Abstract
BACKGROUND & AIMS
Little is known about the incidence of drug-induced liver injury (DILI) and risk factors for adverse outcomes. We evaluated short-term outcomes of a large cohort of patients with DILI enrolled in an ongoing multicenter prospective study.
METHODS
Data were collected from 660 adults with definite, highly likely, or probable DILI. Regression methods were used to identify risk factors for early liver-related death or liver transplantation and chronic liver injury.
RESULTS
Patients’ median age was 51.4 years; 59.5% were female and 59.1% required hospitalization. Within 6 months of DILI onset, 30 patients received liver transplants (4.5%; 95% confidence interval [CI], 3.0%–6.1%) and 32 died (5%; 95% CI, 3.2%–6.5%); 53% of the deaths were liver related. Asian race, itching, lung disease, low serum albumin levels, low platelet counts, and high serum levels of alanine aminotransferase and total bilirubin at presentation were independent risk factors for reduced times to liver-related death or liver transplantation (C-statistic = 0.87). At 6 months after DILI onset, 18.9% of the 598 evaluable subjects had persistent liver damage. African-American race, higher serum levels of alkaline phosphatase, and earlier heart disease or malignancy requiring treatment were independent risk factors for chronic DILI (C-statistic = 0.71).
CONCLUSIONS
Nearly 1 in 10 patients die or undergo liver transplantation within 6 months of DILI onset and nearly 1 in 5 of the remaining patients have evidence of persistent liver injury at 6 months. The profile of liver injury at presentation, initial severity, patient’s race, and medical comorbidities are important determinants of the likelihood of death/transplantation or persistent liver injury within 6 months.
Keywords: Hepatotoxicity, Acute Liver Failure, Transplantation, Causality
Drug-induced liver injury (DILI) is an infrequent cause of liver disease in the general population and accounts for <1% of hospitalized patients presenting with jaundice.1,2 Nonetheless, DILI is a leading reason for regulatory actions involving investigational and approved medications and is also a leading cause of acute liver failure in the United States.3,4 Because of its low incidence and the difficulty in establishing a diagnosis, the natural history of DILI is not well described. One frequently cited report suggested that prolonged medication administration and inadvertent drug rechallenge might be risk factors for developing chronic DILI.5 In addition, DILI patients with cholestatic liver injury might have a slower rate of biochemical improvement and be at increased risk for developing chronic liver disease.6,7 However, the number of patients reported in earlier studies was limited and variable criteria for chronic DILI were used.6-8
The Drug-Induced Liver Injury Network (DILIN) consists of 8 academic medical centers and a data-coordinating center sponsored by the National Institutes of Health. In 2004, a prospective registry study of patients with suspected DILI was initiated to improve our understanding of the etiology, risk factors, and natural history of DILI in the United States.8,9 Subjects with liver injury that persists for at least 6 months after DILI onset are followed for 2 years to better define their long-term outcomes. In the initial analysis of the first 300 patients enrolled in the DILIN database, 15% of the subjects met predefined laboratory, clinical, and/or radiographic criteria for chronic DILI at 6 months after presentation.10 In the current analysis, we set out to determine the short-term (6 month) rates of death, liver transplantation, and chronic DILI of the first 660 evaluable adult patients enrolled in the DILIN prospective study with definite, highly likely, or probable DILI. In addition, we set out to identify risk factors at presentation for early death or liver transplantation as well as for self-limited vs chronic DILI.
Methods
Drug-Induced Liver Injury Network Prospective Study
The protocol for this multicenter observational study was approved by the Institutional Review Boards at each clinical site and all enrolled subjects provided written informed consent. DILI onset was defined as the first date after a subject taking any medication or herbal and dietary supplement (HDS) met the predefined laboratory criteria for study entry. Specifically, all subjects had to have a serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level that exceeded 5× the upper limit of normal (ULN) (or 5× pretreatment baseline if baseline abnormal), a serum alkaline phosphatase (ALP) that exceeded 2× the ULN (or 2× pretreatment baseline if baseline abnormal), a total bilirubin >2.5 mg/dL, or an international normalized ratio (INR) >1.5 on 2 consecutive blood draws. All study participants were older than 2 years of age and had to be enrolled within 6 months of DILI onset. Patients with known or suspected acetaminophen hepatotoxicity, autoimmune hepatitis, or primary sclerosing cholangitis, or a history of a bone marrow or liver transplantation before DILI onset were excluded.
A detailed medical and medication history was obtained at the baseline study visit and additional laboratory and radiologic testing was performed to more fully characterize the DILI event and exclude competing etiologies. All enrolled patients were seen for a follow-up study visit at 6 months after initial enrollment and those with evidence of chronic DILI within 6 months of DILI onset were asked to return for additional follow-up visits at 12 and 24 months. Chronic DILI was defined as having a persistently elevated serum AST, ALT, ALP, or total bilirubin level, histologic evidence of ongoing liver injury, or radiologic evidence of persistent liver injury (ie, ascites on imaging) at 6 months or more after the initial DILI onset date.9,10 The causal relationship between the liver injury episode and the implicated agent(s) was evaluated in a standardized fashion by the DILIN causality committee.11 A DILIN causality score varying from 1 (Definite >95% likelihood), 2 (Very Likely 75%–95% likelihood),3 (Probable 50%–74% likelihood), 4 (Possible 25%–49% likelihood), to 5 (unlikely <25% likelihood) was assigned by consensus agreement of committee members. In subjects with 2 or more implicated drugs, an overall causality score was assigned to the case and then an individual causality score for each drug was given. For cases with multiple implicated HDS products (with or without implicated drugs), an overall causality score was assigned to the case and then a causality score for each individual drug and one overall HDS causality score for all HDS products was assigned. Only the overall causality score for the case was used for reporting. A primary implicated agent was assigned to each case as the agent with the highest individual causality score or the one with higher ranking by site if ties occur. A 3-tiered nested system was used in DILIN to classify the primary implicated agents into primary (overall class), secondary (based on use), and tertiary (based on chemical class). In addition, the severity of the DILI episode was categorized on a 5-point scale from mild (1), moderate (2), moderate-hospitalized (3), severe (4), and fatal (5), where a fatal score was assigned only if the patient died or had liver transplantation due to DILI. Before April 2009, causality was assessed from baseline data and a clinical narrative. After April 16, 2009, accrued 6-month follow-up data were included in the causality assessment, which involves 485 of the 660 patients in this study. All deaths in DILIN patients were classified by clinical site as liver-related or not. The enrolled cases with an overall causality score of “possible” (4) or “unlikely” (5) or not adjudicated yet were excluded from the current analysis, as were children (n = 36). To identify predictors of chronic liver injury, subjects with known pre-existing chronic hepatitis B virus/hepatitis C virus (n = 28) were also excluded from this analysis, as were subjects whose chronic status could not be determined due to dropping out of study before completing their 6-month study visit (n = 77).
Statistical Methods
Descriptive statistics, mean with SD or median with range, were used to describe continuous variables, and frequency and percent were used to describe categorical variables. A nonparametric test, Wilcoxon or Kruskal-Wallis test, was used for comparison of 2 or 3 groups for continuous variables, and χ2 test for comparison between groups for categorical variables. Data for early outcomes (non–liver-related death, liver-related death, or liver transplantation) within 6 months of DILI onset were first described and modeled. Then, data for chronic status at 6 months after DILI onset were described and modeled. Demographic and clinical data at DILI onset or between onset and 6 months from onset were extracted to describe and compare 2 groups with and without 2 early outcomes of interest: death or transplantation within 6 months and chronic DILI at 6 months after DILI onset. Death was subcategorized as liver or non–liver-related and descriptive statistics were used to describe and compare the 3 early adverse events: liver transplantation, liver-related death, and non–liver-related death. Cox regression hazard models were used for time to early event analyses where an early event is defined as death or liver transplantation within 6 month of onset. Clinically, it makes more sense to consider only liver-related early event, rather than lumping all death and liver transplantations together. Time to early liver-related event (liver-related death or liver transplantation) was also performed. Logistic regression was used to model the binary outcomes of chronic status. All model selection used an initial univariate analysis to screen potential candidates for multivariate modeling. Variables with a univariate P value ≤.1 were considered. For variables with known co-linearity or high correlation, clinical judgment was used to select one predictor for additional modeling, for example, jaundice and total bilirubin are highly related and only total bilirubin was used in the multivariate modeling due to its clinical objectivity. Stepwise selection procedure was used to derive the final models and the results reported either as hazard ratio or odds ratio (OR) with 95% confidence interval (CI), C-statistic was used to describe the fit of the final models.
The following potential predictors were considered in the modeling for both outcomes of interest: demographic variables (age, sex, race, weight, body mass index) at baseline visit, signs and symptoms at DILI onset (except jaundice), medical history, latency, duration of primary agent use, laboratory parameters (white blood cell count, absolute eosinophil count, platelets, serum creatinine, antinuclear antibodies, anti-Smith antibodies) at DILI onset, liver biochemistries (ALT, ALP, total bilirubin, Hy’s law, INR, albumin, hemoglobin) at DILI onset. Predictor variables with >50% missing data were not considered further in the modeling. Analyses were carried out on subjects without missing outcomes data, with the assumption that there were no differences between the subjects with and without outcomes data. Subjects with and without known early outcomes were compared in terms of characteristics to assess whether the data are missing at random. Due to multiple comparisons with a large number of variables, we assume that data are missing at random if we observed <5% of significant differences at.05 level. All P values reported are 2-sided and a level of .05 or less is considered statistically significant. All data were analyzed by SAS software (version 9.2, SAS Institute, Inc, Cary, NC).
Results
Patient Population
There were 991 patients enrolled in the DILIN prospective registry from September 2004 through July 31, 2011, which included 801 patients that were adjudicated as definite, highly likely, or probable DILI (Figure 1). Of the 801 DILI patients, an additional 141 patients were excluded from this analysis due to age younger than 18 years (n = 36), pre-existing chronic hepatitis B or C infection (n = 28), or with missing chronic status due to dropping out of the study before 6 months follow-up (n = 77). Of the remaining 660 patients, there were 62 patients who either died (n = 32) or underwent liver transplantation (n = 30) within 6 months of DILI onset. Therefore, 598 total adult DILIN patients had data available at baseline and 6 months after DILI onset for analysis of chronic DILI risk factors. Of note, the clinical and presenting features of the 77 patients with incomplete follow-up were not significantly different from the 660 patients included in this analysis, except that the excluded patients were significantly younger and more likely to be Hispanic (data not shown). Sensitivity analyses assessing impact of missing data in these 77 subjects were not performed.
Figure 1.
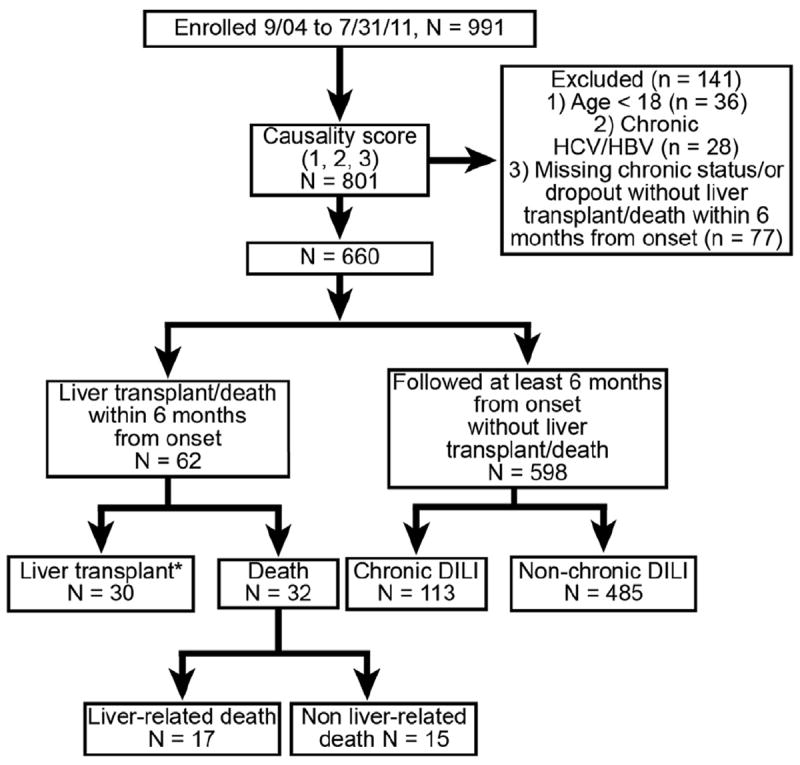
Overview of the study population.
*One subject died after liver transplant
Death and Liver Transplantation Within 6 Months of Drug-Induced Livery Injury Onset
Table 1 provides a descriptive summary of the presenting features of the 62 patients who died or underwent liver transplantation compared with the 598 subjects without these events by month 6. A total of 30 patients (4.5%; 95% CI, 3.0%–6.1%) underwent transplantation and 32 (5%; 95% CI, 3.2%–6.5%) died; 53% of the deaths liver related. Among subjects with an acute hepatocellular injury (ie, R > 5), the percent of early death/transplantation was 13% (95% CI, 9%–17%) compared with 6% (95% CI, 3%–9%) in those with a mixed or cholestatic injury profile. Subjects who died or underwent transplantation were significantly more likely to be Asians and to have underlying diabetes mellitus, lung disease, and various other medical comorbidities. These 62 patients had similar clinical symptoms at presentation compared with those that survived, but their total white blood cell counts at presentation were significantly higher and mean eosinophil count at DILI onset was lower. As expected, subjects who died or underwent transplantation had higher mean serum ALT, total bilirubin, INR, and Model for End-Stage Liver Disease scores compared with those who survived.12 In addition, these patients were more likely to have severe acute hepatocellular injury with a higher median R value at presentation (11.0 vs 5.1; P < .01). In addition, the proportion of patients that met Hy’s law criteria (ie, serum ALT >3× ULN, ALP <2× ULN, and total bilirubin >2× ULN) was greater in those with transplantation/fatal outcomes compared with the survivors (45.8%vs 26.2%; P < .01). Patients with early adverse outcomes were also more likely to have received corticosteroids, and use of ursodeoxycholic acid was similar in both groups. Interestingly, the causality scores of those with early adverse outcomes who did not improve during follow-up were, as a group, lower compared with those who survived.
Table 1.
Characteristics of Patients by 6-Month Outcome Status From Drug-Induced Liver Injury Onset
| Featurea | Death or liver transplantation (n = 62) | Alive with native liver at 6 months (n = 598) | P value |
|---|---|---|---|
| Age, y, mean ± SD | 53.0 ± 15.6 | 50.4 ± 15.9 | .28 |
| Female, % | 58.1 | 59.7 | .89 |
| Race, % | .01 | ||
| White | 67.2 | 81.1 | |
| Black | 16.4 | 10.6 | |
| Asian | 9.8 | 2.5 | |
| Others | 6.6 | 5.9 | |
| Body mass index, mean ± SD | 28.8 ± 7.0 | 27.8 ± 6.6 | .19 |
| Signs and symptoms at DILI onset, % | |||
| Fever | 29.0 | 27.1 | .77 |
| Rash | 17.7 | 27.8 | .10 |
| Itching | 27.4 | 58.5 | <.01 |
| Medical history, % | |||
| Diabetes mellitus | 43.5 | 23.4 | <.01 |
| Heart disease | 29.0 | 19.6 | .10 |
| Kidney disease | 22.6 | 11.5 | .02 |
| Lung disease | 29.0 | 16.9 | .02 |
| Malignancy | 27.4 | 8.5 | <.01 |
| Active malignancy | 17.7 | 3.8 | <.01 |
| HIV+ | 1.6 | 1.8 | 1 |
| Self-reported drug allergies, % | 58.1 | 44.6 | .05 |
| Pre-existing liver disease, %a | 24.2 | 10.7 | <.01 |
| Prior alcohol use, % | 46.7 | 52.5 | .42 |
| Implicated agents | |||
| Medication use, d, median (range) | 67 (2–3112) | 31 (1–6971) | .02 |
| Categories, % | .98 | ||
| Single drug | 56.5 | 55.7 | |
| Single HDS | 8.1 | 8.7 | |
| Multiple drugs and/or HDS | 35.5 | 35.6 | |
| Primary implicated agent class, n (%) | .97 | ||
| Antimicrobial | 27 (43.5) | 270 (45.2) | .89 |
| HDS | 10 (16.1) | 98 (16.4) | 1.00 |
| Cardiovascular | 5 (8.1) | 66 (11.1) | .67 |
| Central nervous system | 6 (9.7) | 45 (7.5) | .61 |
| Antineoplastic | 7 (11.3) | 28 (4.7) | .04 |
| Analgesic | 1 (1.6) | 26 (4.4) | .50 |
| Immunomodulatory | 1 (1.6) | 19 (3.2) | .71 |
| Endocrine | 1 (1.6) | 14 (2.3) | 1.00 |
| Rheumatologic | 2 (3.2) | 8 (1.3) | .24 |
| Other | 2 (3.2) | 23 (3.9) | 1.00 |
| Laboratory parameters at DILI onset | |||
| White blood cell count, 103/mm3, median (range) | 7.9 (0.1–20.2) | 6.3 (0.9–58.6) | <.01 |
| Absolute eosinophil count, μL, median (range) | 65 (0–1612) | 147 (0–7420) | <.01 |
| Eosinophils >500/μL, % | 10.2 | 10.1 | 1.00 |
| Serum creatinine, mg/dL, median (range) | 1.0 (0.5–7.3) | 0.9 (0.1–7.6) | <.01 |
| Antinuclear antibody+, % | 32.8 | 28.2 | .45 |
| Anti-Smith antibody+, % | 25.9 | 20.6 | .38 |
| Liver biochemistries at DILI onset, median (range) | |||
| Serum AST, U/L | 1093 (76–4925) | 287 (26–12,802) | <.01 |
| Serum ALT, U/L | 1051 (23–4212) | 466 (14–15,065) | <.01 |
| ALP, U/L | 192 (94–1004) | 220 (35–1952) | .33 |
| Total bilirubin, mg/dL | 9.5 (0.4–33.4) | 4.9 (0.2–45.7) | <.01 |
| INR | 1.8 (0.9–6.2) | 1.1 (0.8–12.2) | <.01 |
| Albumin, g/dL | 3.0 (1.1–4.2) | 3.7 (1.0–5.8) | <.01 |
| MELD score | 24.0 (6.0–40.0) | 16.0 (6.0–40.0) | <.01 |
| Hy’s law, %b | 45.8 | 26.2 | <.01 |
| R value, median (range) | 11.0 (0.2–82.4) | 5.1 (0.1–426.2) | <.01 |
| Pattern of injury, % | <.01 | ||
| Cholestatic | 14.5 | 25.8 | |
| Mixed | 14.5 | 23.6 | |
| Hepatocellular | 71.0 | 50.7 | |
| Liver biochemistries at peak,c median (range) | |||
| Peak serum AST, U/L | 1494 (80–6924) | 389 (45–12,802) | <.01 |
| Peak serum ALT, U/L | 1329 (70–5831) | 558 (53–15,065) | <.01 |
| Peak ALP, U/L | 308 (119–2414) | 281 (50–2865) | .15 |
| Peak total bilirubin, mg/dL | 25.9 (0.8–59.0) | 9.1 (0.3–63.0) | <.01 |
| Peak INR | 3.4 (1.1–12.9) | 1.1 (0.8–13.1) | <.01 |
| Lowest albumin, g/dL | 2.1 (0.9–3.5) | 3.2 (0.4–4.9) | <.01 |
| Peak R value | 17.1 (0.5–210.4) | 6.6 (0.3–426.2) | <.01 |
| Causality assessment score, n (%)c | <.01 | ||
| Definite | 13 (21.0) | 167 (27.9) | |
| Very likely | 26 (41.9) | 315 (52.7) | |
| Probable | 23 (37.1) | 116 (19.4) | |
| Steroid use, %c | 82.0 | 36.6 | <.01 |
| Ursodeoxycholic acid use, %c | 27.9 | 19.8 | .14 |
| DILIN severity score, n (%)c | <.01 | ||
| Mild | 2 (3.2) | 150 (25.1) | |
| Moderate | 2 (3.2) | 147 (24.6) | |
| Moderate-hospitalized | 2 (3.2) | 187 (31.3) | |
| Severe | 0 (0) | 114 (19.1) | |
| Fatal/transplantation | 56 (90.3) | 0 (0) |
HIV, human immunodeficiency virus; SD, standard deviation.
Per patient self-report. “Malignancy” refers to lifetime history of cancer and “active malignancy” delineates the subgroup of patients with a malignancy requiring treatment at the time of DILI onset.
Hy’s law criteria are subjects with ALT >3× ULN and total bilirubin >2.5 mg/dL at DILI onset with ALP <2× ULN.
Variables observed based on data from onset to the 6-month study visit. All other variables were either observed at DILI onset or at the baseline study visit, where the values would be similar to DILI onset.
Median duration of suspect medication use was significantly longer in those with an early adverse outcome compared with the survivors (67 vs 31 days; P = .02). Although there were many different implicated drugs and HDS products, anti-neoplastic drugs were significantly more commonly reported in those with early adverse outcomes (Table 1).
Among the 62 subjects with an early adverse outcome, 32 patients died; 17 (53%) of the deaths were judged to be liver related, and the remaining 15 were attributed to nonhepatic causes. The Kaplan-Meier curves for time to non–liver-related death, liver-related death, and liver transplantation are presented in Figure 2 with event rates of 2.5%, 2.8%, and 4.8% at 6 months from onset, respectively. Mean time from DILI onset to liver-related death was 56 ± 48 days (range, 5–171 days), to non–liver-related death was 76 ± 51 days (range, 16–171 days), and to liver transplantation was 35 ± 28 (range, 2–131 days). Other important differences between these groups are shown in Table 2. First, the subjects who died of a nonhepatic cause were more likely to have active medical comorbidities, including heart disease and cancer, and a significantly lower body mass index compared with the other 2 groups. Of interest, 33% of these patients were being treated for cancer with an anti-neoplastic drug implicated in the DILI event. In contrast, the patients who underwent liver transplantation were significantly younger (mean age, 47 years; range, 21–73 years) and were less likely to have medical comorbidities. In addition, liver transplant recipients had significantly higher initial and peak serum AST, ALT, and R values compared with the other 2 groups. In fact, 96.7% of transplant recipients had a hepatocellular liver injury pattern at DILI onset compared with 64.7% in those with a liver-related death, and only 26.7% of those who died of nonhepatic causes. In addition, there was a trend toward higher INR and bilirubin levels at presentation compared with the other 2 groups. Not surprisingly, the transplant recipients were more likely to meet Hy’s law criteria compared with the other 2 groups (63.0%vs 35.3% and 26.7%). The DILIN causality scores were similar in the 3 groups. The most frequently implicated drugs in the transplanted patients were antimycobacterial agents, followed by HDS products, which, when combined, accounted for 73% of the transplanted patients (Supplementary Table 1).
Figure 2.
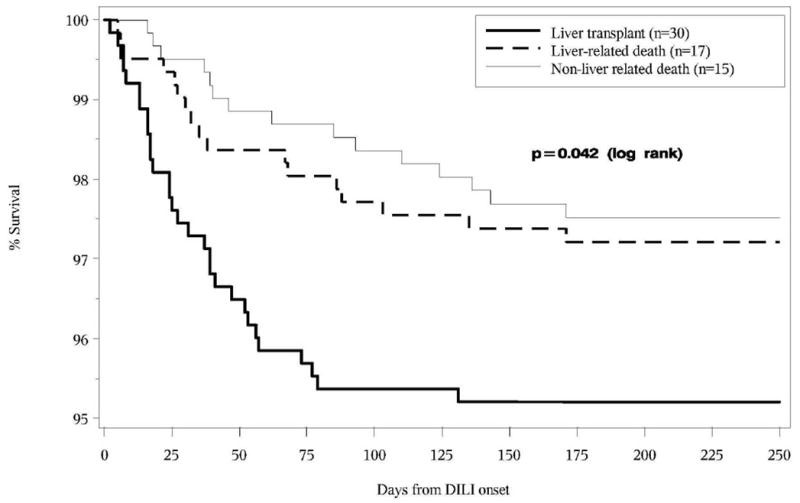
Kaplan–Meier Survival curves for time to each of the three early outcomes
Table 2.
Characteristics of Drug-Induced Livery Injury Patients Who Underwent Liver Transplantation or Died Within 6 Months of Drug-Induced Liver Injury Onset
| Characteristic | Liver transplant recipients (n = 30) | Liver-related death (n = 17) | Nonhepatic death (n = 15) | P valuea |
|---|---|---|---|---|
| Age, y, mean ± SD | 47.0 ± 13.5 | 57.9 ± 15.3 | 59.7 ± 15.9 | .02 |
| Female, % | 66.7 | 52.9 | 46.7 | .38 |
| Race, % | .97 | |||
| White | 62.1 | 76.5 | 66.7 | |
| Black | 17.2 | 11.8 | 20.0 | |
| Asian | 13.8 | 5.9 | 6.7 | |
| Others | 6.9 | 5.9 | 6.7 | |
| Body mass index, mean ± SD | 29.7 ± 7.5 | 30.6 ± 6.7 | 24.7 ± 4.8 | .04 |
| Medical history, % | ||||
| Diabetes mellitus | 33.3 | 52.9 | 53.3 | .27 |
| Heart disease | 20.0 | 23.5 | 53.3 | .08 |
| Kidney disease | 10.0 | 35.3 | 33.3 | .06 |
| Lung disease | 30.0 | 35.3 | 20.0 | .67 |
| Malignancy | 13.3 | 17.6 | 66.7 | <.01 |
| Active malignancy | 3.3 | 11.8 | 53.3 | <.01 |
| HIV+ | 3.3 | 0.0 | 0.0 | 1.00 |
| Self-reported drug allergies | 53.3 | 58.8 | 66.7 | .67 |
| Pre-existing liver disease | 30.0 | 23.5 | 13.3 | .51 |
| Prior alcohol use | 66.7 | 20.0 | 33.3 | <.01 |
| Implicated agents | ||||
| Duration of primary agent use, d, median (range) | 81 (2–2033) | 57 (5–1707) | 46 (3–3112) | .60 |
| Categories, % | .15 | |||
| Single drug | 46.7 | 70.6 | 60.0 | |
| Single HDS | 16.7 | 0.0 | 0.0 | |
| Multiple drug(s) and/or multiple HDS | 36.7 | 29.4 | 40.0 | |
| Primary implicated agent class, n (%) | .42 | |||
| Antimicrobial | 14 (46.7) | 9 (52.9) | 4 (26.7) | .29 |
| HDS | 8 (26.7) | 1 (5.9) | 1 (6.7) | .17 |
| Antineoplastic | 0 (0.0) | 2 (11.8) | 5 (33.3) | <.01 |
| Central nervous system | 3 (10.0) | 1 (5.9) | 2 (13.3) | .87 |
| Cardiovascular | 2 (6.7) | 2 (11.8) | 1 (6.7) | .84 |
| Rheumatologic | 1 (3.3) | 0 (0.0) | 1 (6.7) | .73 |
| Analgesic | 0 (0.0) | 0 (0.0) | 1 (6.7) | .24 |
| Endocrine | 1 (3.3) | 0 (0.0) | 0 (0.0) | 1.00 |
| Gastrointestinal | 1 (3.3) | 0 (0.0) | 0 (0.0) | 1.00 |
| Other | 0 (0.0) | 2 (11.8) | 0 (0.0) | .13 |
| Liver biochemistries at DILI onset | ||||
| Serum AST, U/L | 1478 (254–4925) | 556 (83–2644) | 276 (76–1972) | <.01 |
| Serum ALT, U/L | 1354 (205–4212) | 723 (23–2343) | 375 (123–1895) | <.01 |
| ALP, U/L | 180 (94–688) | 212 (108–942) | 220 (101–1004) | .51 |
| Total bilirubin, mg/dL | 11.7 (0.6–30.0) | 8.8 (0.4–27.5) | 3.7 (0.5–33.4) | .13 |
| INR | 2.1 (1.7–3.9) | 1.8 (1.0–6.2) | 1.2 (0.9–3.6) | .22 |
| Albumin, g/dL | 3.2 (2.0–4.2) | 2.9 (1.8–4.0) | 2.8 (1.1–4.0) | .27 |
| MELD score | 30.0 (7.0–40.0) | 22.0 (7.0–36.0) | 21.0 (6.0–32.0) | <.01 |
| Hy’s law, %b | 63.0 | 35.3 | 26.7 | .05 |
| R value | 17.7 (1.6–82.4) | 7.7 (0.2–27.6) | 3.4 (0.9–41.3) | <.01 |
| Pattern of injury, % | <.01 | |||
| Cholestatic | 3.3 | 29.4 | 20.0 | |
| Mixed | 0.0 | 5.9 | 53.3 | |
| Hepatocellular | 96.7 | 64.7 | 26.7 | |
| Peak liver biochemistriesc | ||||
| Peak serum AST, U/L | 1960 (655–6924) | 1475 (170–3668) | 377 (80–2500) | <.01 |
| Peak serum ALT, U/L | 1580 (551–5831) | 1378 (70–2583) | 504 (123–1895) | <.01 |
| Peak ALP, U/L | 296 (143–1051) | 302 (136–2414) | 672 (119–1484) | .20 |
| Peak total bilirubin, mg/dL | 26.3 (10.3–43.7) | 31.5 (12.0–59.0) | 17.1 (0.8–38.1) | .05 |
| Peak INR | 3.9 (2.0–12.9) | 5.1 (1.4–12.0) | 2.0 (1.1–4.7) | <.01 |
| Lowest albumin, g/dL | 2.1 (1.0–3.1) | 2.1 (1.2–2.8) | 2.0 (0.9–3.5) | .88 |
| Peak MELD score | 37.0 (15.0–40.0) | 36.0 (21.0–40.0) | 31.0 (11.0–34.0) | <.01 |
| Peak R value | 28.8 (8.1–210.4) | 15.9 (0.5–38.3) | 4.1 (1.0–41.5) | <.01 |
| Causality score, n (%)c | .79 | |||
| Definite | 8 (26.7) | 2 (11.8) | 3 (20.0) | |
| Very likely | 11 (36.7) | 8 (47.1) | 7 (46.7) | |
| Probable | 11 (36.7) | 7 (41.2) | 5 (33.3) | |
| Steroid use, %c | 90.0 | 76.5 | 71.4 | .23 |
| Ursodeoxycholic acid use, %c | 26.7 | 23.5 | 35.7 | .81 |
HIV, human immunodeficiency virus; SD, standard deviation.
Comparisons made across 3 patient groups.
Hy’s law criteria are subjects with ALT >3× ULN and total bilirubin >2.5 mg/dL at DILI onset with ALP <2× ULN.
Variables observed based on data from onset to the 6-month study visit. All other variables were either observed at DILI onset or at the baseline study visit where the values would be similar to DILI onset.
Presenting Features Associated With Early Liver-Related Death/Liver Transplantation
Two multivariate models were fit to time to event data, one for time to death/transplantation and the other for time to liver-related death/transplantation. Both final models yielded the same predictors except the malignancy variable was in the final model for time to all death/transplantation, but not in the final model for time to liver-related death/liver transplantation. Due to clinical relevance, only the later model is presented in Table 3. On multivariate analysis, subjects of Asian race and those presenting with lower serum albumin levels, lower platelet counts, higher serum ALT and bilirubin levels, and those with underlying pulmonary disease and without itching were significantly more likely to experience a liver-related death or to undergo transplantation. The C-statistic for the overall model fit was 0.87 (Table 3). Asian race remained an independent risk factor even after considering antimycobacterial agent use because there was no association between Asian race and antimycobacterial agent use.
Table 3.
Predictors for Time to Liver Transplantation or Liver-Related Death Within 6 Months of Drug-Induced Liver Injury Onset
| Parameter | Univariate Cox regression probability model
|
Multivariate Cox regression probability model C-statistic = 0.87 (95% CI: 0.82–0.93)
|
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Race | ||||
| White | Reference | |||
| Black | 1.65 (0.73–3.75) | .231 | 0.82 (0.31–2.17) | .688 |
| Asian | 4.71 (1.83–12.1) | <.01 | 3.18 (1.08–9.30) | .035 |
| Other | 1.34 (0.41–4.37) | .63 | 0.52 (0.12–2.34) | .398 |
| Weight, kg | 1.01 (0.999–1.02) | .07 | ||
| Body mass index | 1.04 (1.00–1.08) | .03 | ||
| Itching | 0.28 (0.15–0.54) | <.01 | 0.33 (0.15–0.700) | <.01 |
| Diabetes/endocrine disorder | 2.07 (1.15–3.70) | .01 | ||
| Pulmonary disease | 2.21 (1.20–4.08) | .01 | 2.86 (1.44–5.66) | <.01 |
| Family history of liver disease | 2.39 (0.95–6.06) | .07 | ||
| ALT at onset, unit = 50 U/L | 1.01 (1.01–1.02) | <.01 | 1.01 (1.01–1.02) | <.01 |
| ALP at onset, unit = 50 U/L | 0.93 (0.86–1.01) | .09 | ||
| Total bilirubin at onset, mg/dL | 1.07 (1.04–1.10) | <.01 | 1.10 (1.06–1.14) | <.01 |
| WBC at onset | 1.05 (1.01–1.09) | .01 | ||
| Platelets at onset | 0.99 (0.99–0.99) | <.01 | 0.99 (0.99–0.997) | <.01 |
| Albumin at onset | 0.36 (0.245–0.52) | <.01 | 0.50 (0.33–0.76) | <.01 |
| Hy’s law | 2.93 (1.62–5.29) | <.01 | ||
NOTE. Predictors based on clinical information at onset or baseline selected by clinical input, univariate Cox regression with P value <.10, and with <50% missing data. Hazard ratio should be interpreted based on 1-unit increase unless otherwise indicated with a magnitude of unit increase.
SD, standard deviation; WBC, white blood cell.
Chronic Drug-Induced Liver Injury Cohort Analysis
Among the 598 patients with available 6-month follow-up data, there were 113 patients (18.9%) who met at least 1 of the 6 protocol-defined criteria for chronic DILI. The 6 specific criteria met in these patients were persistent serum AST, ALT, or ALP levels above baseline in 97 patients, liver function test 1.25× baseline on 2 occasions in 6, portal hypertension after 6 month from DILI onset in 8, radiologic evidence of chronic DILI in 23, histologic evidence of chronic DILI in 6, and clinical evidence of chronic DILI in 36. Forty-three (38.1%) of the subjects met more than 1 criteria for chronic DILI. There were several important baseline differences in those who did and did not go on to develop chronic DILI (Table 4). Although the mean age and percent female were similar, those with chronic DILI were significantly more likely to be African American (18.6%vs 8.7%; P < .01). The clinical symptoms at presentation were similar between the 2 groups, but chronic DILI patients were significantly more likely to have a malignancy requiring treatment and 13 were receiving an anti-neoplastic suspect drug. Median duration of medication use was also significantly longer in those that developed chronic DILI compared with those who did not (51 vs 30 days; P < .01).
Table 4.
Characteristics of Drug-Induced Liver Injury Subjects With and Without Chronic Drug-Induced Liver Injury at 6 Months After Drug-Induced Liver Injury Onset
| Feature | Chronic DILI (n = 113) | Nonchronic DILI (n = 485) | P value |
|---|---|---|---|
| Age, y, mean ± SD | 49.7 ± 15.1 | 50.6 ± 16.1 | .58 |
| Female, % | 67.3 | 57.9 | .07 |
| Race, % | <.01 | ||
| White | 73.5 | 82.9 | |
| Black | 18.6 | 8.7 | |
| Asian | 4.4 | 2.1 | |
| Others | 3.5 | 6.4 | |
| Body mass index, mean ± SD | 27.6 ± 6.8 | 27.8 ± 6.5 | .95 |
| Signs and symptoms at DILI onset, % | |||
| Fever | 32.7 | 25.8 | .16 |
| Rash | 28.3 | 27.6 | .91 |
| Itching | 63.7 | 57.3 | .24 |
| Medical history, % | |||
| Diabetes mellitus | 28.3 | 22.3 | .18 |
| Heart disease | 23.9 | 18.6 | .24 |
| Kidney disease | 15.0 | 10.7 | .19 |
| Lung disease | 15.0 | 17.3 | .68 |
| Malignancy | 15.9 | 6.8 | <.01 |
| Active malignancy | 10.6 | 2.3 | <.01 |
| HIV+ | 2.7 | 1.6 | .44 |
| Self-reported drug allergies, % | 41.6 | 45.4 | .53 |
| Pre-existing liver disease, % | 13.3 | 10.1 | .31 |
| Prior alcohol use, % | 46.4 | 53.9 | .17 |
| Implicated agents | |||
| Duration of primary agent use, d, median (range) | 51 (1–2343) | 30 (1–6971) | <.01 |
| Categories, % | .34 | ||
| Single drug | 50.4 | 56.9 | |
| Single HDS | 8.0 | 8.9 | |
| Multiple drug(s) and/or multiple HDS | 41.6 | 34.2 | |
| Primary implicated agent class, n (%) | .03 | ||
| Antimicrobial | 41 (36.3) | 229 (47.3) | .04 |
| HDS | 18 (15.9) | 80 (16.5) | 1.00 |
| Cardiovascular | 14 (12.4) | 52 (10.7) | .62 |
| Psychotropic | 5 (4.4) | 40 (8.3) | .23 |
| Antineoplastic | 13 (11.5) | 15 (3.1) | <.01 |
| Analgesic | 4 (3.5) | 22 (4.5) | .80 |
| Immunomodulatory | 4 (3.5) | 15 (3.1) | .77 |
| Endocrine | 5 (4.4) | 9 (1.9) | .16 |
| Gastrointestinal | 5 (4.4) | 3 (0.6) | <.01 |
| Other | 4 (3.5) | 19 (3.9) | 1.00 |
| Laboratory parameters at DILI onset | |||
| WBC, 103/mm3, median (range) | 6.2 (0.9–19.4) | 6.4 (0.9–58.6) | .87 |
| Absolute eosinophil count, μL, median (range) | 173 (0.0–4501) | 141 (0–7420) | .14 |
| Eosinophils >500/μL, % | 13.6 | 9.2 | .22 |
| Serum creatinine,mg/dL, median (range) | 0.8 (0.4–6.1) | 0.9 (0.1–7.6) | .45 |
| Antinuclear antibody+, % | 29.4 | 28.0 | .81 |
| Anti-Smith antibody+, % | 24.3 | 19.7 | .29 |
| Liver biochemistries at DILI onset, median (range) | |||
| Serum AST, U/L | 285 (29–3107) | 287 (26–12,802) | .72 |
| Serum ALT, U/L | 383 (14–3748) | 482 (22–15,065) | .10 |
| ALP, U/L | 302 (41–1730) | 208 (35–1952) | <.01 |
| Total bilirubin, mg/dL | 5.8 (0.2–36.2) | 4.7 (0.2–45.7) | .08 |
| INR | 1.1 (0.9–3.5) | 1.1 (0.8–12.2) | .30 |
| Albumin, g/dL | 3.6 (1.7–4.8) | 3.8 (1.0–5.8) | <.01 |
| MELD score | 17.5 (6.0–40.0) | 16.0 (6.0–39.0) | .04 |
| R value | 2.8 (0.1–76.2) | 5.7 (0.2–426.2) | <.01 |
| Pattern of injury, % | <.01 | ||
| Cholestatic | 42.5 | 21.9 | |
| Mixed | 18.6 | 24.7 | |
| Hepatocellular | 38.9 | 53.4 | |
| Peak liver biochemistries,a median (range) | |||
| Peak serum AST, U/L | 389 (57–3229) | 388 (45–12,802) | .63 |
| Peak serum ALT, U/L | 518 (62–3748) | 574 (53–15,065) | .22 |
| Peak ALP, U/L | 467 (65–2865) | 262 (50–2399) | <.01 |
| Peak total bilirubin, mg/dL | 13.7 (0.4–54.8) | 7.9 (0.3–63.0) | <.01 |
| Peak INR | 1.2 (0.9–13.1) | 1.1 (0.8–12.2) | <.01 |
| Lowest albumin, g/dL | 2.9 (0.6–4.9) | 3.2 (0.4–4.8) | <.01 |
| Peak MELD score | 19 (6.0–40.0) | 15 (6.0–40.0) | <.01 |
| Peak R value | 4.1 (0.3–111.3) | 7.4 (0.4–426.2) | <.01 |
| Causality assessment score, n (%)a | <.01 | ||
| Definite | 19 (16.8) | 148 (30.5) | |
| Very likely | 61 (54.0) | 254 (52.) | |
| Probable | 33 (29.2) | 83 (17.1) | |
| Steroid use, %a | 45.5 | 34.4 | .04 |
| Ursodeoxycholic acid use, %a | 24.1 | 18.7 | .23 |
| DILIN severity score, n (%)a | <.01 | ||
| Mild | 19 (16.8) | 131 (27.0) | |
| Moderate | 21 (18.6) | 126 (26.0) | |
| Moderate-hospitalized | 41 (36.3) | 146 (30.1) | |
| Severe | 32 (28.3) | 82 (16.9) | |
| Fatal | 0 (0) | 0 (0) |
HIV, human immunodeficiency virus; MELD, Model for End-Stage Liver Disease; SD, standard deviation; WBC, white blood cell count.
Variables observed based on data from onset to the 6-month study visit. All other variables were either observed at DILI onset or at the baseline study visit where the values would be similar to DILI onset.
The chronic DILI patients were more likely to have cholestatic liver injury at presentation, with significantly higher initial and peak serum ALP levels and lower serum albumin levels compared with those that did not develop chronic DILI (Table 4). Although the presenting total bilirubin and INR levels were similar, the chronic DILI patients had a significantly higher peak total bilirubin and INR levels during follow-up, consistent with a more severe liver injury episode. In support of this, the mean DILIN severity scores were significantly higher (or worse) in those with chronic DILI compared with those without chronic DILI. Not surprisingly, corticosteroids were also more commonly used in those that went on to develop chronic DILI compared with the others.
Presenting Features Associated With Chronic Drug-Induced Liver Injury
Multivariate modeling was used to investigate independent laboratory and clinical features at presentation for developing chronic DILI. When we included potential predictors at presentation that were associated with a greater likelihood of chronic DILI (ie, P < .10), the presenting serum ALP, African-American race, active malignancy, and heart disease, were independent predictors of chronic DILI (C-statistic = 0.71; 95% CI, 0.65–0.76) (Table 5).
Table 5.
Association Between Risk Factors and Chronic Drug-Induced Livery Injury Based on Logistic Regression
| Parameter | Univariate probability model
|
Multivariate logistic regression probability model C-statistic = 0.71 (95% CI: 0.65–0.76)
|
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Female | 1.49 (0.97–2.30) | .07 | ||
| Race | ||||
| White | Reference | |||
| Black | 2.42 (1.360–4.29) | <.01 | 3.10 (1.68–5.72) | <.01 |
| Asian | 2.42 (0.81–7.25) | .12 | 3.12 (0.95–10.22) | .06 |
| Other | 0.62 (0.21–1.81) | .39 | 0.82 (0.28–2.43) | .72 |
| Malignancy | ||||
| No malignancy | Reference | |||
| Inactive malignancy | 1.30 (0.51–3.29) | .58 | 1.34 (0.47–3.83) | .72 |
| Active malignancy | 5.19 (2.22–12.1) | <.01 | 6.37 (2.59–15.7) | <.01 |
| Duration of primary agent use | 1.0 (1.0–1.001) | .06 | ||
| ALP at onset, unit = 50 U/L | 1.07 (1.03–1.11) | <.01 | 1.08 (1.04–1.12) | <.01 |
| Total bilirubin at onset, mg/dL | 1.03 (0.999–1.06) | .06 | ||
| Hemoglobin at onset (±14 days) | 0.83 (0.73–0.95) | <.01 | ||
| Albumin at onset (±14 days)a | 0.64 (0.47–0.86) | <.01 | ||
| Heart disease | ||||
| No heart disease | Reference | |||
| Inactive heart disease | 3.53 (1.50–8.32) | <.01 | 3.36 (1.35–8.39) | <.01 |
| Active heart disease | 1.01 (0.57–1.80) | .96 | 0.77 (0.40–1.49) | .44 |
NOTE. Predictors based on demographic and clinical information at onset or baseline selected by clinical judgment, univariate logistic regression with P value <.10, and with <50% missing data. Odds ratio should be interpreted based on 1-unit increase unless otherwise indicated with a magnitude of unit increase. “Malignancy” refers to lifetime history of cancer and “active malignancy” delineates the subgroup of patients with a malignancy requiring treatment at the time of DILI onset. Similarly, “heart disease” refers to lifetime history of cardiac disease and “active heart disease” refers to subjects with a cardiac condition requiring treatment at the time of DILI onset.
Discussion
This prospective, multicenter observational study provided a unique opportunity to identify presenting clinical and laboratory features associated with the most serious outcomes of DILI (ie, death or liver transplantation), in a large cohort of well-characterized American patients. As noted in Figure 1, thirty-two subjects died and 30 subjects underwent liver transplantation within 6 months of DILI onset. Of note, only 53% of the deaths were attributed to progressive liver injury, and the remaining subjects died of various nonhepatic causes. Liver-related deaths occurred within 1 month of DILI onset in most patients, and the non–liver-related deaths tended to occur at a median of 2 months. These data clearly demonstrate that idiosyncratic DILI in the United States is associated with significant morbidity and mortality. The 9.4% rate of death/transplantation is higher than the rates of adverse outcomes reported from DILI registries in Spain and Sweden (4% and 9.2%, respectively).13,14 The higher observed rates are not likely due to more severe liver injury at enrollment, but rather the prospective tracking of patients enrolled in the DILIN protocol. The suspect agents associated with the 17 liver-related deaths included a multitude of antibacterial antibiotics (47%) and other agents, and the drugs implicated in the 15 subjects with non–liver-related deaths were more likely to be anti-neoplastic agents (33%) (Supplementary Table 1). For unclear reasons, subjects who died or underwent liver transplantation were more likely to be Asian compared with those who survived. Of note, earlier studies have also demonstrated an over-representation of racial/ethnic minorities among subjects with severe DILI.15,16 Whether these observations are due to increased susceptibility, delayed presentation, or impaired liver regeneration among Asians remains unknown, but they are worthy of additional study. Of note, subject age, sex, and recent alcohol consumption did not differ among those with and without early adverse outcomes, suggesting that these factors, which are used in many scoring systems, might not be associated with short-term outcomes. Subjects with early death/transplantation were more likely to have diabetes mellitus and several other medical comorbidities on univariate analyses. The increased mortality among diabetics might be due to the greater prevalence of underlying fatty liver disease or increased susceptibility to infections or other complications of severe acute liver injury.17 However, diabetes and other comorbidities were not significant in the multivariate model, except pulmonary disease (Table 3). The reason for increased mortality in subjects with underlying lung disease is unclear. However, among 15 subjects with lung disease who died or underwent liver transplantation, only 4 received INH and 3 had latent tuberculosis, and only 1 had active tuberculosis.
Our data confirm that DILI patients with laboratory evidence of more severe liver injury at presentation (ie, higher serum ALT and bilirubin levels), as well as during their illness, are more likely to experience adverse outcomes during short-term follow-up. Descriptive summary data showed that those with poor early outcomes were also more likely to have received corticosteroids, presumably in an attempt to salvage their severely damaged livers, although they were not more likely to have evidence of hypersensitivity features (eg, rash, fever, eosinophilia) at presentation. Interestingly, subjects with adverse outcomes were also more likely to have been treated with the suspect agent for a longer period of time compared with those with self-limited DILI, but the specific suspect agents differed in the 2 groups.18,19 It is possible that these individuals might have had liver injury for a longer period of time before discontinuation of the suspect medication. The larger proportion of cases due to antineoplastic agents suggest that these agents might be particularly prone to cause severe DILI.15,16 Our data also confirm that subjects with severe acute hepatocellular injury or those who meet Hy’s law criteria are more likely to experience adverse outcomes. However, 29.4% of those who died of liver-related cause had a cholestatic liver injury pattern at presentation. The lower causality scores in the patients with early death/transplantation might be due, in part, to the lack of improvement in laboratory parameters after drug discontinuation (ie, “positive dechallenge”), which is used as supporting evidence in DILI diagnosis. A similar finding was previously noted in patients with fulminant DILI.20
The 30 subjects who required liver transplantation were significantly younger and less likely to have an active malignancy compared with those who died. These observations are in keeping with other studies in which subjects with an anticipated likelihood of long-term survival are more likely to undergo transplantation. The most commonly implicated agent in the liver transplant recipients was isoniazid, followed by various other agents. Interestingly, nearly one third of the transplantation cases were due to HDS hepatotoxicity without a dominant HDS being implicated (Supplementary Table 1). These data suggest that additional studies of this increasingly recognized cause of severe hepatotoxicity are needed, including a careful analysis of their constitutive ingredients.21
With a minimum of 6 months post-enrollment follow-up, 131 patients were adjudicated as possible DILI, and 48 patients were adjudicated as unlikely DILI, representing 18.1% of the enrolled patients. These data highlight the continued difficulty in making an accurate diagnosis of DILI.8,9 This observation also confirms that a comprehensive series of diagnostic tests should be obtained in all patients with suspected DILI, including tests for acute hepatitis C virus, hepatitis E virus, and Epstein-Barr virus infection.22-24
The 18.9% (95% CI, 15.8%–22.0%) rate of chronic or persistent liver injury at 6 months of follow-up is substantially higher than the 5.7% incidence reported by other groups, which might be due to our longer period of follow-up and high retention rate of enrolled subjects.18 However, the severity of residual liver injury was mild in most patients, with median AST, ALT, and ALP levels of only 63, 75, and 178, respectively, at the 6-month study visit. In addition, only 39.6% of these subjects had a serum bilirubin level >1.0 mg/dL at the 6-month study visit. Of note, the median duration of suspect medication use was longer in those who developed chronic DILI, which is consistent with earlier studies.18,19 In addition, subjects with a higher serum ALP and lower serum albumin levels at presentation were at increased risk of developing chronic DILI.18,19 In addition, the peak ALP, bilirubin, and INR levels were significantly higher in those who developed chronic DILI, indicating that subjects with more severe liver injury were more likely to develop persistent liver injury. This is corroborated by the higher mean DILIN severity score and the larger proportion of chronic DILI patients who required hospitalization compared with those with self-limited injury (66.4%vs 52.6%; P = .008).
On multivariate analysis, African Americans were more likely to develop chronic DILI compared with other racial groups. Earlier studies of anticonvulsant hepatotoxicity have shown a similar trend of poorer outcomes among African Americans, which might be immunologically mediated.25 Subjects with an active malignancy were also more likely to develop chronic DILI, which might be due, in part, to cancer patients being followed more carefully and frequently (detection bias), having liver involvement by the primary cancer, or from continued use of a potentially hepatotoxic medication in a high-risk setting. Finally, subjects with higher serum ALP levels at presentation were more likely to develop chronic DILI. Earlier studies have suggested a slower rate of biochemical improvement in subjects with a cholestatic drug reaction, and our large, prospective dataset strengthens this important observation.19 Of note, subjects with higher bilirubin levels at presentation were also more prone to develop chronic DILI. These observations indicate that those who present with severe cholestatic DILI or require hospitalization might need more frequent and careful follow-up. Chronic DILI patients were also more likely to receive corticosteroids, despite the fact that they did not have more frequent eosinophilia, autoantibodies, or rash at presentation (Table 4). Unfortunately, our observational data do not allow us to determine if corticosteroid use hastened the resolution of liver injury and this important question requires additional study.
Strengths of our study include the fact that this was a prospective observational study carried out at 8 geographically distinct sites. In addition, subjects of varying age, sex, and ethnicity with a broad spectrum of liver injury severity due to a multitude of drugs and HDS products were enrolled, which strengthened the generalizability of our findings. From a diagnostic perspective, all of the subjects underwent a thorough evaluation for competing causes of liver injury and were followed for a minimum of 6 months after DILI onset and a rigorous causality assessment was undertaken in a standardized manner.11 This latter point was particularly important because a modest proportion (18.1%) of the enrolled patients were finally adjudicated as having only possible or unlikely DILI during follow-up. This study also represents the largest cohort of DILI patients reported to date and provided the opportunity to identify independent predictors of early adverse outcomes as well as the risk of developing chronic DILI. However, the 6-month period used to define chronic DILI is somewhat arbitrary and some patients might still be recovering from the injury. Therefore, we are following all of the chronic DILI patients for 24 months after DILI onset to determine how often the liver injury abnormalities persist or resolve. A final limitation of our analysis is the assumption that there were no differences between the subjects with and without outcomes data. Sensitivity analyses, such as multiple imputation approach with assumption of data missing at random, could have been used to investigate the influence of missing data. However, our analyses suggested that the data is likely to be missing at random.
In conclusion, 9.4% of adult American patients with DILI either died or required liver transplantation within 6 months of DILI onset. A multitude of suspect drugs as well as various HDS products were implicated, both in subjects with early adverse outcomes and those who survived. However, Asian race, anti-neoplastic agents, and underlying lung disease were over-represented among subjects with an early adverse outcome. Chronic liver injury developed in 18.8% of the initial survivors, but was generally mild at 6 months of follow-up, and long-term outcomes will require additional study. African Americans, subjects with active malignancy, and those who presented with higher ALP and total bilirubin levels appeared to be at greatest risk for chronic DILI. Although our findings are exploratory, confirmation of these findings in other cohorts might allow for improved prognostication in individual patients and inform the design of future therapeutic trials in patients with clinically significant DILI.
Supplementary Material
Acknowledgments
Thomas Phillips at Duke Clinical Research Institute did most of the programming of the analyses for this article.
Funding
The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases under grants: 2U01-DK065176-06 (Duke University), 2U01-DK065201-06 (University of North Carolina), 2U01-DK065184-06 (University of Michigan), 2U01-DK065211-06 (Indiana University), 5U01DK065193-04 (University of Connecticut), 5U01-DK065238-08 (University of California, San Francisco/California Pacific Medical Center), 1U01-DK083023-01 (University of Texas Southwestern), 1U01-DK083027-01 (Thomas Jefferson Hospital/University of Pennsylvania), 1U01-DK082992-01 (Mayo Clinic), 1U01-DK083020-01 (University of Southern California). Additional funding is provided by Clinical and Translational Science Awards grants: UL1 RR025761 (Indiana University), UL1TR000083 (University of North Carolina), UL1 RR024134 (University of Pennsylvania), UL1 RR024986 (University of Michigan), UL1 RR024982 (University of Texas Southwestern), UL1 RR024150 (Mayo Clinic), and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Abbreviations used in this paper
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- AST
aspartate aminotransferase
- CI
confidence interval
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- HDS
herbal and dietary supplement
- INR
international normalized ratio
- ULN
upper limit of normal
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.03.045.
Conflicts of interest
These authors disclose the following: Robert J. Fontana has received research support from Vertex Pharmaceuticals and Gilead; he has also served as a consultant to Tibotec, Merck, and GlaxoSmithKline in the past year. K. Rajender Reddy has received research support from Merck, Gilead, Abbvie, Bristol-MyersSquibb, Janssen, Ikaria, and Genfit and served as an advisor to Merck, Genetech-Roche, Gilead, BMS, Vertex, Janssen, Idenix, and Abbvie. William M. Lee received research support from BI, BMS, Anadys, Gilead, Vertex, Merck, Roche and has consulted for Lilly, Novartis, and GSK. Naga Chalasani has served as a consultant to Merck, Aegerion, BMS, Abbvie, Lilly, and Salix during the past 12 months and received financial compensation from these entities. He has received research support from Intercept, Cumberland, Gilead, Enterome, and Takeda. The remaining authors disclose no conflicts.
References
- Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102:558–562. doi: 10.1111/j.1572-0241.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Aithal PG, Day CP. The natural history of histologically proved drug-induced liver disease. Gut. 1999;44:731–735. doi: 10.1136/gut.44.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–517. doi: 10.1016/j.jhep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Bjornosson E, Kalaitzakis E, Klintbert AV, et al. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:79–85. doi: 10.1111/j.1365-2036.2007.03355.x. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934e1–14. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Seeff LB, Rochon J, et al. for the US DILIN. Causality assessment in drug induced liver injury using a structured expert opinion process: comparison to RUCAM. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath PS, Kim WR. The Advanced Liver Disease Study group. The Model for End-Stage Liver Disease (MELD) Hepatology. 2007;45:799–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- Bjornosson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Fernandez MC, et al. Drug induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;1299:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Reuben A, Koch DG, Lee, et al. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Gastroenterology. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–517. doi: 10.1016/j.jhep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Lucena M, Camargo R, Andrade R, et al. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123–130. doi: 10.1053/jhep.2001.20645. [DOI] [PubMed] [Google Scholar]
- Navarro VJ, Bonkovsky HL, Hwang SI, et al. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58:2682–2690. doi: 10.1007/s10620-013-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern TJ, Chalasani N, Fontana RJ, et al. for the Drug Induced Liver Injury Network. Role of acute hepatitis E in suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–1672. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal VK, McHutchison JG, Hoofnagle JH for the Drug Induced Liver Injury Network. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8:463–470. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Morris HH. Hypersensitivity syndrome to anti-epileptic drugs: a review including new anticonvulsants. Clev Clin J Med. 1999;66:239–245. doi: 10.3949/ccjm.66.4.239. [DOI] [PubMed] [Google Scholar]
- Galan MV, Potts JA, Silverman AL, et al. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center [corrected] J Clin Gastroenterol. 2005;39:64–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


