Abstract
This work investigated how cold stress induces the appearance of brown adipocytes (BAs) in brown and white adipose tissues (WATs) of adult mice. In interscapular brown adipose tissue (iBAT), cold exposure increased proliferation of endothelial cells and interstitial cells expressing platelet-derived growth factor receptor, α polypeptide (PDGFRα) by 3- to 4-fold. Surprisingly, brown adipogenesis and angiogenesis were largely restricted to the dorsal edge of iBAT. Although cold stress did not increase proliferation in inguinal white adipose tissue (ingWAT), the percentage of BAs, defined as multilocular adipocytes that express uncoupling protein 1, rose from undetectable to 30% of total adipocytes. To trace the origins of cold-induced BAs, we genetically tagged PDGFRα+ cells and adipocytes prior to cold exposure, using Pdgfra-Cre recombinase estrogen receptor T2 fusion protein (CreERT2) and adiponectin-CreERT2, respectively. In iBAT, cold stress triggered the proliferation and differentiation of PDGFRα+ cells into BAs. In contrast, all newly observed BAs in ingWAT (5207 out of 5207) were derived from unilocular adipocytes tagged by adiponectin-CreERT2-mediated recombination. Surgical denervation of iBAT reduced cold-induced brown adipogenesis by >85%, whereas infusion of norepinephrine (NE) mimicked the effects of cold in warm-adapted mice. NE-induced de novo brown adipogenesis in iBAT was eliminated in mice lacking β1-adrenergic receptors. These observations identify a novel tissue niche for brown adipogenesis in iBAT and further define depot-specific mechanisms of BA recruitment.—Lee, Y.-H., Petkova, A. P., Konkar, A. A., Granneman, J. G. Cellular origins of cold-induced brown adipocytes in adult mice.
Keywords: lineage tracing, adipogenesis, innervation, tyrosine hydroxylase, Myf5
Brown adipose tissue (BAT) is a specialized thermoregulatory organ that allows mammals to maintain body temperature without shivering (1–3). BAT has also been proven to be a crucial regulator of energy balance in small mammals (4–6). Importantly, the identification of functional BAT in adult humans has renewed therapeutic interest in increasing its mass and thermogenic activity to combat obesity and to improve insulin sensitivity (7–15). Chronic cold stress increases the functional activity of BAT in humans and laboratory animals (10, 16–18), yet the mechanism by which brown adipocytes (BAs) are recruited by cold is incompletely understood and controversial.
BAs exist in several anatomic locations where they vary with respect to developmental origins and phenotypic characteristics (19). For example, classic BAs compose the parenchyma of the interscapular brown adipose tissue (iBAT) and express high levels of uncoupling protein 1 (UCP1), the prototypic BA marker (1, 20). BAs within iBAT and perirenal BAT are developmentally derived from cells that once expressed myogenic factor 5 (Myf5), a myogenic regulator, suggesting a common progenitor of BAs and myocytes (20). Cold exposure or β-adrenergic stimulation also triggers the appearance of BAs, i.e., multilocular adipocytes that express UCP1, in various white adipose tissue (WAT) depots. These cells generally do not have a history of Myf5 expression (20, 21), and differences in their gene expression pattern have led some to conclude that they are a distinct phenotype (22, 23). In addition, several groups have identified progenitors in WATs of adult mice that have brown adipogenic potential in culture and following transplantation in vivo (22, 24–26). Recent in vivo lineage-tracing studies have identified a subpopulation of WAT stromal cells that express lymphocyte antigen 6 complex (Sca-1), CD34, and platelet-derived growth factor receptor, α polypeptide (PDGFRα) (PDGFRα+ cells) as a major source of inducible BAs appearing in abdominal WAT following β3-adrenergic stimulation (24). However, it is unknown whether a similar cell type gives rise to cold-inducible BAs in different anatomic locations.
Cold-induced BAT thermogenesis is largely under the control of sympathetic (noradrenergic) neuronal activity (1, 18). Furthermore, chronic cold exposure increases BAT mass by recruiting new BAs (27, 28), and this effect also requires intact sympathetic innervation. Although previous work demonstrated a role of the sympathetic nervous system in BAT hyperplasia, the identity and location of the interstitial progenitors have not been established. Furthermore, it is unclear whether cold-induced BAs have distinct origins in classic BAT and WAT depots.
Although the origins of cold-inducible BAs in adult rodents have yet to be established, at least 2 general mechanisms have been suggested: 1) de novo BA generation from progenitor cells, and 2) interconversion of mature unilocular UCP1-negative (UCP1−) adipocytes to multilocular UCP1-positive (UCP1+) BAs. We report below that cold stress rapidly induces de novo brown adipogenesis and angiogenesis in classic BAT. This neogenesis is mediated by sympathetic nerve activity and is highly restricted to the dorsal region of iBAT that interfaces interscapular white adipose tissue (iWAT). Genetic lineage tracing demonstrated that new BAs in iBAT are derived from resident PDGFRα+ progenitors. Infusion of norepinephrine (NE), acting via β1-adrenergic receptors, mimicked the adipogenic and angiogenic effects of cold in warm-adapted mice. In contrast to iBAT, PDGFRα+ progenitors are not recruited during cold-induced “browning” of subcutaneous inguinal white adipose tissue (ingWAT). Rather, genetic-tracing experiments demonstrate that essentially all multilocular BAs that appear following cold exposure in ingWAT are derived from preexisting unilocular adipocytes.
MATERIALS AND METHODS
Mice, lineage tracing, and surgical denervation
C57BL/6J [C57BL/6; stock number (no.) 000664], B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (29) (Rosa26-loxP-STOP-loxP-tdTomato; stock no. 007909), B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (Rosa26-loxP-mT-STOP-loxP-mG; stock no. 007676), and B6.129S4-Myf5tm3(cre)Sor/J (Myf5-Cre; stock no. 007893) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Pdgfra-Cre recombinase estrogen receptor T2 fusion protein (CreERT2) mice (30) were obtained from William Richardson (University College London, London, United Kingdom). Adrb1 knockout (KO) mice were obtained from Brian K. Kobilka and bred as described (31). Adipoq-CreERT2 mice were created by bacterial recombination to insert CreERT2 into the adiponectin translation start site of a bacterial artificial chromosome containing the Adipoq locus (RP24-69M4, BACPAC Resources Center, Children's Hospital Oakland Research Institute, Oakland, CA, USA), and transgenic founders were generated by standard techniques (32). The presence of the Cre-ERT2 transgene was determined by PCR using forward primer 5′-TGAAACAGGGGCAATGGTGCG-3′ and reverse primer 5′-CGGAATAGAGTATGGGGGGCTCAG-3′, which generates a 194-base pair product. All animal protocols were approved by the Institutional Animal Care and Use Committee at Wayne State University.
For cold exposure, C57BL/6 mice (3 per cage) were kept at 4°C for up to 7 d or maintained at colony room temperature (25°C) as controls. To determine proliferation marker expression, ingWAT and iBAT were collected under control conditions or after cold exposure for 1–4 d. Division of iBAT into dorsal and ventral regions was accomplished by inverting whole dissected iBAT and cutting a 2- to 3-mm-thick slice along the anterior-to-posterior axis, parallel to the ventral surface. Attached muscle and WAT were then carefully removed from the bisected iBAT samples. To mark proliferating cells cumulatively, mice were infused with 5-bromo-2′-deoxyuridine (BrdU) (20 μg/h; Sigma-Aldrich, St. Louis, MO, USA) by osmotic minipumps (ALZET Osmotic Pumps; DURECT, Cupertino, CA, USA). For 5-ethynyl-2′-deoxyuridine (EdU) labeling, mice were injected with EdU (2 µmol/mouse, i.p.; Invitrogen, Carlsbad, CA, USA) 3 times with 2 h intervals and sacrificed 6 h after the first injection. Cre recombination in Pdgfra-CreERT2/Rosa26-loxP-STOP-loxP-tdTomato was induced by administering tamoxifen [300 mg/kg in sunflower oil, orally (P.O.); Sigma-Aldrich] on each of 5 consecutive days. Cre recombination in Adipoq-CreERT2/Rosa26-loxP-STOP-loxP-tdTomato, or Adipoq-CreERT2/Rosa26-loxP-mT-STOP-loxP-mG, was induced by administering tamoxifen (100 mg/kg in sunflower oil, P.O.; Sigma-Aldrich) on each of 5 consecutive days. At 10 d after the last tamoxifen administration, mice were infused with BrdU (20 μg/h; Sigma-Aldrich) and kept at 4°C or room temperature for 7 d.
To examine the effect of sympathetic neuronal input on progenitor proliferation, unilateral surgical denervation of iBAT was performed as previously described (33). Ten days postsurgery, mice were infused with BrdU and kept at 4°C or room temperature for 7 d. For NE treatment, mice were infused with NE (1.2 nmol/g per hour, dissolved in 1 mM ascorbic acid; Sigma-Aldrich) or vehicle (1 mM ascorbic acid) by osmotic minipumps for 7 d. The mice were injected with 0.1 mg/g BrdU (in saline solution, i.p.; Sigma-Aldrich) on each of the last 4 d of NE infusion. Tissues were collected 6 h after the last BrdU injection.
Tissue processing and histology
Tissues were processed for histologic sections or whole-mount tissue and subjected to immunohistochemical analysis, as previously described (24). Five-micrometer-thick paraffin sections or 10-μm-thick cryosections of adipose tissue were analyzed for immunostaining or hematoxylin and eosin (H/E) staining. Antibodies used for immunohistochemistry in this study were the following: tdTomato (rabbit, 1:100, or mouse, 1:100; Clontech, Mountain View, CA, USA); PLIN1 [rabbit, 1:500 (34); goat, 1:200, Everest Biotech, Ramona, CA, USA); UCP1 (0.5 μg/ml rabbit; Alpha Diagnostic International, San Antonio, TX, USA); green fluorescent protein (GFP) (goat, 1:200; Genentech, South San Francisco, CA, USA); and BrdU (mouse, 1:50; Invitrogen). Isolectin IB4 Alexa Fluor conjugates (10 µg/ml; Invitrogen) were used for endothelial cell detection. Wheat germ agglutinin (WGA)-Alexa Fluor 647 conjugates (5 μg/ml; Invitrogen) were used for cell membrane staining. HCS LipidTOX Deep Red Neutral Lipid Stain (1:500; Invitrogen) was used for lipid staining. EdU was detected according to the instructions of the manufacturer. Chromogenic immunohistochemistry for BrdU detection was performed using fast red (Sigma-Aldrich), as described previously (35). Species-matched IgGs were used, or the primary antibodies were omitted as nonspecific controls for immunohistochemistry. All quantification of histologic samples was carried out as blind analyses.
Adipocytes and stromovascular cell fractionation and flow cytometry
Stromovascular cell (SVC) fractions were isolated from mouse adipose tissues as previously described (24). Paraformaldehyde (Electron Microscopy Science, Hatfield, PA, USA)-fixed SVCs were processed for EdU detection first, followed by cell surface marker staining using anti-PDGFRα (CD140a)-APC (rat, 1:200; BioLegend, San Diego, CA, USA), F4/80-PE (rat, 1:100; BioLegend), CD31-PE/Cy7 (rat, 1:300; eBioscience, San Diego, CA, USA), Sca1-FITC (rat, 1:200; BioLegend), CD45-PE/Cy7 (rat, 1:300; BioLegend), and hematopoietic lineage cocktail (CD3, Ly-6G/Ly-6C, CD11b, CD45, and TER-119; BioLegend)-Pacific Blue (rat, 1:20; BioLegend). 7-AAD (BioLegend) or DAPI (Invitrogen) was used to gate nucleated cells. Species-matched IgGs (BioLegend) were used as nonspecific controls. Cell sorting and analytic cytometry were performed using BD FACS Vantage SE SORP and BD LSR II (BD Biosciences, San Jose, CA, USA) flow cytometers, respectively. All compensation was performed using single-color controls in BD FACS DiVa (BD Biosciences) software at the time of acquisition. Raw data were processed using FlowJo software (Tree Star, Ashland, OR, USA). F4/80+ macrophages were isolated from SVCs of iBAT of control mice or cold-exposed mice by using anti-F4/80-FITC (BioLegend) and anti-FITC microbeads (Miltenyi Biotec, San Diego, CA, USA).
Western blot and gene expression analyses
Western blot and gene expression analyses were performed as described (24). Western blot was performed using primary antibodies against tyrosine hydroxylase (TH) (MAB318; EMD Millipore, Billerica, MA, USA), UCP1 (UCP11-A; Alpha Diagnostic International, San Antonio, TX, USA), α/β-tubulin (#2148; Cell Signaling, Danvers, MA, USA), and Prohibitin (ab154589; Abcam, Cambridge, MA, USA). Adrb1 cDNA was amplified using primers 5′-CTCGTCCGTCGTCTCCTTCTAC-3′ (forward) and 5′-GTCGATCTTCTTTACCTGTTTTTGG-3′ (reverse); Adrb2 with 5′-TTGCAGTGGATCGCTATGTTG-3′ (forward) and 5′-TGACCACTCGGGCCTTATTCT-3′ (reverse); Adrb3 with 5′-CCTTCAACCCGGTCATCTAC-3′ (forward) and 5′-GAAGATGGGGATCAAGCAAGC-3′ (reverse); Angiopoietin 2 (Angpt2) with 5′-CCGCGGGCAAAATAAGTAGC-3′ (forward) and 5′-CACATGCGTCAAACCACCAG-3′ (reverse); Pdgfb with 5′-GCCTGTGACTAGAAGTCCTG-3′ (forward) and 5′-GTCATGGGTGTGCTTAAACT-3′ (reverse); Pdgfa with 5′-TGTGCCCATTCGCAGGAAGAG-3′ (forward) and 5′-TTGGCCACCTTGACACTGCG-3′ (reverse); Vegfa with 5′-AAGCGCAAGAAATCCCGGTT-3′ (forward) and 5′-CTGCGGATCTTGGACAAACAAATGC-3′ (reverse); Vegfb with 5′-GGCAACACCAAGTCCGAATG-3′ (forward) and 5′-TGTCTGGCTTCACAGCACTC-3′ (reverse); and Vegfc with 5′-ACAGAAGACCGTGTGCGAAT-3′ (forward) and 5′-GGACACAGCGGCATACTTCT-3′ (reverse). All other cDNAs were amplified using primers described previously (36).
Microscopy
Fluorescence and bright-field microscopy was performed as described (37). Raw data were processed using IPLab software (Scanalytics; BD Biosciences), and RGB profiles were analyzed by ImageJ (U.S. NIH, Bethesda, MD, USA). Fiji ImageJ (38) was used to stitch overlapping images.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). Data are presented as mean ± sem. Statistical significance between 2 groups was determined by unpaired t test or Mann-Whitney U test, as appropriate. Comparison among groups was performed using 1-way ANOVA or 2-way ANOVA, with Bonferroni posttests to determine the relevant P values.
RESULTS
Cold stress recruits new BAs at the dorsal edge of iBAT that interfaces iWAT
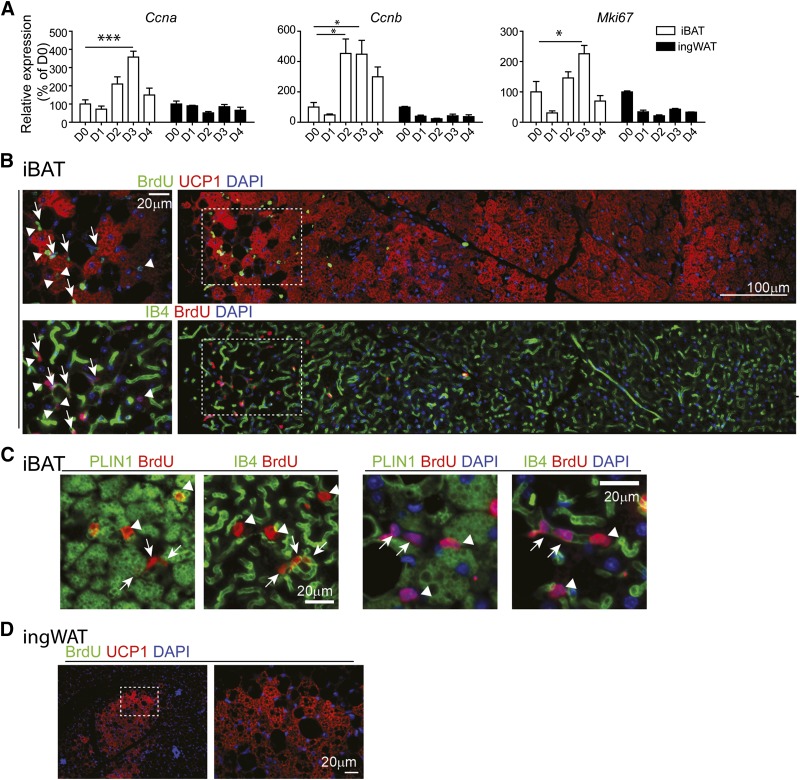
In rats, cold exposure triggers a burst of de novo brown adipogenesis in iBAT during the first 4 d of cold exposure (28, 39). To determine cold-induced mitogenic responses of iBAT and ingWAT of mice, expression levels of cell cycle genes were examined during the course of cold exposure. As expected, cold stress significantly up-regulated expression of the proliferation markers cyclin A2 (Ccna), cyclin B1 (Ccnb), and Mki67 in mouse iBAT, with levels peaking on the third day of exposure (Fig. 1A). In contrast, cold exposure did not affect expression of cell proliferation markers in ingWAT (Fig. 1A).
Figure 1.
Cold exposure induces cell proliferation of endothelial cells and recruits new BAs in BAT. A) Quantitative PCR analysis of proliferation-related genes in iBAT and ingWAT of control B6 mice and mice exposed to 4°C up to 4 d (n = 4–7 per condition; mean ± sem). *P < 0.05; ***P < 0.001. B–D) Representative images of paraffin sections of iBAT (B and C) and ingWAT (D) of mice infused with BrdU during 7 d of cold exposure. Regions of higher magnification (left panels in B and C, right panel in D) are outlined by dashed rectangles. B) Triple fluorescence staining for isolectin-IB4, UCP1, and BrdU indicates the appearance of new BAs (UCP1+BrdU+, arrowheads) and new endothelial cells (IB4+BrdU+, arrows) at the dorsal edge of iBAT, where UCP1+ and UCP1− adipocytes coexist. C) Paraffin sections of iBAT triply stained for isolectin-IB4, PLIN1, and BrdU. Left panels are the merged image of PLIN1 (green) with BrdU (red). Right panels merge images of isolectin-IB4 (green) and BrdU (red) fluorescence. Arrows indicate IB4+BrdU+ endothelial cells, whereas arrowheads indicate PLIN1+BrdU+ new adipocytes. D) Paraffin sections of ingWAT stained for BrdU and UCP1indicate that UCP1+ adipocytes in ingWAT were negative for BrdU. Nuclei were counterstained with DAPI. Scale bars, 20 or 100 µm as indicated.
We next used BrdU to tag and trace cell fate over the course of 7 d of cold exposure (Fig. 1B–D). Cold exposure triggered BrdU incorporation into both BAs and capillary endothelial cells, suggesting that adipogenesis and angiogenesis are coordinated phenomena (Fig. 1B and C). As expected, cold exposure greatly increased the number of multilocular adipocytes expressing UCP1 in ingWAT (Fig. 1D and Supplemental Fig. S1; see below); however, cold exposure did not affect BrdU incorporation in ingWAT (Fig. 1D), indicating that the mechanisms of BA recruitment differ in ingWAT and iBAT.
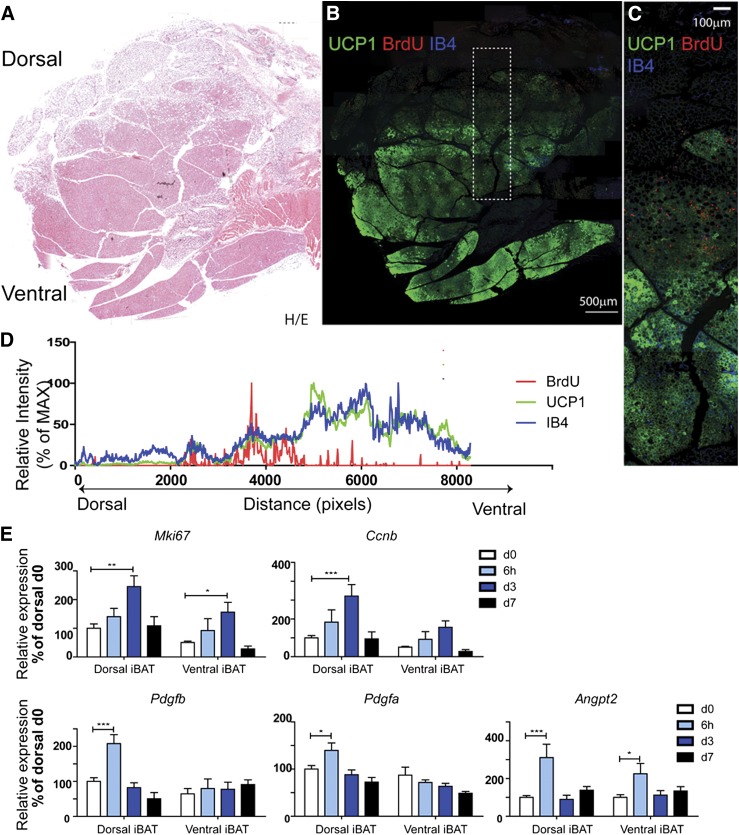
Whether cold-induced brown adipogenesis occurs in a specific region of BAT has not been previously addressed; however, we noted that most proliferation was observed in a region containing a mixture of brown and white adipocytes, and few BrdU+ BAs or endothelial cells were observed within the BAT parenchyma (Fig. 1B). To gain further insight into the histologic organization of de novo adipogenesis in iBAT, we examined proliferation along the entire dorsal/ventral axis that included iWAT and the iBAT parenchyma. The BAT parenchyma was readily distinguished by eosin staining, intense UCP1 immunofluorescence, and greater capillary density (isolectin-GS-IB4 fluorescent intensity) (Fig. 2A–C). Analysis of BrdU staining demonstrated that cell proliferation was highly concentrated near the dorsal edge of iBAT that interfaces iWAT (Fig. 2B–D). To confirm the distinct pattern of mitogenic response, expression levels of cell cycle genes were determined by quantitative PCR. As expected from the histology, induction of Mki67 and Ccnb was greatest in dorsal BAT (Fig. 2E). Cold stress rapidly induced expression of proangiogenic genes, including PDGFR ligands and Angpt2, which was prominent in dorsal, but not ventral, iBAT (Fig. 2E).
Figure 2.
Cold stress triggers cell proliferation at the dorsal edge of BAT that interfaces suprascapular WAT. A and B) Histologic analysis of BrdU incorporation in iBAT during cold exposure. Adjacent paraffin sections were stained for H/E (A), or BrdU, isolectin-IB4, and UCP1 (B). Scale bar, 500 µm. C) Magnified view of boxed region from (B). Scale bar, 100 µm. D) Analysis of fluorescence intensity of (C) indicated that cell proliferation (BrdU-Red) concentrated at the dorsal edge of BAT, where UCP1 expression and vascular density drop. MAX, maximum. E) Quantitative PCR analysis of proliferation- and angiogenesis-related gene expression in dorsal and ventral BAT of control mice and mice exposed to 4°C up to 7 d (n = 6, mean ± sem). *P < 0.05; **P < 0.01;***P < 0.001.
Cold exposure induces proliferation of PDGFRα+ cells in iBAT
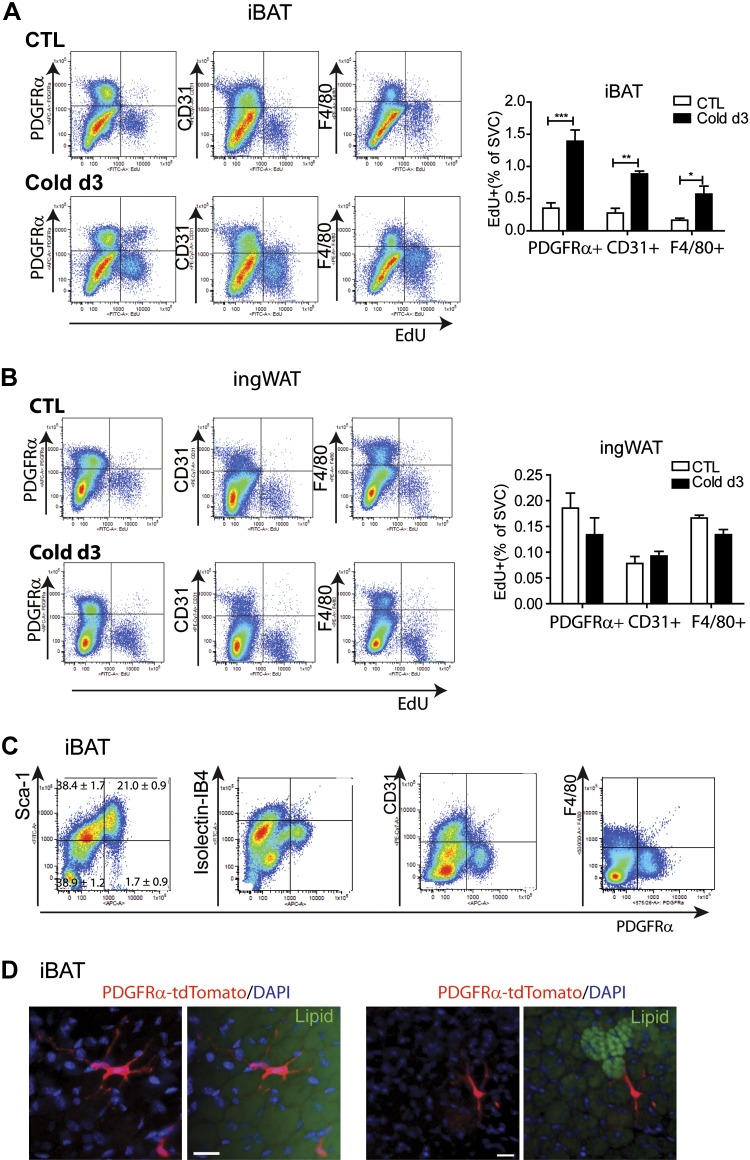
As mentioned above, brown adipogenesis in abdominal WAT involves proliferation of PDGFRα+ progenitors and resident macrophages (36, 40). To determine the cell types undergoing cold-induced proliferation, we examined EdU incorporation by flow cytometry after 3 d of cold exposure (Fig. 3 and Supplemental Fig. S2). Consistent with histologic analysis, cold exposure increased EdU incorporation in PDGFRα+ cells by 4-fold in iBAT (Fig. 3A), and these cells accounted for 60.6 ± 3.1% of proliferating nonhematopoietic cells (Supplemental Fig. S2A). Cold exposure also increased proliferation of endothelial cells and macrophages (Fig. 3A). In contrast to iBAT, cold stress did not affect proliferation of PDGFRα+, CD31+, or F4/80+ cells in WAT depots, including ingWAT, which exhibited prominent induction of UCP1 expression (Fig. 3B and Supplemental Fig. S2D–F).
Figure 3.
Effect of cold exposure on cell proliferation in various adipose depots. A and B) Flow cytometric analysis of proliferating cell populations in various adipose depots. Representative flow cytometric profile of EdU incorporation by cells expressing PDGFRα, CD31, and F4/80 along with quantification of 3–4 independent experiments (mean ± sem). *P < 0.05; **P < 0.01; ***P < 0.001. CTL, control. Cold exposure induced the highest mitogenic responses of PDGFRα+ cells and endothelial cells in iBAT, whereas cold exposure had no effect on cell proliferation in ingWAT. C) Flow cytometric analysis of cell surface marker expression in PDGFRα+ cells in SVCs obtained from iBAT. D) Representative images of PDGFRα+ cells in whole-mount iBAT from tamoxifen-induced Pdgfra-CreERT2/Rosa26-loxP-stop-loxP-tdTomato mice. Lipid (green) was stained with HCS LipidTOX. Scale bars, 20 µm.
PDGFRα+ cells in iBAT constituted 20% of SVCs and 35% of cells expressing Sca1, a marker commonly used to identify preadipocytes (Fig. 3C). The PDGFRα+ subpopulation did not express endothelial cell markers (CD31 and isolectin-GS-IB4) or hematopoietic lineage markers (CD45 and F4/80) (Fig. 3C). Thus, PDGFRα+ cells in iBAT shared a similar cell surface marker profile (Fig. 3C), dendritic morphology (Fig. 3D) (24, 36, 41), and in vitro adipogenic potential with preadipocytes isolated from WAT depots (24).
Lineage tracing demonstrates that proliferating PDGFRα+ cells give rise to BAs during cold exposure
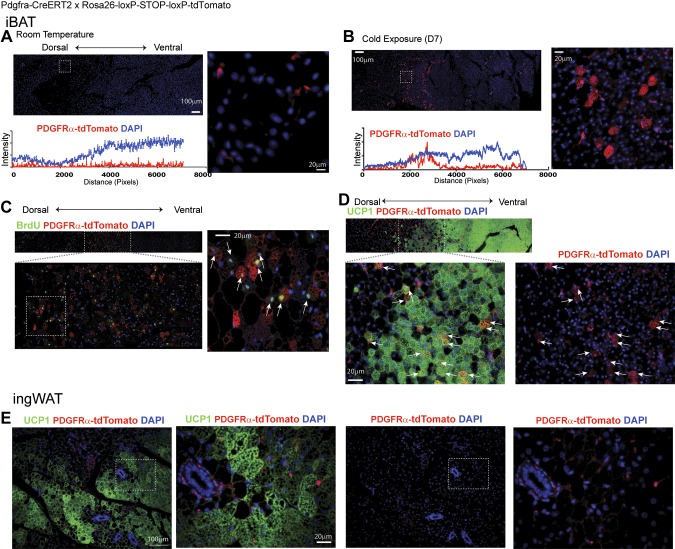
To determine the contribution of PDGFRα+ cells to BAT expansion during cold, we performed genetic lineage tracing of Pdgfra-CreERT2/tdTomato double-transgenic mice (24). tdTomato reporter expression was induced in PDGFRα+ cells with tamoxifen 10–15 d prior to cold exposure. Mice were then maintained at room temperature or exposed to cold for 7 d while infused with BrdU. In control mice, tdTomato+ progenitors were evenly distributed throughout iBAT, and tdTomato+ adipocytes were very rare (Fig. 4A). In contrast, cold exposure generated numerous tdTomato+ multilocular BAs near the dorsal boundary of iBAT (Fig. 4B–D), and 60 ± 5% of tdTomato+ BAs (n = 4, mean ± sem) had incorporated BrdU during the week of cold exposure. Immunostaining for UCP1 confirmed the BA phenotype of tdTomato+ adipocytes (Fig. 4D). Although cold exposure greatly increased the number of multilocular UCP1+ BAs in ingWAT, none was derived from PDGFRα+ progenitors (Fig. 4E). This observation, and the fact that cold stress did not induce proliferation in ingWAT, are consistent with the conclusion that emergent BAs in ingWAT are derived from postmitotic, differentiated adipocytes.
Figure 4.
Lineage tracing demonstrates that proliferating PDGFRα+ cells give rise to BAs during cold exposure. Representative images of paraffin sections of iBAT (A–D) and ingWAT (E) of Pdgfra-CreERT2/Rosa26-loxP-stop-loxP-tdTomato induced with tamoxifen and kept at room temperature (A) and 4°C (B–E) for 7 d. High-magnification images of boxed regions are provided in each panel. A) tdTomato+ staining of iBAT from mice housed at room temperature (RT) identified PDGFRα+ progenitors in iBAT. B) Cold exposure triggered the appearance of numerous tdTomato+ multilocular adipocytes near the dorsal edge of iBAT. Analysis of fluorescence intensity indicated that PDGFRα+ progenitors are evenly distributed throughout the tissue. A high-magnification view of the boxed region clearly demonstrates multilocular morphology of tdTomato+ adipocytes. C) Representative images of paraffin sections stained for BrdU and tdTomato. Arrows in high magnification indicate BrdU+tdTomato+ adipocytes. D and E) Representative images of paraffin sections stained for UCP1 and tdTomato. D) Arrows in high-magnification image indicate UCP1+tdTomato+ adipocytes in iBAT. E) UCP1+ adipocytes in ingWAT were negative for tdTomato. Nuclei were counterstained with DAPI (n = 4 per condition). Scale bars, 20 or 100 µm as indicated.
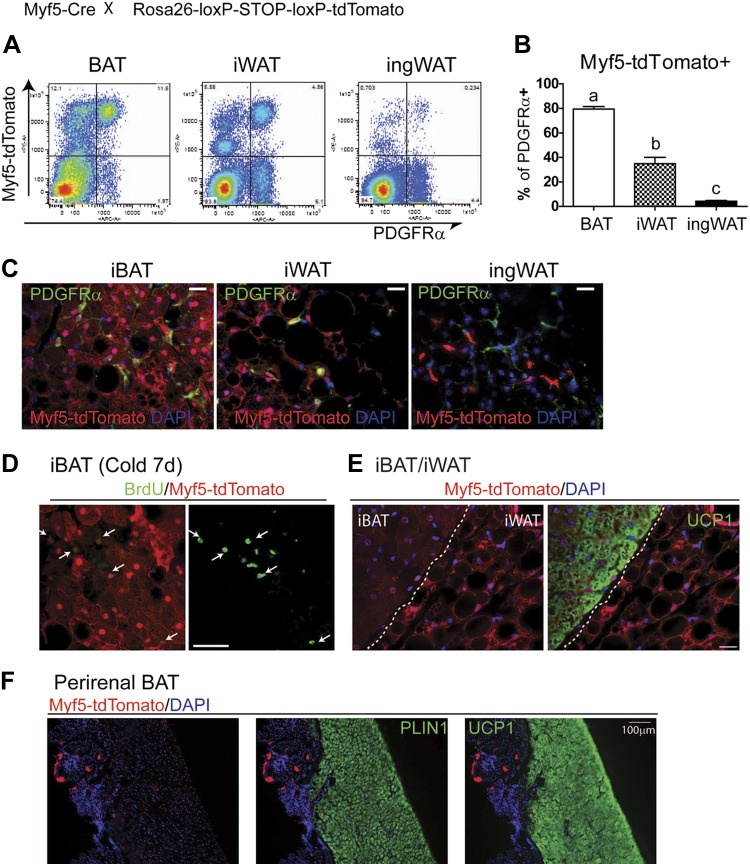
Previous work using constitutive Myf5-Cre tracing found that interscapular and perirenal BAs and myocytes are derived from cells with a history of Myf5 expression (20), and it has been proposed that Myf5 defines an immediate common developmental progenitor of myocytes and BAs, but not white adipocytes (20). We found that PDGFRα progenitors (Fig. 5A–C) and BAs generated by cold stress (BrdU+ multilocular adipocytes) (Fig. 5D) all had histories of Myf5 expression. However, the interpretation of this result is complicated by the observation that white adipocytes in adjacent iWAT also had histories of Myf5 expression (Fig. 5E), as did a substantial fraction of stromal cells that were not preadipocytes (i.e., PDGFRα−CD45− cells) (Fig. 5A). Conversely, the majority of BAs in perirenal BAT did not previously express Myf5 (Fig. 5F).
Figure 5.
Lineage tracing of Myf5-derived cells in various adipose tissue depots. A and B) Flow cytometric analysis of contribution of Myf5+ lineage-derived cells to PDGFRα expressing cells in iBAT, iWAT, and ingWAT from Myf5Cre/Rosa26-loxP-STOP-loxP-tdTomato mice (n = 3). Significant differences (P < 0.001) are indicated by different letters (a, b, and c). C) Representative images of cryosections of iBAT, iWAT, and ingWAT of Myf5Cre/Rosa26-loxP-STOP-loxP-tdTomato mice stained for PDGFRα. D and E) Cryosections of BAT and WAT from Myf5Cre/Rosa26-loxP-STOP-loxP-tdTomato mice. D) New BAs labeled by BrdU were positive for previous Myf5 expression 7 d after cold exposure. E) Uniform Myf5 reporter (tdTomato) expression in UCP1+ BAs in iBAT and UCP1− white adipocytes in adjacent iWAT. F) Absence of Myf5 reporter expression in most UCP1+ BAs in perirenal adipose tissue. Scale bars, 20 µm.
Adipocyte tracing confirms that new BAs form in iBAT, but not in ingWAT
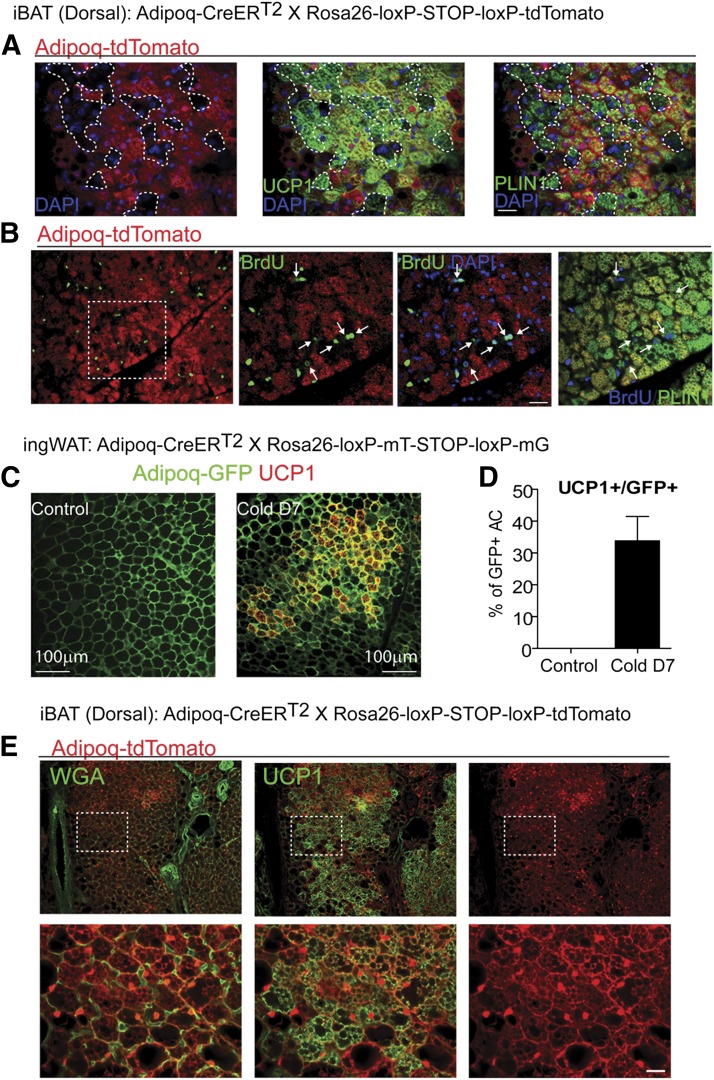
The source of cold-induced BAs in ingWAT is controversial (42–44); however, the fact that this recruitment does not involve proliferation or differentiation of known progenitors (Fig. 4E) suggested that the appearance of BAs involves reinstatement of UCP1 expression in existing unilocular white adipocytes. To test this hypothesis directly, we traced the fate of existing mature adipocytes with respect to UCP1 expression following cold stress. For this purpose, we crossed transgenic mice with tamoxifen-sensitive Cre recombinase under the control of the adiponectin promoter (Adipoq-CreERT2) with the Rosa26-loxP-stop-loxP-tdTomato or Rosa26-loxP-mT-loxP-mG reporter strains. In this system, tamoxifen induces reporter expression specifically in differentiated adipocytes. Recombination specificity was confirmed by exclusive expression of fluorescent reporter protein in adipocyte fractions and adipose tissue (Supplemental Fig. S3A), and recombination efficiency in dissociated adipocytes was 97.2 ± 1.4% (n = 3, mean ± sem) (Supplemental Fig. S3B).
In this experimental design, new BAs that are derived from progenitors will appear as tdTomato−, BrdU+ multilocular adipocytes, whereas those derived from preexisting fat cells will appear as tdTomato+, UCP1+ adipocytes. As expected, 7 d of cold exposure induced the appearance of tdTomato− BAs near the dorsal boundary of iBAT (Fig. 6A and Supplemental Fig. S3C). These newly generated adipocytes had incorporated BrdU (Fig. 6B), confirming that they came from proliferating progenitors.
Figure 6.
Tracking of mature adipocytes confirms that BA recruitment in BAT involves de novo adipogenesis, whereas new BAs in ingWAT are derived from preexisting unilocular adipocytes. A and B) Representative images of paraffin sections of iBAT of Adipoq-CreERT2/Rosa26-loxP-stop-loxP-tdTomato mice induced with tamoxifen and kept at 4°C for 7 d, stained for tdTomato, Plin1, and UCP1 (A) or BrdU (B). Numerous tdTomato− multilocular adipocytes (tdTomato−Plin1+UCP1+ and tdTomato−Plin+BrdU+) appeared at the dorsal edge of iBAT. tdTomato− adipocyte clusters are surrounded by dotted lines in (A) or indicated by arrows in (B). C) Representative images of paraffin sections of iBAT of Adipoq-CreER/Rosa26-loxP-mT- STOP-loxP-mG induced with tamoxifen and kept at room temperature or 4°C for 7 d, stained for UCP1 and GFP. D) Quantification of UCP1+ adipocytes (Control, n = 3; Cold d7, n = 5; mean ± sem). E) Representative images of paraffin sections of ingWAT of Adipoq-CreER T2/Rosa26-loxP-stop-loxP-tdTomato induced with tamoxifen and kept at room temperature or 4°C for 7 d, stained for UCP1 and tdTomato. Plasma membranes were stained with WGA. All UCP1+ adipocytes were derived from preexisting adipocytes that previously expressed adiponectin. Scale bars, 20 or 100 µm as indicated.
Under control conditions, virtually all tdTomato+ adipocytes in ingWAT were unilocular and lacked detectible UCP1 expression, and cold exposure increased the number of UCP1+ multilocular adipocytes to >30% of total adipocytes (Fig. 6C and D). To definitively identify cell phenotypes in cold-exposed mice, we colocalized the BA phenotypic marker (UCP1) with the genetic reporter (tdTomato) and defined individual cell borders with fluorescent WGA. We found that 100% of multilocular UCP1+ adipocytes were also tdTomato+ (5207 out of 5207; n = 3 mice), demonstrating that these inducible BAs were derived from preexisting unilocular adipocytes (Fig. 6D and E). This conclusion was confirmed using mTmG reporter mice in which the genetic reporter was targeted to the plasma membrane (Supplemental Fig. S3D).
Surgical denervation eliminates cold-induced de novo brown adipogenesis in iBAT
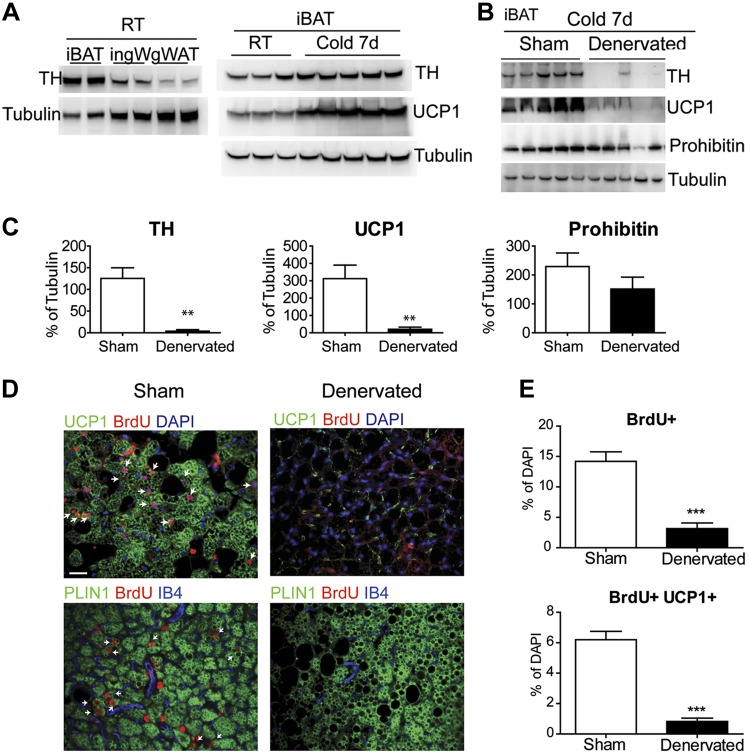
BA activity is thought to be mainly controlled by its sympathetic innervation, although it has been recently suggested that macrophages are an independent source of adrenergic control during cold stress (45). We found that the level of sympathetic innervation, assessed by TH, was much greater in iBAT compared to ingWAT and gonadal WAT, and TH levels in iBAT increased somewhat after 7 d of cold stress (Fig. 7A). To determine the potential role of sympathetic nerve activity in progenitor proliferation, we surgically denervated iBAT unilaterally and used the sham-operated contralateral pad as the intact control. As expected, denervation virtually eliminated TH protein in iBAT and abolished UCP1 induction during cold stress (Fig. 7B and C). Denervation did not affect prohibitin protein levels, indicating that mitochondrial content was largely maintained after denervation (Fig. 7B and C). Importantly, surgical denervation dramatically reduced the number of new BAs induced by cold (Fig. 7D and E). Although cold stress slightly increased macrophage proliferation (Fig. 3A), TH could not be detected in macrophages isolated from cold-exposed mice, even when collected in >10-fold excess of that present in iBAT (Supplemental Fig. S4). These results indicate that macrophages are an insignificant source of BAT TH, the rate-limiting enzyme of catecholamine biosynthesis, and that these cells cannot substitute for the sympathetic innervation in mediating cold-induced brown adipogenesis or UCP1 expression.
Figure 7.
Surgical denervation prevents cold-induced BA recruitment in iBAT. A) TH protein levels in iBAT, ingWAT, and gonadal WAT (gWAT) in mice housed at room temperature (RT). Effect of 7 day cold exposure on TH and UCP1 levels in iBAT. B) Surgical denervation of iBAT abolished TH and UCP1 induction during cold stress. RT, room temperature. C) Quantification of protein levels from (B). D) Immunofluorescence staining of paraffin sections of iBAT of mice kept at 4°C for 7 d, stained for BrdU and UCP1 or PLIN1. Arrows indicate double-positive cells. E) Quantification of BrdU+ cells in paraffin sections. Scale bar, 20 µm.
NE, acting via β1-adrenergic receptors (Adrb1), triggers de novo brown adipogenesis in warm-adapted mice
Sympathetic nerves release a mixture of catecholamine and peptide transmitters. Based on previous work in rats (27), we suspected that NE would mimic the effects of cold in warm-adapted mice. Because selective β3-adrenergic receptor agonists do not stimulate de novo adipogenesis in iBAT (24, 46) and PDGFRα progenitors express β1-adrenergic receptors, but not β3-adrenergic receptors (Supplemental Fig. S5), we felt it was likely that the effects of NE would be mediated by β1-adrenergic receptors. Indeed, β1-adrenergic receptor signaling stimulates proliferation of BAT SVCs in vitro (47, 48); however, the role of Adrb1 signaling in adipogenesis and angiogenesis has not been established in vivo. To test the effects of NE on brown adipogenesis in vivo, wild-type and Adrb1 KO mice were infused with ascorbic acid vehicle or NE for 7 d. All mice underwent unilateral surgical denervation and were coinfused with BrdU to tag proliferating cells.
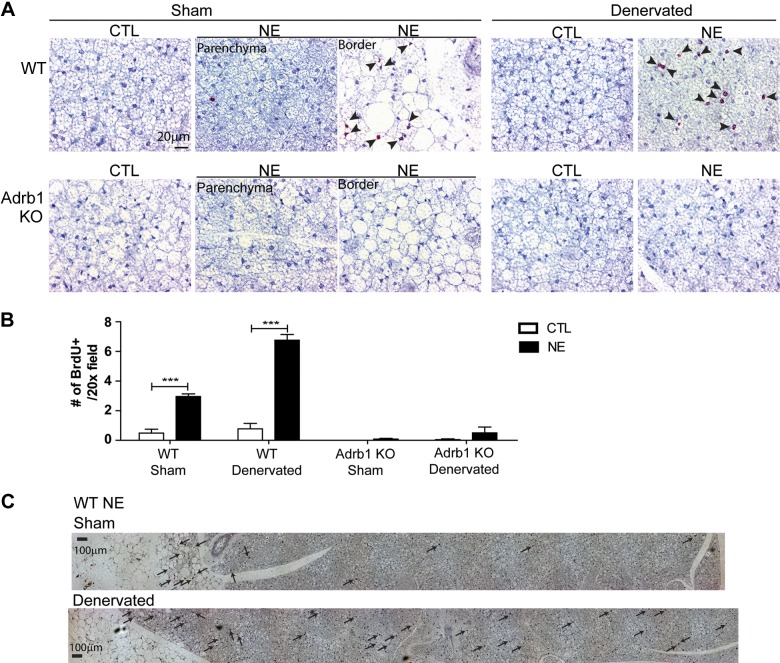
In wild-type mice, NE infusion induced highly significant de novo adipogenesis and angiogenesis in neurally intact BAT, and this effect was significantly greater in denervated BAT (Fig. 8A, top, and B). In contrast, Adrb1 KO mice had very low levels of basal proliferation, and NE infusion failed to stimulate tissue expansion. The differential effect of NE on intact and denervated iBAT prompted us to investigate the tissue location of de novo adipogenesis (Fig. 8C). As with cold stress, we found that brown adipogenesis in neurally intact iBAT was heavily concentrated near the border of the BAT parenchyma and iWAT. In contrast, NE-induced adipogenesis in the denervated pad extended throughout the tissue parenchyma.
Figure 8.
Adrb1 is required for new BA recruitment from progenitor proliferation in iBAT. A) Representative images of paraffin sections of iBAT from wild-type mice and Adrb1 KO mice infused with NE or vehicle for 7 d. NE treatment significantly increased cell proliferation in control and surgically denervated iBAT of wild-type (WT) mice, whereas NE treatment failed to induce cell proliferation in intact and denervated iBAT of Adrb1 KO mice. B) Quantification of BrdU+ cells in paraffin sections (WT CTL, n = 6; WT NE, n = 4; KO CTL, n = 6; KO NE, n = 5; mean ± sem). ***P < 0.001. C) Representative images of paraffin sections of intact or denervated iBAT from wild-type mice infused with NE for 7 d. Arrows indicate location of BrdU+ cells. Scale bars, 20 or 100 µm as indicated.
DISCUSSION
Cold stress induces the de novo adipogenesis in classic iBAT (28, 39, 48) and the appearance of multilocular adipocytes that express UCP1 in certain subcutaneous WAT depots. The present work used fate tracing and inducible genetic tagging to establish the identity of BA progenitors, and the location and mechanism of cold-induced de novo brown adipogenesis in classic iBAT. In addition, we used positive and negative genetic tracing to demonstrate that the BAs that appear in ingWAT following cold stress are derived predominantly, if not exclusively, from preexisting “white” adipocytes.
Consistent with work performed in rats (28, 39), our results demonstrate that classic iBAT of mice contains a population of BA progenitors that proliferates and differentiates during the first few days of cold exposure. Previous work, performed by analysis of cell frequencies at the ultrastructural level, suggested that BAs are recruited from “interstitial cells” during cold stress (28, 39). The present work establishes that the progenitors in iBAT share similar surface antigens and morphologic characteristics with those that contribute to de novo brown adipogenesis in WAT (24). Specifically, the cells are a subpopulation of Sca1+ stromal cells that expresses PDGFRα. These cells are morphologically and immunologically distinct from closely associated endothelial cells, which also proliferate in response to cold stress. The PDGFRα+ BA progenitors also have a dendritic morphology that appears to be characteristic of adipocyte progenitors in various fat pads (24). These cells had a history of Myf5 expression; however, this history may reflect the expression in the paraxial mesoderm (49) because adjacent white adipocytes and nonadipogenic stromal cells were also positive for this reporter. The exclusive contribution of Myf5 lineage to BAs has also been challenged by demonstrations of white adipocytes with histories of Myf5 expression in several adipose tissue depots (21, 50). On the other hand, endothelial cells were negative for the Myf5 reporter, further indicating that these cells do not contribute significantly to cold-induced brown adipogenesis (51, 52).
Unexpectedly, we found that cold-induced brown adipogenesis was largely restricted to a narrow (∼2 mm) band near the dorsal aspect of the iBAT pad, with very little neogenesis occurring in the tissue parenchyma. Progenitor recruitment coincided with significant angiogenesis that was also tightly restricted to the dorsal edge. The mechanisms that restrict proliferation to this specific region or niche are not known but could involve several factors such as density of PDGFRα+ cells and the presence of gap junctions in the BAT parenchyma (53), which might restrict proliferation and integration of new BAs. In addition, denervation/infusion experiments suggest that the relative level of exposure to tissue NE may play an important role. Neuronal reuptake is the major mechanism of neurotransmitter inactivation, and we speculate that the action of neuronal or infused NE is limited by the dense innervation of the BAT parenchyma. Thus, proliferation within intact BAT was concentrated at the tissue “leading edge” where NE inactivation might be least and neuronal spillover greatest. Consistent with this interpretation, denervation, which eliminates presynaptic inactivation, allowed infused NE-induced proliferation to occur throughout the parenchyma. We believe it is unlikely that the effect of denervation involves postsynaptic (vs. presynaptic) supersensitivity because NE treatment began at the time of denervation.
The origin of BAs in ingWAT has been controversial (42–44, 54); however, with the exception of one report (43), independent lines of investigation strongly indicate that most, if not all, BAs that appear in ingWAT following cold stress are derived from existing unilocular adipocytes. Adipocytes in ingWAT develop as typical multilocular BAs in the first 3 wk of life (55–57). By adulthood, cells within this pad become unilocular; however, UCP1 expression can be observed within 3 h of cold stress (55), and detailed ultrastructural studies by Cinti and coworkers (42, 58) strongly indicate that cold exposure reinstates the brown phenotype in existing unilocular adipocytes. A direct demonstration of conversion between unilocular UCP1− and multilocular UCP1+ adipocytes was recently provided by Rosenwald et al. (44), using inducible and constitutive Cre recombinase to tag and trace cells that express UCP1. Our results strengthen these conclusions by showing that 1) the appearance of BAs in ingWAT does not involve proliferation or the recruitment of PDFRα+ preadipocytes, and 2) essentially all cold-induced BAs were derived from adipocytes that were tagged 10–17 d earlier. Our conclusions are based upon both positive and negative tracing techniques using fixable markers that defined cell phenotypes, cell origins, and cell borders. Importantly, we validated the use of tamoxifen to transiently induce reporter expression by showing that essentially all BAs generated by de novo adipogenesis (i.e., UCP1+ BrdU+ adipocytes) were negative when the reporter was induced by adiponectin-CreER.
In contrast to the above analysis, a recent report by Wang et al. (43) suggested that cold stress induces rapid and widespread de novo brown adipogenesis in ingWAT and white adipogenesis in gonadal WAT. In this study, adiponectin-reverse tetracycline transactivator was used to induce expression of β galactosidase in existing fat cells, and the absence of the formazan product of β galactosidase in adipocytes was interpreted as de novo adipogenesis. However, there are critical limitations of this approach that prevent firm conclusions to be drawn from such negative tracing results. First, tracing at the cellular level requires uniform penetration of substrate, which can be difficult to achieve in thick mounts of fat tissue, and clear definition of cell borders with independent tracers. Furthermore, the formazan product that is used to identify cells can be extracted during paraffin embedding, as evidenced by the appearance of blue formazan in the centers of extracted lipid droplets, tens of microns from possible sites of production. Importantly, the conclusion of de novo adipogenesis using β galactosidase activity rests upon defined cells lacking formazan. In this regard, Wang et al. (43) report that unlabeled cells are observed within hours of cold stress and first appear as fully formed unilocular adipocytes, which then become multilocular with extended cold exposure. That multilocular BAs derive from unilocular adipocytes in ingWAT seems well established; however, the conclusion that these unilocular cells represent de novo adipogenesis should be weighed against detailed ultrastructural studies (42, 58), positive lineage-tracing experiments (44), and by the lack of positive identification of the putative progenitor.
Although BAs arise from a similarly committed progenitor in various fat depots, their mode of recruitment reflects critical differences in tissue microenvironments. Cold-induced adipogenesis in iBAT requires neural activation and, under physiologic conditions, is limited to a niche at the tissue’s dorsal edge. Infusion of NE mimics the effects of cold with respect to the magnitude and dorsal location of brown adipogenesis, and these effects require β1-adrenergic receptors. In contrast, selective activation of β3-adrenergic receptors does not trigger de novo brown adipogenesis in iBAT, despite triggering intense thermogenesis and metabolic activation (42, 59). The differential effect of NE vs. β3-adrenergic receptor agonists on de novo adipogenesis is likely explained by the fact that PDGFRα+ and CD31+ cells express β1-adrenergic, but not β3-adrenergic, receptors. On the other hand, β3-adrenergic receptor agonists trigger de novo brown adipogenesis in abdominal WAT (24, 46). Here, however, the primary targets of β3-adrenergic receptor agonists are widely dispersed mature white adipocytes that succumb to agonist treatment. Under these conditions, progenitor proliferation is driven by close interactions with macrophages that clear dead fat cells, and BA differentiation appears to be driven by local signals or by β3-adrenergic receptors that are expressed in activated progenitors (36, 40). We note in this regard that β3-adrenergic receptor agonists do not trigger adipocyte death or macrophage recruitment in iBAT or ingWAT, which likely explains why progenitors remain quiescent in these locations during agonist treatment (24, 36). Finally, neither cold nor direct β3-adrenergic receptor activation triggered de novo brown adipogenesis in subcutaneous WAT. Rather, essentially all new adipocytes were derived from existing fat cells that appear to be white yet can rapidly reinstate the BA phenotype upon adrenergic activation.
Supplementary Material
Acknowledgments
We thank Dr. W. Richardson for providing Pdgfra-CreER mice, Dr. B. Kobilka for providing Adrb1 KO mice, and members of CIMER for discussions. We also thank E. Van Buren and J. Back for technical support with FACS analyses and Dr. D. Burk for the insight to use WGA to define cell borders. This study was supported by U.S. National Institutes of Health (NIH) Grants RO1DK62292 and RO1DK76629 (to J.G.G.). The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center Grant P30CA022453 to The Karmanos Cancer Institute, Wayne State University, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Wayne State University.
Glossary
- Angpt2
Angiopoietin 2
- BA
brown adipocyte
- BAT
brown adipose tissue
- BrdU
5-bromo-2′-deoxyuridine
- CreERT2
Cre recombinase estrogen receptor T2 fusion protein
- EdU
5-ethynyl-2′-deoxyuridine
- GFP
green fluorescent protein
- H/E
hematoxylin and eosin
- iBAT
interscapular brown adipose tissue
- ingWAT
inguinal white adipose tissue
- iWAT
interscapular white adipose tissue
- KO
knockout
- Myf5
myogenic factor 5
- NE
norepinephrine
- no.
number
- PDGFRα
platelet-derived growth factor receptor, α polypeptide
- P.O.
orally
- SVC
stromovascular cell
- TH
tyrosine hydroxylase
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- WGA
wheat germ agglutinin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 2.Gesta S., Tseng Y. H., Kahn C. R. (2007) Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 [DOI] [PubMed] [Google Scholar]
- 3.Himms-Hagen J. (1990) Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 4, 2890–2898 [PubMed] [Google Scholar]
- 4.Stanford K. I., Middelbeek R. J. W., Townsend K. L., An D., Nygaard E. B., Hitchcox K. M., Markan K. R., Nakano K., Hirshman M. F., Tseng Y.-H., Goodyear L. J. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., Eychmüller A., Gordts P. L., Rinninger F., Bruegelmann K., Freund B., Nielsen P., Merkel M., Heeren J. (2011) Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 [DOI] [PubMed] [Google Scholar]
- 6.Tseng Y.-H., Cypess A. M., Kahn C. R. (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 9, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 8.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 9.Muzik O., Mangner T. J., Granneman J. G. (2012) Assessment of oxidative metabolism in brown fat using PET imaging. Front. Endocrinol. (Lausanne) 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., Saito M. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Lans A. A., Hoeks J., Brans B., Vijgen G. H., Visser M. G., Vosselman M. J., Hansen J., Jörgensen J. A., Wu J., Mottaghy F. M., Schrauwen P., van Marken Lichtenbelt W. D. (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., Carpentier A. C. (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cypess A. M., Chen Y.-C., Sze C., Wang K., English J., Chan O., Holman A. R., Tal I., Palmer M. R., Kolodny G. M., Kahn C. R. (2012) Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. USA 109, 10001–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zingaretti M. C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. (2009) The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120 [DOI] [PubMed] [Google Scholar]
- 15.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster D. O., Frydman M. L. (1979) Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 57, 257–270 [DOI] [PubMed] [Google Scholar]
- 17.Blondin D. P., Labbé S. M., Tingelstad H. C., Noll C., Kunach M., Phoenix S., Guérin B., Turcotte E. E., Carpentier A. C., Richard D., Haman F. (2014) Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab. 99, E438–E446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himms-Hagen J. (1969) The role of brown adipose tissue in the calorigenic effect of adrenaline and noradrenaline in cold-acclimated rats. J. Physiol. 205, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms M., Seale P. (2013) Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 [DOI] [PubMed] [Google Scholar]
- 20.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Gurmaches J., Hung C. M., Sparks C. A., Tang Y., Li H., Guertin D. A. (2012) PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W.D., Hoeks J., Enerbäck S., Schrauwen P., Spiegelman B. M. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldén T. B., Hansen I. R., Timmons J. A., Cannon B., Nedergaard J. (2012) Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31 [DOI] [PubMed] [Google Scholar]
- 24.Lee Y. H., Petkova A. P., Mottillo E. P., Granneman J. G. (2012) In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz T. J., Huang T. L., Tran T. T., Zhang H., Townsend K. L., Shadrach J. L., Cerletti M., McDougall L. E., Giorgadze N., Tchkonia T., Schrier D., Falb D., Kirkland J. L., Wagers A. J., Tseng Y. H. (2011) Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 108, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2010) Chronic peroxisome proliferator-activated receptor γ (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Géloën A., Collet A. J., Bukowiecki L. J. (1992) Role of sympathetic innervation in brown adipocyte proliferation. Am. J. Physiol. 263, R1176–R1181 [DOI] [PubMed] [Google Scholar]
- 28.Bukowiecki L. J., Géloën A., Collet A. J. (1986) Proliferation and differentiation of brown adipocytes from interstitial cells during cold acclimation. Am. J. Physiol. 250, C880–C887 [DOI] [PubMed] [Google Scholar]
- 29.Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., Lein E. S., Zeng H. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivers L. E., Young K. M., Rizzi M., Jamen F., Psachoulia K., Wade A., Kessaris N., Richardson W. D. (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konkar A. A., Zhai Y., Granneman J. G. (2000) β1-adrenergic receptors mediate β3-adrenergic-independent effects of CGP 12177 in brown adipose tissue. Mol. Pharmacol. 57, 252–258 [PubMed] [Google Scholar]
- 32.Seibler J., Zevnik B., Küter-Luks B., Andreas S., Kern H., Hennek T., Rode A., Heimann C., Faust N., Kauselmann G., Schoor M., Jaenisch R., Rajewsky K., Kühn R., Schwenk F. (2003) Rapid generation of inducible mouse mutants. Nucleic Acids Res. 31, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granneman J. G., Campbell R. G. (1984) Effects of sucrose feeding and denervation on lipogenesis in brown adipose tissue. Metabolism 33, 257–261 [DOI] [PubMed] [Google Scholar]
- 34.Moore H.-P. H., Silver R. B., Mottillo E. P., Bernlohr D. A., Granneman J. G. (2005) Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J. Biol. Chem. 280, 43109–43120 [DOI] [PubMed] [Google Scholar]
- 35.Granneman J. G., Li P., Zhu Z., Lu Y. (2005) Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 289, E608–E616 [DOI] [PubMed] [Google Scholar]
- 36.Lee Y. H., Petkova A. P., Granneman J. G. (2013) Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granneman J. G., Moore H.-P. H., Krishnamoorthy R., Rathod M. (2009) Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J. Biol. Chem. 284, 34538–34544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preibisch S., Saalfeld S., Tomancak P. (2009) Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukowiecki L., Collet A. J., Follea N., Guay G., Jahjah L. (1982) Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am. J. Physiol. 242, E353–E359 [DOI] [PubMed] [Google Scholar]
- 40.Lee Y.-H., Thacker R. I., Hall B. E., Kong R., Granneman J. G. (2014) Exploring the activated adipogenic niche: interactions of macrophages and adipocyte progenitors. Cell Cycle 13, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y.-H., Granneman J. G. (2012) Seeking the source of adipocytes in adult white adipose tissues. Adipocyte 1, 230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 43.Wang Q. A., Tao C., Gupta R. K., Scherer P. E. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenwald M., Perdikari A., Rülicke T., Wolfrum C. (2013) Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen K. D., Qiu Y., Cui X., Goh Y. P., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R. M., Chawla A. (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himms-Hagen J., Melnyk A., Zingaretti M. C., Ceresi E., Barbatelli G., Cinti S. (2000) Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 279, C670–C681 [DOI] [PubMed] [Google Scholar]
- 47.Bronnikov G., Bengtsson T., Kramarova L., Golozoubova V., Cannon B., Nedergaard J. (1999) β1 to β3 switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct β-adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology 140, 4185–4197 [DOI] [PubMed] [Google Scholar]
- 48.Bronnikov G., Houstĕk J., Nedergaard J. (1992) Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J. Biol. Chem. 267, 2006–2013 [PubMed] [Google Scholar]
- 49.Gensch N., Borchardt T., Schneider A., Riethmacher D., Braun T. (2008) Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development 135, 1597–1604 [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Gurmaches J., Guertin D. A. (2014) Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran K.V., Gealekman O., Frontini A., Zingaretti M. C., Morroni M., Giordano A., Smorlesi A., Perugini J., De Matteis R., Sbarbati A., Corvera S., Cinti S. (2012) The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry R., Rodeheffer M. S. (2013) Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider-Picard G., Carpentier J. L., Orci L. (1980) Quantitative evaluation of gap junctions during development of the brown adipose tissue. J. Lipid Res. 21, 600–607 [PubMed] [Google Scholar]
- 54.Guerra C., Koza R. A., Yamashita H., Walsh K., Kozak L. P. (1998) Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 102, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Master S. R., Hartman J. L., D’Cruz C. M., Moody S. E., Keiper E. A., Ha S. I., Cox J. D., Belka G. K., Chodosh L. A. (2002) Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol. Endocrinol. 16, 1185–1203 [DOI] [PubMed] [Google Scholar]
- 56.Gouon-Evans V., Pollard J. W. (2002) Unexpected deposition of brown fat in mammary gland during postnatal development. Mol. Endocrinol. 16, 2618–2627 [DOI] [PubMed] [Google Scholar]
- 57.Xue B., Rim J. S., Hogan J. C., Coulter A. A., Koza R. A., Kozak L. P. (2007) Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res. 48, 41–51 [DOI] [PubMed] [Google Scholar]
- 58.Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Pénicaud L., Casteilla L. (1992) Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 103, 931–942 [DOI] [PubMed] [Google Scholar]
- 59.Jimenez M., Barbatelli G., Allevi R., Cinti S., Seydoux J., Giacobino J.-P., Muzzin P., Preitner F. (2003) β 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 270, 699–705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.