.). D.B. was supported by the Visual Sciences Training Program grant (T32 EY007157). The authors delare no conflict of interest.
Glossary
- 11-RAL
11-cis-retinal
- 11-RE
11-cis-retinyl ester
- 11-ROL
11-cis-retinol
- at-RAL
all-trans-RAL
- at-RE
all-trans-retinyl ester
- at-ROL
all-trans-retinol
- CRALBP
cellular retinal binding protein
- dpf
day(s) postfertilization
- LRAT
lecithin retinol acyltransferase
- MPM
multiphoton excitation fluorescence microscopy
- OKR
optokinetic response
- PBST
PBS with Tween 20
- POS
photoreceptor outer segment
- Ret-NH2
retinylamine
- Rho
rhodopsin
- RPE
retinal pigment epithelium
- TPM
trusted platform module
- XM
XOPS:mCFP
REFERENCES
- 1.Rodieck R. W. (1998) The First Steps In Seeing, Sinauer Associates, Sunderland, MA [Google Scholar]
- 2.Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns M. E., Baylor D. A. (2001) Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu. Rev. Neurosci. 24, 779–805 [DOI] [PubMed] [Google Scholar]
- 4.McBee J. K., Van Hooser J. P., Jang G. F., Palczewski K. (2001) Isomerization of 11-cis-retinoids to all-trans-retinoids in vitro and in vivo. J. Biol. Chem. 276, 48483–48493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb T. D., Pugh E. N. Jr (2004) Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23, 307–380 [DOI] [PubMed] [Google Scholar]
- 6.Wald G. (1968) The molecular basis of visual excitation. Nature 219, 800–807 [DOI] [PubMed] [Google Scholar]
- 7.von Lintig J. (2012) Metabolism of carotenoids and retinoids related to vision. J. Biol. Chem. 287, 1627–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein P. S., Law W. C., Rando R. R. (1987) Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc. Natl. Acad. Sci. USA 84, 1849–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rando R. R. (1991) Membrane phospholipids and the dark side of vision. J. Bioenerg. Biomembr. 23, 133–146 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz A., Winston A., Lim Y. H., Gilbert B. A., Rando R. R., Bok D. (1999) Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 274, 3834–3841 [DOI] [PubMed] [Google Scholar]
- 11.Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. (2005) Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. (2005) RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA 102, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. (2005) Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. USA 102, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyubarsky A. L., Savchenko A. B., Morocco S. B., Daniele L. L., Redmond T. M., Pugh E. N. Jr (2005) Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry 44, 9880–9888 [DOI] [PubMed] [Google Scholar]
- 15.Wenzel A., Oberhauser V., Pugh E. N. Jr., Lamb T. D., Grimm C., Samardzija M., Fahl E., Seeliger M. W., Remé C. E., von Lintig J. (2005) The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J. Biol. Chem. 280, 29874–29884 [DOI] [PubMed] [Google Scholar]
- 16.Perry R. J., McNaughton P. A. (1991) Response properties of cones from the retina of the tiger salamander. J. Physiol. 433, 561–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baylor D. A., Nunn B. J., Schnapf J. L. (1984) The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J. Physiol. 357, 575–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnapf J. L., Nunn B. J., Meister M., Baylor D. A. (1990) Visual transduction in cones of the monkey Macaca fascicularis. J. Physiol. 427, 681–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mata N. L., Radu R. A., Clemmons R. C., Travis G. H. (2002) Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 36, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mata N. L., Ruiz A., Radu R. A., Bui T. V., Travis G. H. (2005) Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry 44, 11715–11721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleisch V. C., Neuhauss S. C. (2010) Parallel visual cycles in the zebrafish retina. Prog. Retin. Eye Res. 29, 476–486 [DOI] [PubMed] [Google Scholar]
- 22.Malchow R. P., Yazulla S. (1986) Separation and light adaptation of rod and cone signals in the retina of the goldfish. Vision Res. 26, 1655–1666 [DOI] [PubMed] [Google Scholar]
- 23.Bilotta J., Saszik S., Sutherland S. E. (2001) Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev. Dyn. 222, 564–570 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S. C., Bleckert A., Williams P. R., Takechi M., Kawamura S., Wong R. O. (2013) Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc. Natl. Acad. Sci. USA 110, 15109–15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadool J. M. (2003) Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev. Biol. 258, 277–290 [DOI] [PubMed] [Google Scholar]
- 26.Fleisch V. C., Schonthaler H. B., von Lintig J., Neuhauss S. C. (2008) Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J. Neurosci. 28, 8208–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonthaler H. B., Lampert J. M., Isken A., Rinner O., Mader A., Gesemann M., Oberhauser V., Golczak M., Biehlmaier O., Palczewski K., Neuhauss S. C., von Lintig J. (2007) Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur. J. Neurosci. 26, 1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., von Lintig J. (2008) RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isken A., Holzschuh J., Lampert J. M., Fischer L., Oberhauser V., Palczewski K., von Lintig J. (2007) Sequestration of retinyl esters is essential for retinoid signaling in the zebrafish embryo. J. Biol. Chem. 282, 1144–1151 [DOI] [PubMed] [Google Scholar]
- 30.Westerfield M. (1994) The Zebrafish Book: A Guide For The Laboratory Use Of Zebrafish (Brachydanio rerio), M. Westerfield, Eugene, OR [Google Scholar]
- 31.Morris A. C., Schroeter E. H., Bilotta J., Wong R. O., Fadool J. M. (2005) Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Invest. Ophthalmol. Vis. Sci. 46, 4762–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golczak M., Kuksa V., Maeda T., Moise A. R., Palczewski K. (2005) Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. USA 102, 8162–8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauptmann G., Gerster T. (1994) Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 10, 266. [DOI] [PubMed] [Google Scholar]
- 34.Rinner O., Rick J. M., Neuhauss S. C. (2005) Contrast sensitivity, spatial and temporal tuning of the larval zebrafish optokinetic response. Invest. Ophthalmol. Vis. Sci. 46, 137–142 [DOI] [PubMed] [Google Scholar]
- 35.Schonthaler H. B., Lampert J. M., von Lintig J., Schwarz H., Geisler R., Neuhauss S. C. (2005) A mutation in the silver gene leads to defects in melanosome biogenesis and alterations in the visual system in the zebrafish mutant fading vision. Dev. Biol. 284, 421–436 [DOI] [PubMed] [Google Scholar]
- 36.Van Hooser J. P., Liang Y., Maeda T., Kuksa V., Jang G. F., He Y. G., Rieke F., Fong H. K., Detwiler P. B., Palczewski K. (2002) Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 277, 19173–19182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palczewska G., Maeda T., Imanishi Y., Sun W., Chen Y., Williams D. R., Piston D. W., Maeda A., Palczewski K. (2010) Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat. Med. 16, 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saszik S., Bilotta J., Givin C. M. (1999) ERG assessment of zebrafish retinal development. Vis. Neurosci. 16, 881–888 [DOI] [PubMed] [Google Scholar]
- 39.Clark D. (1981) Visual Responses in Developing Zebrafish(Brachydanio Rerio), University of Oregon, Portland, OR [Google Scholar]
- 40.Schmitt E. A., Dowling J. E. (1999) Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J. Comp. Neurol. 404, 515–536 [PubMed] [Google Scholar]
- 41.Kiser P. D., Golczak M., Palczewski K. (2014) Chemistry of the retinoid (visual) cycle. Chem. Rev. 114, 194–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaner W. S., Das S. R., Gouras P., Flood M. T. (1987) Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J. Biol. Chem. 262, 53–58 [PubMed] [Google Scholar]
- 43.Mata J. R., Mata N. L., Tsin A. T. (1998) Substrate specificity of retinyl ester hydrolase activity in retinal pigment epithelium. J. Lipid Res. 39, 604–612 [PubMed] [Google Scholar]
- 44.Baylor D. A., Lamb T. D., Yau K. W. (1979) The membrane current of single rod outer segments. J. Physiol. 288, 589–611 [PMC free article] [PubMed] [Google Scholar]
- 45.Seeliger M. W., Grimm C., Ståhlberg F., Friedburg C., Jaissle G., Zrenner E., Guo H., Remé C. E., Humphries P., Hofmann F., Biel M., Fariss R. N., Redmond T. M., Wenzel A. (2001) New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat. Genet. 29, 70–74 [DOI] [PubMed] [Google Scholar]
- 46.Wenzel A., von Lintig J., Oberhauser V., Tanimoto N., Grimm C., Seeliger M. W. (2007) RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest. Ophthalmol. Vis. Sci. 48, 534–542 [DOI] [PubMed] [Google Scholar]
- 47.Winston A., Rando R. R. (1998) Regulation of isomerohydrolase activity in the visual cycle. Biochemistry 37, 2044–2050 [DOI] [PubMed] [Google Scholar]
- 48.Stecher H., Gelb M. H., Saari J. C., Palczewski K. (1999) Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J. Biol. Chem. 274, 8577–8585 [DOI] [PubMed] [Google Scholar]
- 49.Saari J. C., Nawrot M., Kennedy B. N., Garwin G. G., Hurley J. B., Huang J., Possin D. E., Crabb J. W. (2001) Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 739–748 [DOI] [PubMed] [Google Scholar]
- 50.Schonthaler H. B., Fleisch V. C., Biehlmaier O., Makhankov Y., Rinner O., Bahadori R., Geisler R., Schwarz H., Neuhauss S. C., Dahm R. (2008) The zebrafish mutant lbk/vam6 resembles human multisystemic disorders caused by aberrant trafficking of endosomal vesicles. Development 135, 387–399 [DOI] [PubMed] [Google Scholar]
- 51.Driessen C. A., Winkens H. J., Hoffmann K., Kuhlmann L. D., Janssen B. P., Van Vugt A. H., Van Hooser J. P., Wieringa B. E., Deutman A. F., Palczewski K., Ruether K., Janssen J. J. (2000) Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol. Cell. Biol. 20, 4275–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto H., Simon A., Eriksson U., Harris E., Berson E. L., Dryja T. P. (1999) Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat. Genet. 22, 188–191 [DOI] [PubMed] [Google Scholar]
- 53.Nakamura M., Hotta Y., Tanikawa A., Terasaki H., Miyake Y. (2000) A high association with cone dystrophy in Fundus albipunctatus caused by mutations of the RDH5 gene. Invest. Ophthalmol. Vis. Sci. 41, 3925–3932 [PubMed] [Google Scholar]
- 54.Cideciyan A. V., Haeseleer F., Fariss R. N., Aleman T. S., Jang G. F., Verlinde C. L., Marmor M. F., Jacobson S. G., Palczewski K. (2000) Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis. Neurosci. 17, 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]