Abstract
In Leishmania mexicana parasites, a unique glucose transporter, LmxGT1, is selectively targeted to the flagellar membrane, suggesting a possible sensory role that is often associated with ciliary membrane proteins. Expression of LmxGT1 is down-regulated ∼20-fold by increasing cell density but is up-regulated ∼50-fold by depleting glucose from the medium, and the permease is strongly down-regulated when flagellated insect-stage promastigotes invade mammalian macrophages and transform into intracellular amastigotes. Regulation of LmxGT1 expression by glucose and during the lifecycle operates at the level of protein stability. Significantly, a ∆lmxgt1 null mutant, grown in abundant glucose, undergoes catastrophic loss of viability when parasites deplete glucose from the medium, a property not exhibited by wild-type or add-back lines. These results suggest that LmxGT1 may function as a glucose sensor that allows parasites to enter the stationary phase when they deplete glucose and that in the absence of this sensor, parasites do not maintain viability when they run out of glucose. However, alternate roles for LmxGT1 in monitoring glucose availability are considered. The absence of known sensory receptors with defined ligands and biologic functions in Leishmania and related kinetoplastid parasites underscores the potential significance of these observations.—Rodriguez-Contreras, D., Aslan, H., Feng, X., Tran, K., Yates, P. A., Kamhawi, S., Landfear, S. M. Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor.
Key Words: environmental sensing, Leishmania parasites, protein expression, transceptor, TaV2A peptide
Leishmania species are parasitic protozoa that cause a spectrum of diseases, ranging from disfiguring cutaneous to fatal visceral leishmaniases that constitute a major global health problem (1). These parasites exist as extracellular, motile, flagellated promastigotes within the sandfly vector that transmits the infection and as intracellular amastigotes, which maintain a short, nonmotile flagellum (2, 3), within phagolysosomal vesicles (also called parasitophorous vacuoles) of mammalian host macrophages. Leishmania parasites express a plethora of membrane-transport proteins on their surface (4, 5) that are involved in the uptake of nutrients from either the alimentary tract of the sandfly vector or the macrophage parasitophorous vacuole. Notably, a number of membrane proteins in Leishmania and related kinetoplastid protozoa localize selectively to the flagellar membrane (6–12). Several such proteins are likely to be involved in signal transduction and environmental sensing (13), including putative receptor adenylate cyclases (7), calcium-binding proteins (14), protein tyrosine phosphatases (12), and others. However, in general, it has not been determined what these proteins may sense in the environment or what biologic functions they affect. The observation of putative-sensing proteins in the flagella of a kinetoplastid parasite is consistent with the established role of cilia and flagella as sensors of the extracellular environment in a wide variety of eukaryotes (15, 16). In addition, several membrane transport proteins, including those for glucose (8, 11) and calcium (12), and some channels, such as an aquaglyceroporin (9) and a calcium channel (12), are present selectively in the flagellar membrane of different kinetoplastid parasites. Studies on the flagellar aquaporin 1 from Leishmania major (9) indicate that it is involved in sensing osmolarity and cell-volume control, also consistent with a role in environmental sensing.
In Leishmania mexicana, three distinct, broad, substrate specificity (17), hexose-transporter isoforms are encoded by clustered genes (18), designated LmxGT1, LmxGT2, and LmxGT3 [historically named “glucose transporters” (GTs) for their highest affinity hexose ligand]. LmxGT2 and LmxGT3 are expressed in the pellicular plasma membrane (PPM) that surrounds the promastigote cell body and are excluded from the flagellar membrane. LmxGT1 is present largely in the flagellar membrane (11, 19) but populates the PPM sparsely if at all. The unique N-terminal hydrophilic domain of LmxGT1 is responsible for flagellar membrane targeting, and a sequence segment (11) within this domain as well as an interacting protein, KHARON1 (20), have been identified as critical for flagellar targeting. Despite this strikingly distinct organellar targeting, a specific biologic function for the LmxGT1 permease remains to be determined. Furthermore, the regulation of this permease by physiologic conditions or during the parasite lifecycle is also poorly understood.
Here, we show that LmxGT1 plays a critical role in adaptation and survival of promastigotes when they deplete glucose from their growth medium. In the absence of this flagellar permease, as in the ∆lmxgt1 null mutant, promastigotes, growing in abundant glucose, undergo a catastrophic decline in cell viability as they run out of this nutrient, whereas wild-type or “add-back”-complemented null mutants that express LmxGT1 are able to adapt, enter a stable stationary phase, and survive glucose depletion. These observations support a role for this flagellar GT in responding to extracellular glucose levels and hence, in sensing the physiologic environment, a property in common with that of other flagellar or ciliary membrane proteins. The results also suggest a vital function for LmxGT1 in the biology of Leishmania parasites.
MATERIALS AND METHODS
Parasite culture and media
Promastigotes of WT L. mexicana (strain MNYZ/BZ/62/M379) or various GT null mutants derived from this WT strain were cultured at 26°C in glucose-free RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA), pH 7.4, supplemented with 10% heat-inactivated fetal bovine serum (Thermo Scientific Hyclone, Logan, UT, USA), 0.1 mM xanthine, and 5 mg/ml hemin (low sugar condition of growth). For high glucose conditions, those used for routine culture of promastigotes, 1.8 g/l glucose (10 mM) was added to the medium. Cell lines complemented with episomal GT genes were grown in the presence of 80 µg/ml G418 (Life Technologies, Carlsbad, CA, USA). Growth curves were determined by counting triplicate samples on a hemacytometer grid. Axenic culture from amastigotes was grown at 32.5°C in DMEM, modified for Leishmania (21), pH 5.5, supplemented with 30 mM 2-(N-morpholino)ethanesulfonic acid instead of HEPES, 20% heat-inactivated fetal calf serum, and 10 mM glucose.
Generation of tagged LmxGT1 fusion proteins, recombinant DNA constructs, and cell lines
The GT1-influenza virus hemagglutinin epitope tag (HA)3 open-reading frame (ORF) was cloned from pX63NEO[GT1-HA3] DNA [described in ref. (20)] by use of a forward primer containing an SfiIA site (underlined), 5′-GAGGCCACCTAGGCCATGAGCAACTCTTCCACAAAGCATCC-3′, and a reverse primer with SfiIB site, 5′-GAGGCCACGCAGGCCCTAACTAGTGGCGTAGTCG-3′. The GT1 3′-UTR immediately after the STOP codon was cloned by use of forward and reverse primers containing SfiIC and SfiID sites, respectively (underlined): 5′-GAGGCCTCTGTGGCCGCAGAATTAGGAAGACGCTGCACTGGTC-3′ and 5′-GAGGCCTGACTGGCCGAAGTGTATTCGTCTAGTGGGGTGATCGC-3′ (22). These two DNA fragments were used to generate the GT1::HA::TaV2A::luciferase (LUC)::BSD::GT1[3′-UTR] fusion, as described previously (20). Subsequently, the GT1::HA::TaV2A::LUC fusion gene was amplified by use of forward primer 5′-GATCGGATCCATGAGCAACTCTTCCACAAAGCATCC-3′ and reverse primer 5′-CATGAATTCTCAGGTCTGCTCGTTCTTCAGCACGCGCTC-3′ and cloned into the BamHI and EcoRI sites (underlined) of the Leishmania expression vector pX63NEO-RI (23). For simplicity, the fusion ORF is referred to henceforth as LmxGT1-TaV2A [or LmxGT1 GT fused at its C terminus to the TaV2A peptide (GT1-2A)]-Luc. All constructs were confirmed by sequencing at the Oregon Health & Science University (OHSU) Sequencing Core Facility (Portland, OR, USA).
To generate ∆lmxgt1-3[pGT1-GFP] and ∆lmxgt1[pGT1-GFP], ∆lmxgt1-3[pGT1-HA3] and ∆lmxgt1[pGT1-HA3], or ∆lmxgt1-3[pGT1] and ∆lmxgt1[pGT1] cell lines, ∆lmxgt1-3 (19) and ∆lmxgt1 (24) promastigotes were transfected with plasmid DNA from the Leishmania expression vector pXG−′GFP+ containing the GFP-tagged LmxGT1 sequence (11) or pX63NEO vector containing the HA3-tagged (20) or untagged (24) LmxGT1 sequence, respectively. Promastigotes were transfected according to previously described electroporation techniques by use of a Gene Pulser Xcell (Bio-Rad Laboratories, Hercules, CA, USA) (25). Other cell lines used are: ∆lmxgt1-3[pGT2-GFP] (19); WT[pGT1-GFP] (11); WT[pGT1-HA3] (20), where WT represents the WT genetic background.
Uptake assays
Assays for uptake of radiolabeled substrates were performed by an oil-stop method, as reported (26), and protein determination for normalization was performed by use of the DC Protein Assay Kit (Bio-Rad Laboratories). Time courses for uptake were performed by use of 100 µM [6-3H]d-glucose (Moravek Biochemicals, Brea, CA, USA), and transport rates were calculated by linear regression.
Quantitative real-time PCR
Purification of mRNA and quantitative real-time PCR (qRT-PCR) were performed as described previously (27) by use of the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Forward and reverse primers used for GT1 and GT2 genes were as follows: GT1-RTF1, 5′-CGCTGAAACCCAAAAAAGTG-3′; GT1-RTR1, 5′-GGGTAAGATGTCGTTTTGATGG-3′; GT2-RTF1, 5′-CTCTAGGTCCGAAAAGGAGCC-3′; and GT2-RTR1, 5′-TAACCCTGGCATTGCTCGCC-3′.
Mouse infection and parasite recovery
Female BALB/c mice (age 5–6 wk) were purchased from Charles River Laboratories (Wilmington, MA, USA). Stationary-phase L. mexicana WT, ∆lmxgt1 (24), or ∆lmxgt1[pGT1] was washed with Dulbecco’s PBS (Life Technologies) and resuspended at 2.5 × 108 cells/ml. For each cell line, two animals were challenged with subcutaneous inoculation of 5 × 106 parasites (20 µl) into the hind footpads. Two and 4 weeks after infection, animals were killed, and parasites were aspirated from the infected foot by use of a 1 ml syringe with a 27 gauge needle, filled with 0.5 ml RPMI-1640 medium. The recovered cells were cultured in the promastigote culture medium described above, and the transformed promastigotes were then stored frozen in liquid nitrogen until use.
Macrophage infections
The THP-1 human acute leukemia monocyte cell line was cultivated at 37°C and 5% CO2, as described (20). The cultures were diluted every 3 d to prevent cell density from exceeding 1 × 106 cells/ml. Cells were kept for a maximum of eight subculture cycles. THP-1 cells were differentiated with 100 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich) in a 2-well Lab-Tek II chambered coverglass (Nalge Nunc International, Rochester, NY, USA) for localization studies or in 82 mm Petri dishes for amastigote purification for 48 h at 37°C, 5% CO2. Seeded macrophages were infected with promastigotes at stationary phase (10:1 parasite:macrophage ratio) for 6 h, washed extensively with PBS, and then incubated in medium at 37°C, 5% CO2. For amastigote purification, infected macrophages were harvested 4 d after infection and amastigotes were isolated as described previously (18). Infections of primary bone marrow-derived macrophages from BALB/c mice with stationary-phase promastigotes (2:1 and 5:1 parasite:macrophage ratio) were performed as described previously (17).
Immunodetection
For immunofluorescence, parasites were treated as described previously (20). Coverslips were mounted onto microscope slides by use of Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). For immunoblotting, promastigotes or amastigotes were washed with PBS, and cell pellets were lysed in 1× LDS sample buffer (Life Technologies) containing 5 mM DTT. Samples were resolved by electrophoresis, transferred, and developed as described previously (20). Molecular-weight markers were the PageRuler prestained protein ladder (Thermo Scientific Pierce, Rockford, IL, USA). Antibodies, dilutions, and sources are as follows: mouse anti-GFP (JL-8), 1:8000 (Clontech Laboratories, Mountain View, CA, USA); mouse anti-α-tubulin (B-5-1-2), 1:3000, and rabbit anti-HA peptide, 1:1000 (Sigma-Aldrich); mouse anti-Renilla Luc, 1:1000 (Millipore, Burlington, MA, USA), and rabbit anti-TaV2A peptide, 1:3000 (Molecular Probes, Eugene, OR, USA); rabbit anti-GFP, 1:1000, and anti-rabbit IgG Alexa Fluor-488 and anti-mouse IgG Alexa Fluor-594, 1:1000 (Molecular Probes); and anti-mouse and anti-rabbit HRP-conjugated secondary antibodies, 1:20,000 (Thermo Scientific Pierce). Blots were developed by use of the SuperSignal luminescence detection kit (Thermo Scientific Pierce), followed by detection on an ImageQuant LAS 4000 scanner (GE Healthcare, Pittsburgh, PA, USA) and quantification by use of Adobe Photoshop CS4 software.
Cycloheximide treatment
To measure the half-life of the LmxGT1-TaV2A protein, ∆lmxgt1-3 promastigotes were transfected with the pX63NEO-RI expression vector (23), encompassing the LmxGT1-TaV2A/Luc ORF that is cotranslationally cleaved to generate separate LmxGT1-Tv2A and Luc polypeptides. Promastigotes of these transgenic parasites were inoculated at a density of 1 × 105 parasites/ml into RPMI-1640 medium containing either high or low glucose, plus 80 µg/ml G418. Following 60 h growth, when cells had reached a density of 3.5 × 106 cells/ml, parasites were resuspended in the same medium at a density of 1 × 107 cells/ml, and cycloheximide (10 µg/ml) was added to inhibit translation (28). Samples of 2.5 × 106 parasites were withdrawn from 0–8 h for immunoblotting and detection of LmxGT1-TaV2A and Luc polypeptides.
Deconvolution microscopy
Deconvolution microscopy was carried out at the Advanced Light Microscopy Core at The Jungers Center, OHSU. Immunofluorescence images were acquired on a high-resolution, wide-field DeltaVision Core system (Applied Precision, Issaquah, WA, USA) by use of an Olympus IX71 inverted microscope. The camera was a Nikon CoolSNAP ES2 HQ. Each image was acquired as Z-stacks in a 640 × 640 format with a 60× 1.42 numerical aperture PlanApo lens with an additional magnification of ×1.6, which is equivalent to 93×, by use of softWoRx acquisition software version 5.5.1 (Applied Precision). Images were deconvolved in softWoRx.
Infection of sandflies
Colony-bred, 2- to 4-d-old Lutzomya longipalpis females were infected by artificial feeding (3 h in the dark) through a chick skin membrane on heparinized rabbit blood (Spring Valley Laboratories, Sykesville, MD, USA) containing 350 U/ml penicillin, 350 µg/ml streptomycin, and 5 × 106 cells/ml early procyclic forms (P2-P3) from three L. mexicana cell lines that had been isolated previously from BALB/c footpad lesions (WT, ∆lmxgt1 null mutants, and ∆lmxgt1[pGT1] add-back parasites). Flies were then separated to insure that only fully fed flies were kept for follow-up. Flies were given 30% sucrose solution daily and maintained at 26°C and 75% humidity. For the add-back group, 100 µg/ml G418 was added to the sucrose solution. Ten to 13 flies from each group were dissected at different days after infection, and each gut was placed into a microcentrifuge tube in 50 µl PBS, macerated with a plastic pestle (Kimble Chase, Vineland, NJ, USA), and the number of metacyclic and nonmetacyclic forms, distinguished by morphology and movement, was determined by counting on a hemacytometer (29).
Statistical analysis
Statistical evaluation of the means of experimental groups from sandfly infections was done by use of one-way ANOVA, followed by the Tukey-Kramer posttest. Student’s unpaired t test was used to determine the statistical significance in other experiments.
RESULTS
Regulation of flagellar GT expression by cell density and glucose
To understand the unique functions of the flagellar LmxGT1, we have compared various properties of this flagellar permease with LmxGT2, the major mediator of hexose uptake that is expressed in the PPM of promastigotes (24). Expression of several transporters in Leishmania species has been shown to be regulated by increasing cell density in culture and by the presence or absence of ligands for the permease [e.g., refs. (30–33)]. Hence, we examined the expression of LmxGT1 and LmxGT2 transporters in culture-form promastigotes over a range of cell densities and in the presence of high and low glucose in the medium. For these studies, fusion proteins between each permease and GFP were expressed from an episomal expression vector pXG−′GFP+ (34) that contained the relevant ORF but that provided exogenous 5′- and 3′-UTRs. Hence, the regulation detected is inherent to the permease ORF or protein and cannot be ascribed to transcriptional control or to alteration of mRNA half-life regulated by UTRs of the cognate mRNA.
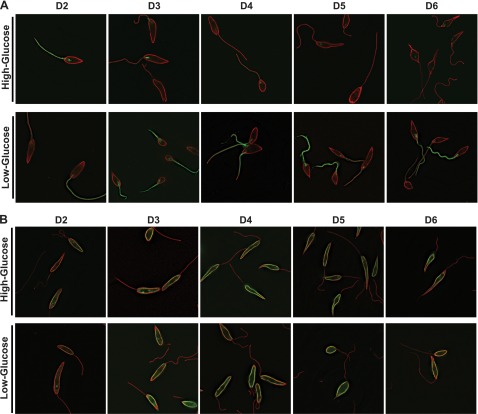
Figure 1A shows deconvolution fluorescence images of ∆lmxgt1-3 null mutant promastigotes expressing the LmxGT1-GFP fusion protein, captured on days 2–6 of the growth curve (cell densities shown in Fig. 2A) in medium containing high glucose (10 mM glucose on day 0) and low glucose (no glucose added to the medium; ∼0.5 mM glucose provided by the serum on day 0). Whereas LmxGT1-GFP is visible in the flagellar membrane of parasites on day 2 of growth in high glucose, the fluorescence signal decays rapidly thereafter. In contrast, LmxGT1-GFP fluorescence is robust in parasites cultured in low glucose through day 6. On the contrary, for parasites expressing LmxGT2-GFP (Figs. 1B and 2E), fluorescence signal is present at similar levels on the PPM from days 2 to 6 in both high and low glucose. Hence, expression of LmxGT1 is responsive to both cell density and the concentration of glucose in the medium.
Figure 1.
Immunofluorescence images of ∆lmxgt1-3 null mutant parasites expressing LmxGT1-GFP (A) or LmxGT2-GFP (B) from an episomal expression vector. Parasites were inoculated at a density of 1 × 105 cells/ml and grown for 6 d in RPMI 1640 medium containing high (10 mM) or low (0.5 mM) initial glucose. Samples were withdrawn at days 2, 3, 4, 5, and 6 (D2–D6) for fixation and imaging by deconvolution microscopy. Promastigotes were stained with anti-α-tubulin and Alexa Fluor 594-conjugated antibodies (red) to visualize the subpellicular microtubule network. Green represents GFP.
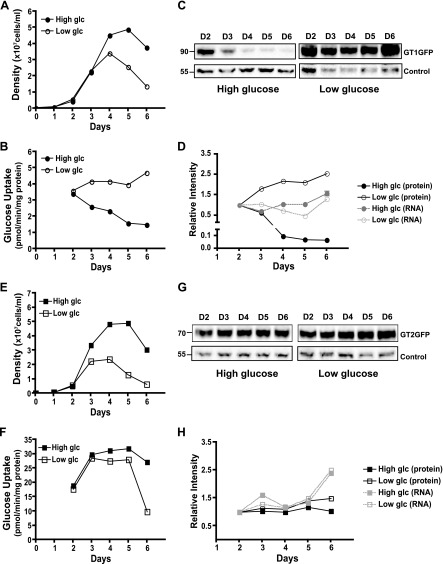
Figure 2.
Expression of LmxGT1-GFP (A–D) and LmxGT2-GFP (E–H) as a function of days in culture and in the presence of high (10 mM) or low (0.5 mM) glucose. Each GFP-tagged transporter gene was expressed in a ∆lmxgt1-3 null mutant background. A and E) Cell density for each transgenic null mutant was quantified and plotted as the average of triplicate determinations. glc, glucose. B and F) The rate of uptake of 100 µM [3H]d-glucose by each transgenic line as a function of days of growth and high or low glucose. C and G) Western blot of total lysates probed with anti-GFP antibody. Control represents a cross-reacting band and was used for normalization. Numbers to the left of each blot in this and other figures represent molecular-weight markers (in kilodaltons). D and H) Quantification of the Western blot signal for each GFP fusion protein (protein) and for the corresponding mRNA (RNA). For protein, the intensity of the Western blot signal was normalized to that of the loading control for each sample. RNA was quantified by qRT-PCR of total RNA from each sample and normalized to 18 S rRNA. Samples from day 2 were set to a relative intensity value of 1.0, and other samples were normalized to those on day 2. Culture aliquots used in all panels for A–D and E–H were withdrawn from the same cultures to ensure sample uniformity across different assays.
To quantify the above results, we also performed uptake assays by use of 100 µM [3H]d-glucose. Indeed, one reason for expressing the fusion proteins in the ∆lmxgt1-3 null mutant is that all hexose uptake activity in these lines is provided by the episomally expressed permease so that functional transporter expression can be monitored directly as glucose uptake. As anticipated (Fig. 2B), glucose uptake by LmxGT1-GFP dropped rapidly, as cell density increased when parasites were cultured in a high-glucose medium, but uptake was maintained at a constant level when parasites were grown in a low-glucose medium. To establish that these results were not affected by the GFP tag, these experiments were also performed with the untagged LmxGT1 permease, and identical results were obtained (Supplemental Fig. 1). In contrast, uptake by LmxGT2-GFP increased somewhat from days 2 to 3 but was constant thereafter (except for day 6 in the low-glucose sample, where cell viability dropped markedly; Fig. 2E) in either high or low glucose (Fig. 2F). Further quantification was obtained by monitoring expression of LmxGT1-GFP (Fig. 2C, D) and LmxGT2-GFP (Fig. 2G, H) by immunoblotting, again demonstrating the precipitous drop (∼20-fold) in LmxGT1-GFP but not LmxGT2-GFP expression, with increasing cell density only in parasites cultured in high glucose medium. Indeed, under low glucose conditions, LmxGT1-GFP increased ∼2.5-fold during parasite growth (Fig. 2D), representing an ∼50-fold increase on LmxGT1-GFP for low glucose compared with high glucose at day 6. Finally, the levels of LmxGT1-GFP and LmxGT2-GFP mRNAs were also monitored by use of qRT-PCR. All mRNA levels remained constant during growth in high or low glucose, indicating that the decreased LmxGT1-GFP expression at high cell density in high glucose does not reflect changes in the level of mRNA and, thus, must be a result of the decreased level of the transporter itself, controlled at the level of its synthesis or turnover. Overall, these results indicate that flagellar LmxGT1 expression is translationally and/or posttranslationally regulated by both cell density and the concentration of glucose in the medium but that the level of the more abundant PPM GT LmxGT2 is not regulated by either physiologic condition.
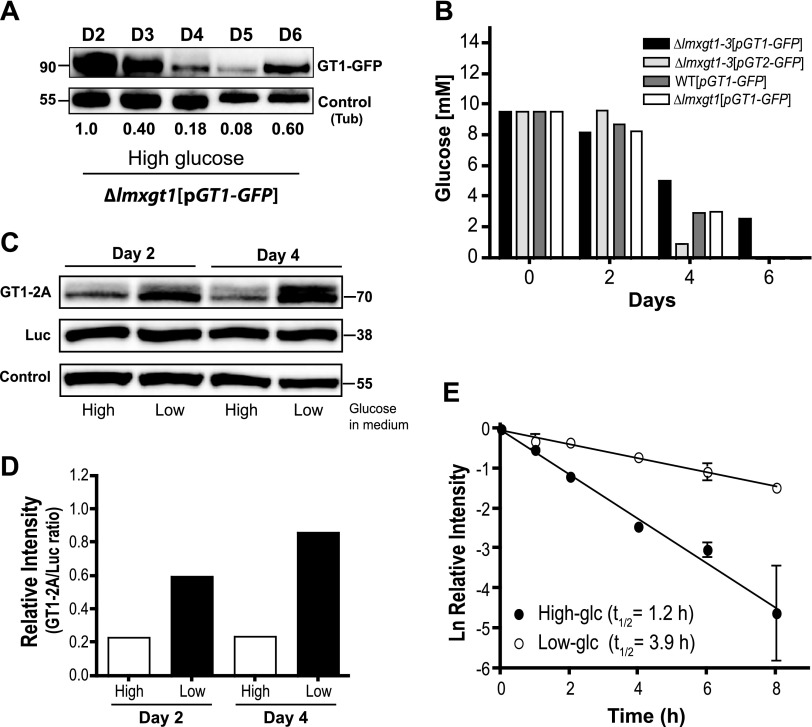
To determine whether the above results were influenced by the ∆lmxgt1-3 null mutant background, fluorescence microscopy and immunoblots were performed on similar parasite lines expressing LmxGT1-GFP in the ∆lmxgt1 null mutant (Fig. 3A) and in the WT backgrounds (Supplemental Fig. 2). These cell lines still express endogenous LmxGT2 and LmxGT3 permeases and, thus, deplete glucose from the medium more rapidly (Fig. 3B, white and dark-gray bars; days 4 and 6) than the ∆lmxgt1-3 mutants (Fig. 3B, black bars). Notably, for transgenic parasites in the ∆lmxgt1 and WT backgrounds, growing in high glucose, the LmxGT1-GFP signal decreased abruptly but then began to re-emerge by day 6 (Fig. 3A and Supplemental Fig. 2B, C). This re-emergence coincided with depletion of glucose from the medium (Fig. 3B). Hence, even in parasites initially growing in high glucose, expression of LmxGT1-GFP is induced by glucose depletion.
Figure 3.
Regulation of LmxGT1 expression by glucose. A) Expression of LmxGT1-GFP in ∆lmxgt1 null mutant background as a function of days in culture in the presence of high glucose. Parasites were inoculated at a density of 1 × 105 cells/ml and grown for 6 d. Western blot represents total cell lysates probed with anti-GFP antibody. The control band represents α-tubulin (Tub). The numbers below each lane indicate the relative intensity of the GT1-GFP signal on each day. The GT1-GFP signal was first normalized to the intensity of the tubulin control in the same lane, and all numbers were then renormalized to the signal on day 2, which was set at a value of 1.0. B) Quantification of glucose in the medium for each indicated parasite line grown in high glucose medium. Parasites were removed from 1.5 ml culture by centrifugation, followed by filtration through a Millex-GP Filter Unit (0.22 µm; Millipore). The glucose content in the culture supernatants was determined by use of a glucose assay kit (Eton Bioscience, San Diego, CA, USA) (35). Values plotted represent the average of triplicate determinations. C and D) Quantification of the relative expression of GT1-2A and Luc polypeptides in parasites encoding the LmxGT1-TaV2A-Luc ORF on an episome and cultured in high and low glucose. Samples were collected at 2 and 4 d after inoculation and analyzed by Western blotting, probed with anti-TaV2A and anti-Luc antibodies. C) Western blot developed with anti-horseradish peroxidase antibody. Control represents the signal from a cross-reacting band. Positions of molecular-weight markers (∼38 and ∼70 kDa) are indicated. D) Quantification of relative intensity of GT1-2A and Luc signals as a function of high (High) or low (Low) glucose in the medium. The blot in C was digitized at several different exposures, and the ratio of the band intensities was calculated (GT1-2A:Luc ratio). Data represent the average of the ratios from the different scans. E) Quantification of half-life (t1/2) for LmxGT1-TaV2A protein in high (High-glc) and low (Low-glc) glucose. Parasites expressing this fusion protein and Luc from an episome were treated with cycloheximide, and samples were withdrawn for immunoblotting at the times indicated. The intensity of the LmxGT1-TaV2A signal for each sample was normalized to that of Luc, a stable protein with a half-life of days, and these normalized intensities were then renormalized to the value of 1.0, assigned to the t = 0 intensity to give relative intensity. Data represent the average and range of two replicate experiments. Ln, the logarithm to the base of each relative intensity value.
Expression of a LmxGT1-TaV2A fusion protein establishes that regulation of LmxGT1 occurs at the level of protein turnover
To determine whether glucose regulates expression of LmxGT1 at the level of translation or protein turnover, we used a novel expression system [developed Yates and co-workers (20), and unpublished data] that encodes a single fusion polypeptide (LmxGT1-TaV2A-Luc) consisting of the LmxGT1 ORF, viral TaV2A peptide that induces a cotranslational intraribosomal cleavage (36), and Renilla Luc ORF. When expressed from the episomal vector, LmxGT1-TaV2A-Luc is translated as a single ORF, but the cleavage following translation of the TaV2A peptide generates a LmxGT1-TaV2A fusion protein and a separate Luc polypeptide. Hence, if glucose deprivation stimulates translation of LmxGT1, both the LmxGT1-TaV2A and Luc polypeptides would be up-regulated similarly, as they are translated from the same fused ORF. In contrast if LmxGT1 protein is stabilized against degradation by glucose limitation, then the steady-state level of the LmxGT1-TaV2A fusion protein will be up-regulated by glucose deprivation; however, the Luc polypeptide will not be similarly up-regulated because it is released as a separate protein during translation. Hence, the monitoring of the relative expression of the LmxGT1-TaV2A and Luc polypeptides following glucose limitation distinguishes between translational control and regulation at the level of protein half-life.
Parasites expressing the LmxGT1-TaV2A-Luc episomal construct were cultured in high and low glucose, and expression of the LmxGT1-TaV2A and Luc polypeptides was monitored by Western blotting at days 2 and 4 of growth (Fig. 3C). Comparison of the ratio of each polypeptide detected under the high and low glucose culture conditions revealed that the LmxGT1-TaV2A protein was up-regulated considerably more by glucose limitation than was Luc (Fig. 3D). Thus, the ratio of intensities of the LmxGT1-TaV2A band to the Luc band increased, in low glucose compared with high glucose, from 0.2 to 0.6 (3-fold) on day 2 and from 0.2 to 0.8 (4-fold) on day 4. This result indicates that the steady-state level of LmxGT1 is up-regulated by glucose deprivation, largely by decreasing the rate of protein turnover.
To confirm these results by use of a conventional method for measuring protein half-life, the transgenic parasites expressing the LmxGT1-TaV2A and Luc polypeptides were treated with cycloheximide to inhibit translation (28), and the levels of the two proteins were determined from 0 to 8 h thereafter by immunoblotting (Fig. 3E). The half-life of the LmxGT1-TaV2A permease was 1.2 h for parasites cultured in high glucose, but it increased to 3.9 h when parasites were cultured in low glucose medium.
Regulation of LmxGT1 expression during the transformation of promastigotes to intracellular amastigotes
To determine whether expression of the LmxGT1 ORF is regulated during transformation of promastigotes to intracellular amastigotes, ∆lmxgt1-3 promastigotes expressing LmxGT1-GFP were used to infect THP-1 macrophages, and the GFP signal was monitored from 1 to 6 d after infection (Fig. 4A). For comparison, a similar experiment was done for parasites expressing the LmxGT2-GFP fusion protein, a polypeptide that has been demonstrated to be down-regulated in amastigotes compared with promastigotes (32). For the LmxGT1 construct, GFP was visible in the region of the small flagellum and flagellar pocket, 1 d after infection (Fig. 4A, D1), but this signal decayed and was barely detectable 4 d after infection (Fig. 4A, D4, B) representing a >5-fold decrease in expression. Similar results were obtained when LmxGT1-GFP was expressed in the ∆lmxgt1 background, and the level and time course of expression were the same regardless of whether the infecting promastigotes were cultured in high or low glucose medium (Fig. 4B). Likewise, LmxGT2-GFP fluorescence signal, present in the PPM and flagellar pocket, decayed with a time course comparable with that of LmxGT1-GFP (Fig. 4A). These results suggest 1) that the steady-state level of both permeases decreases dramatically when promastigotes convert to amastigotes and 2) that this developmental down-regulation operates at the level of the protein synthesis or stability, as each GFP-tagged transporter was encoded by an episome that encompassed only the relevant GT ORF and not associated cognate mRNA UTR sequences. Furthermore, previous studies (18) established that whereas endogenous LmxGT2 mRNA is strongly down-regulated in amastigotes compared with promastigotes, LmxGT1 mRNA is expressed at similar levels in both lifecycle stages. Thus, any lifecycle regulation detected for LmxGT1 should be dictated by the ORF and not the mRNA. In summary, LmxGT1 is expressed much more robustly in promastigotes compared with amastigotes, and the regulation of transporter expression occurs at the level of protein synthesis and/or stability.
Figure 4.
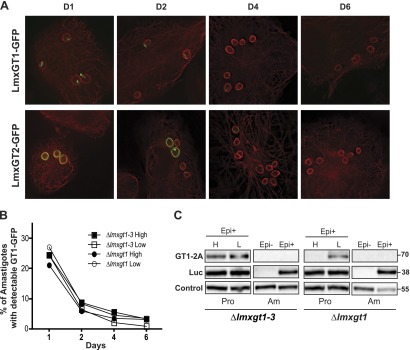
Expression of LmxGT1-GFP and LmxGT2-GFP following infection of macrophages. A) THP-1 macrophages were infected with ∆lmxgt1-3 null mutants expressing each GFP fusion protein, and deconvolution fluorescence images were recorded for cells at days 1, 2, 4, and 6 postinfection. Green represents GFP, and red represents fluorescence from an anti-α-tubulin antibody. B) The percentage of amastigotes with detectable GFP signal was plotted vs. days postinfection for ∆lmxgt1-3 and ∆lmxgt1 null mutants expressing LmxGT1-GFP from an episomal expression vector in high and low glucose. Typically, 80–-150 amastigotes were counted per sample. C) Loss of LmxGT1-TaV2A signal when parasites transform from promastigotes to amastigotes. Lines encoding the LmxGT1-TaV2A-Luc ORF on an episomal vector were prepared in the ∆lmxgt1-3 and ∆lmxgt1 null mutant backgrounds. Expression of GT1-2A and Luc from the cotranslationally cleaved translation product was monitored by Western blot by use of anti-TaV2A and anti-Luc antibodies. For amastigotes (Am), lysates were prepared from parasites without the episome (Epi–) as a negative control and from parasites encompassing the LmxGT1-TaV2A-Luc-encoding episome (Epi+). For promastigotes (Pro), lysates were prepared from early logarithmic-phase parasites (day 2 of growth) of the episome-carrying (Epi+) line, cultured in the presence of high glucose (H) or low glucose (L). In this experiment, the LmxGT1-TaV2A protein apparently began to down-regulate in the high glucose-cultured promastigotes in the ∆lmxgt1 line (fifth lane from the left) more rapidly than it did in the ∆lmxgt1-3 line (first lane from the left). The band marked Control refers to a cross-reacting signal used as a loading control.
To confirm the above results and to demonstrate that down-regulation of LmxGT1 expression in amastigotes was not simply a result of instability of the GFP component of the fusion protein, we performed similar experiments by use of LmxGT1 fused to the HA epitope tag. Indeed, the LmxGT1-HA3 and LmxGT1-GFP fusion proteins were similarly down-regulated when promastigotes were converted into axenic amastigotes (37) (Supplemental Fig. 3).
To distinguish between control at the translational and protein half-life level, the expression of the LmxGT1-TaV2A and Luc polypeptides, encoded on an episome as the cotranslationally cleaved LmxGT1-TaV2A-Luc ORF, was also monitored during transformation of promastigotes to intracellular amastigotes (Fig. 4C). Experiments were done by use of the ∆lmxgt1-3 and ∆lmxgt1 null mutants as the genetic background. Signal for LmxGT1-TaV2A and Luc proteins is present in the episome-containing promastigotes (Epi+). In contrast, the LmxGT1-TaV2A polypeptide is not detectable in amastigotes of the episome-carrying line (Epi+), whereas Luc polypeptide is clearly detectable. As expected, neither protein is present in the episome-negative lines (Epi−). These results confirm that expression of LmxGT1 is strongly down-regulated when parasites transform from promastigotes to intracellular amastigotes. Stage-specific regulation is achieved by increasing the rate of LmxGT1 turnover in amastigotes, as the Luc marker protein, whose translation is initiated from the LmxGT1 start codon, is still expressed robustly.
∆lmxgt1 null mutants undergo catastrophic loss of viability at high cell density
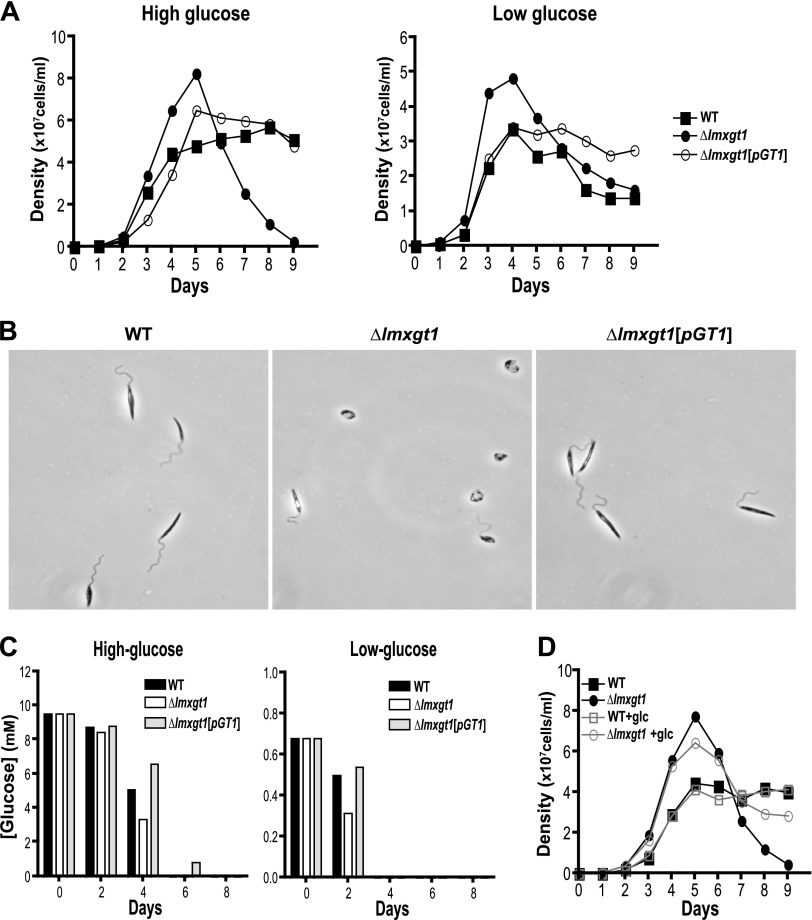
To explore potential functions of the LmxGT1 transporter, WT, ∆lmxgt1, and ∆lmxgt1[pGT1] lines were first passed through BALB/c mice. We then examined growth curves of ∆lmxgt1 null mutants compared with WT and ∆lmxgt1[pGT1] add-back parasites (Fig. 5). In high glucose medium, WT and add-back parasites grew logarithmically and then entered stationary phase between 4 and 6 × 107 cells/ml. ∆lmxgt1 null mutants somewhat surpassed this density, reaching ∼8 × 107 cells/ml, but in striking contrast to WT parasites, they did not enter stationary phase but rather experienced a catastrophic loss of viability shortly thereafter (Fig. 5A, left, filled circles). By day 8 of growth, ∆lmxgt1 parasites were largely dead, whereas WT and add-back lines were still vital (Fig. 5B), and by day 9, very few null mutant parasites remained alive. Notably, loss of viability for ∆lmxgt1 parasites initiated after day 5 when glucose in the medium became depleted (Fig. 5C), suggesting that these two events could be linked for the null mutant but not for WT parasites.
Figure 5.
Viability of WT, ∆lmxgt1 null mutants, and ∆lmxgt1[pGT1] add-back lines as a function of days of growth in high and low glucose. A) Growth curves for each cell line in high (left) and low (right) glucose. Values plotted represent the average of triplicate measurements. B) Phase-contrast microscopic images of parasites from each line at day 8 of growth in high glucose medium. C) Quantification of glucose in the medium for each parasite line grown in high or low glucose medium. Measurements were made as in Fig. 3B. D) Cell density of WT and ∆lmxgt1 (∆lmgt1) null mutants grown without or with (+glc) daily supplementation of the medium with 2.5 mM glucose. Data were plotted as the mean of the three replicate measurements.
When similar growth curves were measured in low glucose medium, ∆lmxgt1 null mutants still attained a higher cell density than WT or add-back parasites, and they experienced a substantial decline in viability when glucose was depleted from the medium. However, under these conditions, the null mutants persisted considerably longer than those grown in high glucose medium (Fig. 5A, right), maintaining similar density (∼1.5 × 107 cells/ml) to WT parasites on day 9. These results show that the LmxGT1 permease is critical for viability of L. mexicana promastigotes when they are initially growing in abundant glucose but then experience a precipitous depletion of this nutrient. In contrast, for parasites growing under limited glucose availability, there is a significant loss of cell density in the ∆lmxgt1 null mutants, but the catastrophic loss of viability leading to cell obliteration does not occur.
To explore this phenomenon further, we repeated growth curves for WT and ∆lmxgt1 null mutants in high glucose medium, to which additional glucose (2.5 mM) was or was not added daily (Fig. 5D). Under conditions of daily glucose supplementation, ∆lmxgt1 parasites retained substantial viability after reaching the highest cell density (Fig. 5D, open circles), maintaining a density of ∼3 × 107 cells/ml by day 9, close to the density attained by WT parasites. This result indicates that the catastrophic loss of viability in ∆lmxgt1 null mutants at high cell density is indeed dependent on depletion of glucose from the medium.
One significant technical detail is that catastrophic cell death upon acute glucose depletion was only observed once the ∆lmxgt1 null mutants had been passaged through mice. This phenotype was observed in two separate ∆lmxgt1 lines that had been passaged through different animals. Whereas we do not know why this phenotype is lost upon extended cultivation in vitro, this observation raises the caution that passage of mutants through animals may be a desirable practice before analysis of phenotypes.
Phenotype of the ∆lmxgt1 null mutant in sandfly infections
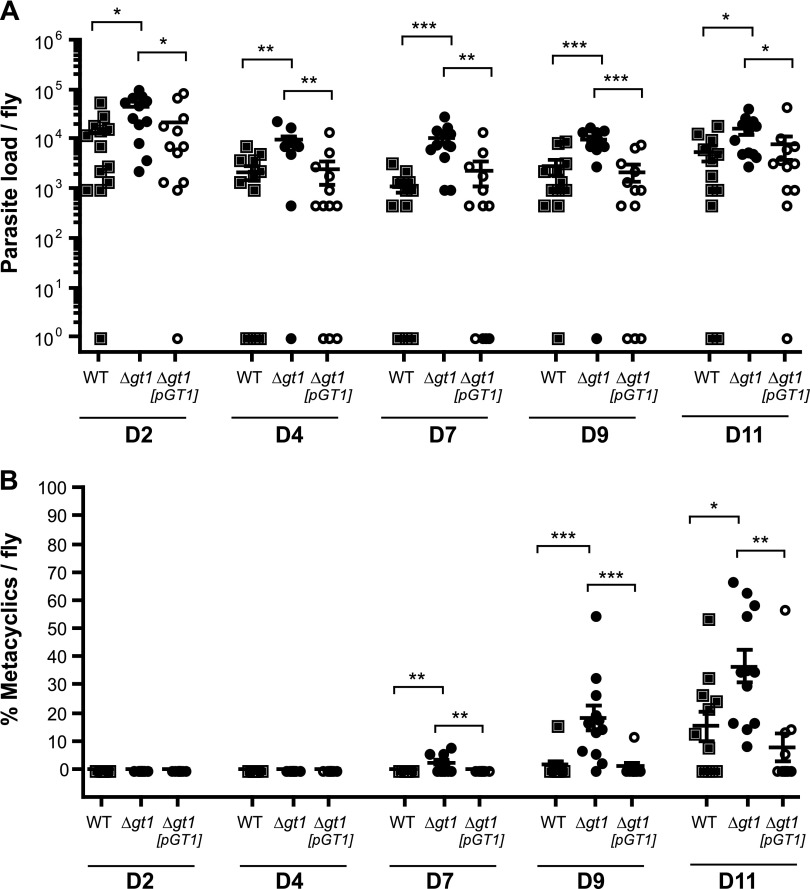
To investigate a possible role for LmxGT1 in promastigotes within their natural biologic environment, L. longipalpis sandflies were fed blood meals containing WT, ∆lmxgt1 null mutants, and ∆lmxgt1[pGT1] add-back parasites that had been passed through mice to ensure retention of virulence. The number of parasites present within the insect gut was determined between 2 and 11 d postfeeding (Fig. 6). Whereas there was variation in the number of parasites present in different flies fed upon the same parasite lines, as is typically observed in sandfly infections, the average parasite burden in flies infected with the ∆lmxgt1 null mutant was significantly higher (P<0.05–P<0.001) than that of flies infected with WT or add-back parasites (Fig. 6A), indicating that the ∆lmxgt1 null mutants grew to a somewhat higher density than WT or add-back parasites within the alimentary tract of the vector. The ability of the ∆lmxgt1 null mutant to grow to higher density in sandflies was consistent with its ability to grow to higher density in culture; however, in sandflies, the null mutants retained viability through day 11. As standard conditions for rearing sandflies in the laboratory provide continual access to sucrose, this in vivo situation is probably similar to growth of promastigotes in vitro in the presence of daily glucose supplementation (Fig. 5D), conditions in which the ∆lmxgt1 parasites also exhibit continued viability into stationary phase.
Figure 6.
Infection of L. longipalpis sandflies by WT (filled squares), ∆lmxgt1 (filled circles), and ∆lmxgt1[pGT1] (open circles) promastigotes. Sandflies were dissected from days 2, 4, 7, 9, and 11 postinfection, and the parasite load (A) and percent metacyclic forms (B) in the midgut were quantified. Error bars represent the geometric mean ± sem. In both graphs, WT, ∆gt1, and ∆gt1[pGT1] represent WT, ∆lmxgt1 null mutants, and ∆lmxgt1[pGT1] add-back promastigotes, respectively. Similar results were obtained from two independent experiments. Significant differences are shown as *P < 0.05; **P < 0.01; and ***P < 0.001.
Furthermore, morphologic characterization of development into infective-stage metacyclics in sandflies, which have considerably shorter cell bodies and longer flagella than procyclic promastigotes (38), indicated that ∆lmxgt1 null mutants exhibit enhanced metacyclogenesis compared with the other two parasite lines, as metacyclic forms appeared earlier in the ∆lmxgt1 null mutant and were present in significantly higher percentage (Fig. 6B). To evaluate whether the absence of LmxGT1 increased parasite virulence in mammals, murine bone marrow-derived macrophages were infected with the same number of WT or ∆lmxgt1 promastigotes at stationary phase, and the number of intracellular parasites was quantified at 2 h and 1 and 5 d postinfection. There were no significant differences in the number of amastigotes between ∆lmxgt1 and WT parasites after 5 d of infection (Supplemental Fig. 4), suggesting that the absence of LmxGT1 in these parasites neither enhanced nor impaired parasite infectivity in the mammalian host. Thus, LmxGT1 is not necessary for metacyclogenesis or infectivity to mammals.
DISCUSSION
LmxGT1 as a potential glucose transceptor
The observation that Leishmania parasites express three distinct glucose/hexose transporters suggests that each permease mediates distinct biologic functions, despite the large degree of sequence conservation and shared substrate specificity and affinity. Hence, the elucidation of functional differences between these proteins is crucial for understanding their unique roles in parasite biology. The observation that LmxGT1 localizes selectively to the parasite flagellum has led to the suspicion that it might have a role in glucose sensing, consistent with the established sensory functions of many ciliary and flagellar proteins in a wide range of eukaryotes (15, 16).
In this study, we have demonstrated that ∆lmxgt1 null mutants, grown in medium containing abundant glucose, experience catastrophic loss of viability, concomitant with the depletion of glucose in the medium. This profound loss of viability is not observed in WT or add-back parasites or in null mutants grown in low glucose or supplemented continually with glucose. Furthermore, the steady-state level of LmxGT1 is relatively low in glucose-rich medium, but the protein is stabilized and accumulates to a higher level once glucose is depleted. Together, these results imply that LmxGT1 likely plays a nutrient-sensing role that detects glucose depletion, spares parasite vitality, and allows entry of promastigotes into stable stationary phase. Whereas growth to high density in glucose-replete medium initially results in strong down-regulation of LmxGT1, this permease is reinduced at high cell density when parasites deplete glucose from the medium (Fig. 3A and Supplemental Fig. 2B, C). Hence, the protein is present in the flagellar membrane at the time when the critical transition from growth to stationary phase occurs and could thus play a role in this process.
Various membrane transporters or transporter-like proteins have established roles as nutrient sensors (39–42), and these proteins have been designated “transceptors” (40). Some of these sensors, such as the Snf3 and Rgt2 glucose sensors in Saccharomyces cerevisiae (39), bind their cognate nutrients but do not transport them, whereas others, such as the amino acid permease Gap1, the phosphate transporter Pho84, and the ammonium channel Mep2 (41), serve both sensory and transport functions. Features shared by many transceptors include extended N- or C-terminal hydrophilic domains compared with paralogs that are transporters but not sensors, low levels of expression, and induction of expression by depletion of the nutrient ligand in the growth medium. All of these properties are exhibited by LmxGT1, consistent with the idea that it could function as a glucose transceptor that both transports and senses glucose. However, another potential explanation, which we cannot rule out currently, is that delivery of glucose to the flagellum by LmxGT1 per se is the critical role that is mediated by this permease in monitoring glucose availability. Intraflagellar glucose or one of its metabolites might then bind to another component of the flagellum that acts as the primary sensor. In this scenario, LmxGT1 would play a role in glucose sensing as a result of its location in the flagellum, but it would not itself be a glucose sensor. The definitive distinction between these two models for LmxGT1 involvement in glucose sensing will require an additional substantial body of work.
It is notable that three nontransporting transceptors in yeast—Snf3, Rgt2, and the amino acid transceptor Ssy1—interact with associated proteins via their extended N or C termini [reviewed in ref. (40)]. These associated proteins constitute the initial components in a signal transduction pathway that controls transcription of bona fide glucose or amino acid transporter genes. As transcriptional control is essentially absent in kinetoplastid protozoa (43), we do not anticipate that LmxGT1 would be involved in this level of regulation. However, other transceptors stimulate PKA (40) or TOR kinase (42) signaling pathways to regulate a variety of cellular processes. Hence, one possibility is that the extended N terminus of LmxGT1 may interact with accessory proteins to initiate a signal transduction cascade. Notably, the KHARON1 protein interacts with this N-terminal domain to mediate flagellar targeting of LmxGT1, but other candidate-interacting proteins were also detected in these protein–protein interaction experiments (20), and some of these might mediate transmission of a glucose-responsive signal.
It is notable that a study on the three TOR kinase genes of L. major revealed that a null mutant in LmTOR3 acquired glucose sensitivity (44). Specifically, the null mutant exhibited significant cell death upon transfer from a glucose-replete to glucose-deficient medium, but this sensitivity was not exhibited by WT or add-back parasites. This phenotype is reminiscent of the cell death phenotype shown by ∆lmxgt1 null mutants when they exhaust glucose in the medium. Given the well-established role of TOR kinases in sensing nutrient status in many organisms (45, 46) and their control over growth, cell size, and autophagy, a potential role for these proteins downstream of LmxGT1 is worthy of investigation. It is also noteworthy, albeit of uncertain significance, that the TOR1 gene is located almost immediately upstream from the GT1 gene on chromosome 36 in various species of Leishmania (TriTrypDB database, http://tritrypdb.org/tritrypdb/), with these two genes separated by only one intervening gene. Whereas clustering of functionally related genes is not the rule in the Leishmania genome, it does occur occasionally, as for the genes encoding pyrimidine biosynthetic enzymes (http://tritrypdb.org/tritrypdb/), thus raising the possibility that the apposition of GT1 and TOR1 genes could reflect a functional linkage. Nonetheless, potential differences between the poorly understood signal transduction systems of Leishmania and those of better-studied eukaryotes could present a challenge for identifying relevant mechanisms of signal transduction by LmxGT1. Hence, a focus on LmxGT1-interacting partners may be the most judicious approach for initiating the elucidation of potential glucose-sensing pathways in these parasites.
Catastrophic cell death in ∆lmxgt1 null mutants
What induces rapid cell death in ∆lmxgt1 null mutants that deplete glucose from the medium is currently uncertain. However, a potentially interesting parallel occurs in tumor cells, many of which are especially susceptible to nutrient starvation. In these cells, the phosphatidylinositol-3 kinase pathway, which includes mammalian TOR as one of its components, is often constitutively activated, leading to enhancement of cell metabolism, protein synthesis, and cell growth and down-regulation of autophagy, even when nutrients are limiting. This activated metabolic wiring can lead to “metabolic catastrophe” (47) via induction of apoptosis or necrosis when such tumor cells are exposed to nutrient stress. The observation that ∆lmxgt1 null mutants initially overgrow somewhat the level of WT or add-back parasites is consistent with such growth-promoting metabolic wiring. Apparently, the failure to switch from a growth to a stationary-phase mode results in cell death. It is also intriguing that ∆lmxgt1 null mutants that are growing on limiting glucose evade catastrophic cell death (Fig. 4A, right), suggesting that they may be metabolically preadapted to the consequences of glucose limitation and are thus not susceptible to metabolic catastrophe. Thus, the determination of the relative use of various metabolic pathways in WT and ∆lmxgt1 cells during growth, stationary phase, and catastrophic death phases and in the presence of abundant and limiting glucose may be important for dissecting biochemical mechanisms for the observed phenotype of the null mutant.
In the context of sandfly infections, ∆lmxgt1 null mutant promastigotes populate the sandfly midgut more densely than WT promastigotes (Fig. 6). As sandflies had access to a continual supply of sucrose and hence, of glucose in these experiments, the parasites presumably do not encounter severe glucose depletion, and thus, an abrupt loss of viability was not seen in these in vivo experiments. However in the natural state, parasites are likely to experience depletion of glucose within the sandfly when the insect has not ingested a recent sugar meal. Hence, the ability of LmxGT1 to sense sugar depletion and promote a switch to stable stationary phase is probably important for parasite viability and transmission in the wild. Although the ∆lmxgt1 null mutants transformed into metacyclic parasites earlier than WT promastigotes, we do not currently have a mechanistic explanation for this phenotypic difference.
Regulation of LmxGT1 expression
In this study, we have established that LmxGT1 is regulated by both glucose concentration and during the transformation of promastigotes to amastigotes, primarily at the level of LmxGT1 protein turnover. Furthermore, we have introduced a novel approach for determining whether a protein is regulated at the level of translation or protein turnover, two mechanisms of posttranscriptional regulation that likely play important roles in gene regulation in an organism that does not exhibit transcriptional regulation (43). This method relies on measuring the relative level of the protein of interest, LmxGT1, in this case, and of the Luc polypeptide, both of which are translated initially as part of a single polyprotein but are subsequently cleaved cotranslationally at the intervening TaV2A sequence. Coregulation of LmxGT1 and Luc would be diagnostic of translational control, whereas the disparate levels of expression observed here (Figs. 3C, D and 4C) indicate differential rates of protein turnover. This approach circumvents the difficulties of carrying out cumbersome pulse-chase experiments, measurements that are typically not feasible for membrane proteins of low abundance, such as LmxGT1, where the inability to incorporate sufficient radiolabel during a short pulse is a serious limitation. The method is also superior to measurements of protein half-lives following treatment with cycloheximide (Fig. 3E), as it does not require incubation with drugs that can alter cellular physiology.
An important mechanism for regulating expression of many transporters and receptors involves ubiquitination, followed by internalization and degradation in the lysosome (48). Whereas we have not investigated the specific mechanism for regulating turnover of LmxGT1, a ubiquitin-dependent process is a likely explanation. Indeed, a previous study (32) on LmxGT2, LmxGT3, and the myoinositol transporter LmxMIT confirmed that these permeases are targeted to the multivesicular tubular lysosome in stationary-phase promastigotes and in amastigotes and that constitutive targeting to this organelle occurs for fusion proteins in which each permease is linked to a ubiquitin ORF.
In conclusion, we have presented evidence consistent with a role for the flagellar GT LmxGT1 as a glucose sensor. These results provide the first phenotype for the ∆lmxgt1 null mutant and offer insight into the likely function in the parasite lifecycle of this unique permease. The inability of promastigotes to survive without LmxGT1 under conditions of acute glucose depletion underscores the critical importance of this transporter during the parasite lifecycle. Additional studies will be required to define further the potential sensory role of LmxGT1, including the search for other proteins that may associate with this membrane protein and transduce a signal mediated by the presence or absence of the glucose ligand in its binding site. Possible implication of other signaling cascades that might operate downstream of LmxGT1, such as TOR kinase or PKA pathways, will also be important topics for future investigations.
Although kinetoplastid parasites must sense many changes in environmental conditions to progress through their lifecycles (1), there is currently a remarkable paucity of knowledge about how this occurs. These parasites do not encode GPCRs or tyrosine kinase receptors in their genomes (49), and there are, to our knowledge, no known sensory membrane proteins with identified ligands and defined biologic functions, with the possible exception of LmaAQP1 that functions as a flagellar aquaglyceroporin channel and is involved in osmotaxis and osmoregulation (9). Hence, the implication of LmxGT1 as a potential glucose sensor that controls the switch from growth to stationary phase represents a novel opportunity to dissect a sensory pathway critical to the parasite lifecycle.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NIH) Grants AI25920 (to S.M.L.) and AI023682 (to P.A.Y.). This work was partially supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, NIH. This project was supported by Shared Instrumentation Grant S10-RR023432 from the National Center for Research Resources (NCRR), a component of NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The authors thank Claudio Meneses for maintaining the sandfly colony used in this work. The authors acknowledge Aurelie Snyder and the Advanced Light Microcopy Core at The Jungers Center for technical assistance.
Glossary
Abbreviations:
- GFP
green fluorescent protein
- GT
glucose transporter
- GT1-2A
LmxGT1 glucose transporter fused at its C terminus to the TaV2A peptide or LmxGT1-TaV2A
- HA
influenza virus hemagglutinin epitope tag
- Luc
luciferase
- NCRR
National Center for Research Resources
- OHSU
Oregon Health & Science University
- ORF
open-reading frame
- PPM
pellicular plasma membrane
- qRT-PCR
quantitative real-time PCR
- TOR
target of rapamycin
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Rodrigues J. C., Godinho J. L., and de Souza W. (2014) Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell. Biochem. 74, 1–42 [DOI] [PubMed] [Google Scholar]
- 2.Gluenz E., Ginger M. L., and McKean P. G. (2010) Flagellum assembly and function during the Leishmania life cycle. Curr. Opin. Microbiol. 13, 473–479 [DOI] [PubMed] [Google Scholar]
- 3.Gluenz E., Höög J. L., Smith A. E., Dawe H. R., Shaw M. K., and Gull K. (2010) Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 24, 3117–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landfear S. M. (2011) Nutrient transport and pathogenesis in selected parasitic protozoa. Eukaryot. Cell 10, 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naderer T., and McConville M. J. (2008) The Leishmania-macrophage interaction: a metabolic perspective. Cell. Microbiol. 10, 301–308 [DOI] [PubMed] [Google Scholar]
- 6.Engman D. M., Krause K.-H., Blumin J. H., Kim K. S., Kirchhoff L. V., and Donelson J. E. (1989) A novel flagellar Ca2+-binding protein in trypanosomes. J. Biol. Chem. 264, 18627–18631 [PubMed] [Google Scholar]
- 7.Paindavoine P., Rolin S., Van Assel S., Geuskens M., Jauniaux J. C., Dinsart C., Huet G., and Pays E. (1992) A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol. Cell. Biol. 12, 1218–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper R. C., Xu X., Russell D. G., Little B. M., and Landfear S. M. (1995) Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J. Cell Biol. 128, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figarella K., Uzcategui N. L., Zhou Y., LeFurgey A., Ouellette M., Bhattacharjee H., and Mukhopadhyay R. (2007) Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol. Microbiol. 65, 1006–1017 [DOI] [PubMed] [Google Scholar]
- 10.Tull D., Vince J. E., Callaghan J. M., Naderer T., Spurck T., McFadden G. I., Currie G., Ferguson K., Bacic A., and McConville M. J. (2004) SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol. Biol. Cell 15, 4775–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran K. D., Rodriguez-Contreras D., Shinde U., and Landfear S. M. (2012) Both sequence and context are important for flagellar targeting of a glucose transporter. J. Cell Sci. 125, 3293–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberholzer M., Langousis G., Nguyen H. T., Saada E. A., Shimogawa M. M., Jonsson Z. O., Nguyen S. M., Wohlschlegel J. A., and Hill K. L. (2011) Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol. Cell. Proteomics 10, M111.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maric D., Epting C. L., and Engman D. M. (2010) Composition and sensory function of the trypanosome flagellar membrane. Curr. Opin. Microbiol. 13, 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan K. T., Ames J. B., Asfaw S. H., Wingard J. N., Olson C. L., Campana P. T., Araújo A. P., and Engman D. M. (2005) A flagellum-specific calcium sensor. J. Biol. Chem. 280, 40104–40111 [DOI] [PubMed] [Google Scholar]
- 15.Pazour G. J., and Witman G. B. (2003) The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 15, 105–110 [DOI] [PubMed] [Google Scholar]
- 16.Berbari N. F., O’Connor A. K., Haycraft C. J., and Yoder B. K. (2009) The primary cilium as a complex signaling center. Curr. Biol. 19, R526–R535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Contreras D., Feng X., Keeney K. M., Bouwer H. G., and Landfear S. M. (2007) Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana. Mol. Biochem. Parasitol. 153, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchmore R. J., and Landfear S. M. (1998) Differential regulation of multiple glucose transporter genes in Leishmania mexicana. J. Biol. Chem. 273, 29118–29126 [DOI] [PubMed] [Google Scholar]
- 19.Burchmore R. J. S., Rodriguez-Contreras D., McBride K., Merkel P., Barrett M. P., Modi G., Sacks D., and Landfear S. M. (2003) Genetic characterization of glucose transporter function in Leishmania mexicana. Proc. Natl. Acad. Sci. USA 100, 3901–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran K. D., Rodriguez-Contreras D., Vieira D. P., Yates P. A., David L., Beatty W., Elferich J., and Landfear S. M. (2013) KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J. Biol. Chem. 288, 22721–22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iovannisci D. M., and Ullman B. (1983) High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J. Parasitol. 69, 633–636 [PubMed] [Google Scholar]
- 22.Fulwiler A. L., Soysa D. R., Ullman B., and Yates P. A. (2011) A rapid, efficient and economical method for generating leishmanial gene targeting constructs. Mol. Biochem. Parasitol. 175, 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdés R., Vasudevan G., Conklin D., and Landfear S. M. (2004) Transmembrane domain 5 of the LdNT1.1 nucleoside transporter is an amphipathic helix that forms part of the nucleoside translocation pathway. Biochemistry 43, 6793–6802 [DOI] [PubMed] [Google Scholar]
- 24.Feng X., Rodriguez-Contreras D., Polley T., Lye L. F., Scott D., Burchmore R. J., Beverley S. M., and Landfear S. M. (2013) 'Transient' genetic suppression facilitates generation of hexose transporter null mutants in Leishmania mexicana. Mol. Microbiol. 87, 412–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapler G. M., Coburn C. M., and Beverley S. M. (1990) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 10, 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seyfang A., and Landfear S. M. (2000) Four conserved cytoplasmic sequence motifs are important for transport function of the Leishmania inositol/H+ symporter. J. Biol. Chem. 275, 5687–5693 [DOI] [PubMed] [Google Scholar]
- 27.Feng X., Feistel T., Buffalo C., McCormack A., Kruvand E., Rodriguez-Contreras D., Akopyants N. S., Umasankar P. K., David L., Jardim A., Beverley S. M., and Landfear S. M. (2011) Remodeling of protein and mRNA expression in Leishmania mexicana induced by deletion of glucose transporter genes. Mol. Biochem. Parasitol. 175, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz D., Valdés R., Sanchez M. A., Hayenga J., Elya C., Detke S., and Landfear S. M. (2010) Purine restriction induces pronounced translational upregulation of the NT1 adenosine/pyrimidine nucleoside transporter in Leishmania major. Mol. Microbiol. 78, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamhawi S., Modi G. B., Pimenta P. F., Rowton E., and Sacks D. L. (2000) The vectorial competence of Phlebotomus sergenti is specific for Leishmania tropica and is controlled by species-specific, lipophosphoglycan-mediated midgut attachment. Parasitology 121, 25–33 [DOI] [PubMed] [Google Scholar]
- 30.Seyfang A., and Landfear S. M. (1999) Substrate depletion upregulates uptake of myo-inositol, glucose and adenosine in Leishmania. Mol. Biochem. Parasitol. 104, 121–130 [DOI] [PubMed] [Google Scholar]
- 31.Richard D., Leprohon P., Drummelsmith J., and Ouellette M. (2004) Growth phase regulation of the main folate transporter of Leishmania infantum and its role in methotrexate resistance. J. Biol. Chem. 279, 54494–54501 [DOI] [PubMed] [Google Scholar]
- 32.Vince J. E., Tull D., Landfear S., and McConville M. J. (2011) Lysosomal degradation of Leishmania hexose and inositol transporters is regulated in a stage-, nutrient- and ubiquitin-dependent manner. Int. J. Parasitol. 41, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittra B., Cortez M., Haydock A., Ramasamy G., Myler P. J., and Andrews N. W. (2013) Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 210, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha D. S., Schwarz J. K., Turco S. J., and Beverley S. M. (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77, 57–64 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W. W., McCall L. I., and Matlashewski G. (2013) Role of cytosolic glyceraldehyde-3-phosphate dehydrogenase in visceral organ infection by Leishmania donovani. Eukaryot. Cell 12, 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heras S. R., Thomas M. C., García-Canadas M., de Felipe P., García-Pérez J. L., Ryan M. D., and López M. C. (2006) L1Tc non-LTR retrotransposons from Trypanosoma cruzi contain a functional viral-like self-cleaving 2A sequence in frame with the active proteins they encode. Cell. Mol. Life Sci. 63, 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bates P. A. (1993) Axenic culture of Leishmania amastigotes. Parasitol. Today (Regul. Ed.) 9, 143–146 [DOI] [PubMed] [Google Scholar]
- 38.Bates P. A. (1994) The developmental biology of Leishmania promastigotes. Exp. Parasitol. 79, 215–218 [DOI] [PubMed] [Google Scholar]
- 39.Özcan S., and Johnston M. (1999) Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63, 554–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holsbeeks I., Lagatie O., Van Nuland A., Van de Velde S., and Thevelein J. M. (2004) The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem. Sci. 29, 556–564 [DOI] [PubMed] [Google Scholar]
- 41.Thevelein J. M., and Voordeckers K. (2009) Functioning and evolutionary significance of nutrient transceptors. Mol. Biol. Evol. 26, 2407–2414 [DOI] [PubMed] [Google Scholar]
- 42.Hundal H. S., and Taylor P. M. (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am. J. Physiol. Endocrinol. Metab. 296, E603–E613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton C., and Shapira M. (2007) Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol. Biochem. Parasitol. 156, 93–101 [DOI] [PubMed] [Google Scholar]
- 44.Madeira da Silva L., and Beverley S. M. (2010) Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc. Natl. Acad. Sci. USA 107, 11965–11970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarbassov D. D., Ali S. M., and Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 46.Wullschleger S., Loewith R., and Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 47.Jin S., DiPaola R. S., Mathew R., and White E. (2007) Metabolic catastrophe as a means to cancer cell death. J. Cell Sci. 120, 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda M., and Sorkin A. (2007) Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol. Interv. 7, 157–167 [DOI] [PubMed] [Google Scholar]
- 49.El-Sayed N. M., Myler P. J., Bartholomeu D. C., Nilsson D., Aggarwal G., Tran A. N., Ghedin E., Worthey E. A., Delcher A. L., Blandin G., Westenberger S. J., Caler E., Cerqueira G. C., Branche C., Haas B., Anupama A., Arner E., Aslund L., Attipoe P., Bontempi E., Bringaud F.,, Burton P., Cadag E., Campbell D. A., Carrington M., Crabtree J., Darban H., da Silveira J. F., de Jong P., Edwards K., Englund P. T., Fazelina G., Feldblyum T., Ferella M., Frasch A. C., Gull K., Horn D., Hou L., Huang Y., Kindlund E., Klingbeil M., Kluge S., Koo H., Lacerda D., Levin M. J., Lorenzi H., Louie T., Machado C. R., McCulloch R., McKenna A., Mizuno Y., Mottram J. C., Nelson S., Ochaya S., Osoegawa K., Pai G., Parsons M., Pentony M., Pettersson U., Pop M., Ramirez J. L., Rinta J., Robertson L., Salzberg S. L., Sanchez D. O., Seyler A., Sharma R., Shetty J., Simpson A. J., Sisk E., Tammi M. T., Tarleton R., Teixeira S., Van Aken S., Vogt C., Ward P. N., Wickstead B., Wortman J., White O., Fraser C. M., Stuart K. D., and Andersson B. (2005) The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309, 409–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.