Abstract
(−)-Epigallocatechin-3-gallate (EGCG), the major polyphenol in green tea, has been reported to inhibit the Wnt/β-catenin pathway, which is aberrantly up-regulated in colorectal cancers, but its precise mechanism of action remains unclear. Here, we used a sensitive cell-based system to demonstrate that EGCG suppresses β-catenin response transcription (CRT), activated by Wnt3a-conditioned medium (Wnt3a-CM), by promoting the degradation of intracellular β-catenin. EGCG induced β-catenin N-terminal phosphorylation at the Ser33/37 residues and subsequently promoted its degradation; however, this effect was not observed for oncogenic forms of β-catenin. Pharmacological inhibition or depletion of glycogen synthase kinase-3β (GSK-3β) did not abrogate the EGCG-mediated β-catenin degradation. EGCG did not affect the activity and expression of protein phosphatase 2A (PP2A). Consistently, the phosphorylation and degradation of β-catenin was found in adenomatous polyposis coli (APC) mutated colon cancer cells after EGCG treatment. EGCG repressed the expression of cyclin D1 and c-myc, which are β-catenin/T-cell factor-dependent genes, and inhibited the proliferation of colon cancer cells. Our findings suggest that EGCG exerts its cancer-preventive or anticancer activity against colon cancer cells by promoting the phosphorylation and proteasomal degradation of β-catenin through a mechanism independent of the GSK-3β and PP2A.
Keywords: (−)-epigallocatechin-3-gallate (EGCG), Wnt/β-catenin signaling, phosphorylation, proteasomal degradation, colon cancer
1. Introduction
(−)-Epigallocatechin-3-gallate (EGCG), a polyphenolic compound present at high concentrations in green tea (Camellia sinensis), has been proposed to have activities that can be used for prevention or alleviation of several chronic diseases including cancer, heart disease, obesity, diabetes, and neurodegenerative diseases [1]. Studies in animal models have shown cancer preventive activity of EGCG in different organs such as the liver, stomach, skin, lung, mammary glands and colon [2, 3]. In studies with cell lines, EGCG possesses activities related to cancer prevention such as the inhibition of various protein kinases, DNA methyltransferase, and epidermal growth factor receptor signaling [4–7].
The Wnt/β-catenin pathway, which is triggered by the association of Wnt ligands (Wnt1, Wnt3a, and Wnt8) with receptor Frizzed (Fz) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors, is essential for cell proliferation, differentiation, and tumorigenesis [8, 9]. The functions of this pathway depend upon the level of intracellular β-catenin, which is regulated by its phosphorylation and subsequent degradation [10]. In the absence of Wnt, casein kinase 1 (CK1) and glycogen synthase kinase-3β (GSK-3β) sequentially catalyze β-catenin phosphorylation at residues Ser45, Thr41, Ser37, and Ser33 in a complex with adenomatous polyposis coli (APC) and axin [11, 12]. Phosphorylated β-catenin is then recognized by the E3 ubiquitin ligase F-box β-transducin repeat-containing protein (β-TrCP), which leads to its ubiquitination and degradation [13, 14]. Wnt stimulation leads to the inhibition of β-catenin phosphorylation and degradation by negatively regulating GSK-3β in the destruction complex (CK1/GSK-3β/axin/APC) [15].
The development and progression of colorectal cancer is related to the accumulation of a series of genetic and epigenetic alteration [16, 17]. Molecular lesions in the APC gene are observed in the majority of sporadic colorectal cancer cases, as well as in familial adenomatous polyposis (FAP), and they appear early in the progression of this cancer [18]. In addition, the N-terminal phosphorylation motif of β-catenin is frequently mutated in colorectal cancer [19]. These alterations lead to the accumulation of β-catenin in the nucleus, where it forms a complex with T-cell factor/lymphocyte enhancer factor (TCF/LEF) family transcription factors, and then activates the target genes, such as c-myc, cyclin D1, metalloproteinase-7, and peroxisome proliferation-activated receptor-δ, which play important roles in colorectal tumorigenesis and metastasis [20–23]. Thus, the inhibition of the Wnt/β-catenin pathway, which is aberrantly up-regulated in colorectal cancer, is a potential strategy for the prevention or treatment of colorectal cancer. In the present study, we demonstrated that EGCG induces the phosphorylation of β-catenin at Ser33/37 residues through a GSK-3β- and PP2A-independent mechanism and subsequently promotes its degradation, thereby suppressing the growth of colon cancer cells.
2. Materials and Methods
2.1. Cell Culture, Reporter Assay, and Chemicals
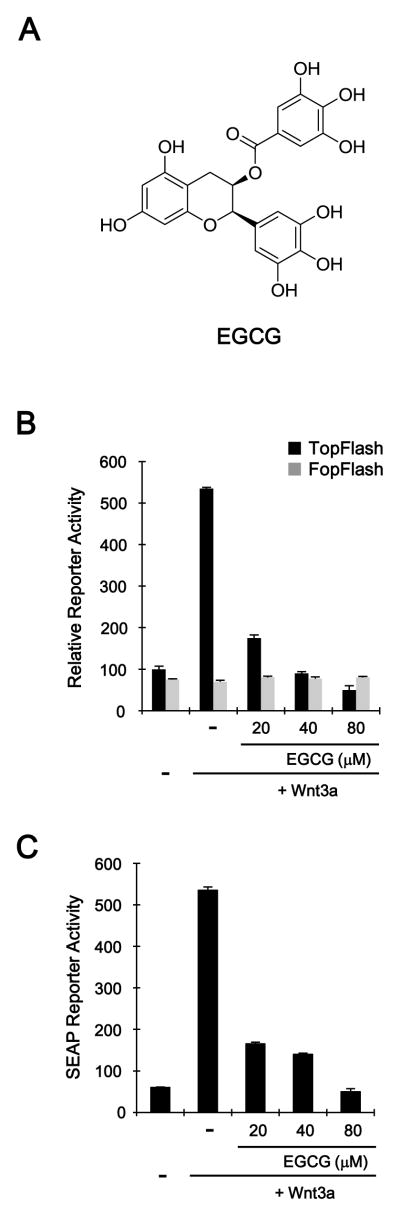
HEK293, SW480, HCT116, and Wnt3a-secreting L cells were obtained from American Type Culture Collection and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 120 μg/ml penicillin, and 200 μg/ml streptomycin. Wnt3a-conditioned medium (Wnt3a-CM) was prepared as previously described [24]. The HEK293 reporter (TOPFlash) and control (FOPFlash), and HEK293-SEAP reporter cells were established as previously described [24]. The luciferase assay was performed using the Dual Luciferase Assay Kit (Promega, Madison, WI) and the secreted alkaline phosphatase assay was performed using a Phospha-Light™ Assay kit (Applied Biosystems, CA). LiCl and MG-132 were purchased from Sigma-Aldrich (St. Louis, MO). EGCG (Fig. 1A) was provided by Mitsui Norin Co. Ltd. (Tokyo, Japan). EGCG was dissolved in double-deionized filter-sterilized water. For treatment, the cells were incubated with EGCG in a medium supplemented with 2% FBS, SOD (5 U/ml), and catalase (30 U/ml) to prevent the auto-oxidation of EGCG and production of superoxide and hydrogen peroxide [25].
Fig. 1.
Inhibition of the Wnt/β-catenin pathway by EGCG. A: Chemical structure of EGCG. B and C: Concentration-dependent inhibition of CRT. HEK293-FL, HEK293-SEAP reporter and control cells were incubated with indicated concentrations of EGCG in the presence of Wnt3a-CM. After 15 h, luciferase activity (B) or SEAP activity (C) was determined. The results represent the average of three experiments, and the bars indicate standard deviations.
2.2. Plasmids, siRNA and Transfection
Human Frizzled-1 (hFz-1) cDNA was cloned as previously described [24]. Reporter plasmids containing cyclin D1 promoters were prepared by amplifying the promoter regions, which harbored TCF-4 response elements, by PCR and inserting them into pRL-null vectors to yield pCyclinD1-RL. The pTOPFlash and pFOPFlash reporter plasmids were obtained from Upstate Biotechnology (Lake Placid, NY). The dominant negative β-TrCP (β-TrCP) expression plasmid was a gift from M. Davis (Hebrew University-Hadassah Medical School, Israel). pCMV-RL and pSV-FL plasmids were purchased from Promega. siRNA targeting GSK-3β (5′-GUAAUCCACCUCUGGCUAC-3′) was synthesized by Invitrogen (Valenica, CA). Negative control siRNA (Silencer™) was purchased from Ambion. Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
2.3. Western Blotting and Antibodies
The cytosolic fraction was prepared as previously described [26]. Proteins were separated by SDS-PAGE in a 4–12% gradient gel (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% nonfat milk and probed with anti-β-catenin (BD Transduction Laboratories, Lexington, KY), anti-GSK-3β (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cyclin D1 (Santa Cruz Biotechnology), anti-myc (Santa Cruz Biotechnology) and anti-actin antibodies (Cell Signaling Technology). The membranes were then incubated with horseradish-peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotechnology) or anti-rabbit IgG (Santa Cruz Biotechnology), and they were visualized using the ECL system (Santa Cruz Biotechnology).
2.4. RNA Extraction and Semi-quantitative RT-PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions. cDNA synthesis, reverse transcription, and PCR were performed as previously described [27]. The amplified DNA was separated on 2% agarose gels and stained with ethidium bromide.
2.5. Cell Viability Assay
Cells were inoculated into 96-well plates and incubated with EGCG in a medium supplemented with 2% FBS, SOD (5 U/ml), and catalase (30 U/ml) for 48 h. The cell viability of each treated sample was measured in triplicate using a Celltiter-Glo assay kit (Promega, Madison, WI) according to the manufacturer’s instructions. To calculate the inhibition of cell growth, the value at time 0 was subtracted.
2.6. in vitro Phosphatase Assay
Recombinant β-catenin proteins were phosphorylated at their N-terminal residues by purified CK1 and GSK-3β as previously described [28] and then these phosphorylated β-catenin proteins were incubated with the purified catalytic subunit of PP2A. The proteins were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. The transferred proteins were analyzed using Western blotting with an anti-phospho-β-catenin antibody (Cell Signaling Technology).
3. Results
3.1. EGCG Inhibits the Wnt/β-catenin Pathway
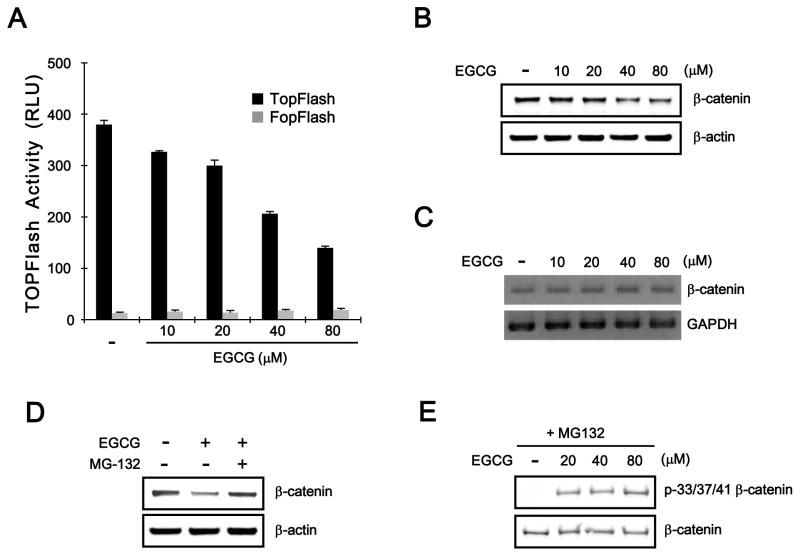
Several studies have reported that EGCG inhibits the proliferation of colon cancer cells, which are characterized by the aberrant up-regulation of Wnt/β-catenin signaling. To determine the mechanism underlying EGCG-mediated inhibition of the Wnt/β-catenin pathway, we used HEK293-FL reporter cells that were stably transfected with a synthetic β-catenin/Tcf-dependent firefly luciferase (FL) reporter and hFz-1 expression plasmid. When HEK293-FL reporter cells were treated with Wnt3a-conditioned medium (Wnt3a-CM), FL activity increased (Fig. 1B). Incubation with EGCG resulted in a concentration-dependent decrease in β-catenin response transcription (CRT) activated by Wnt3a-CM; 80 μM EGCG induced near-complete inhibition of CRT relative to the control treatment (Fig. 1B). In contrast, EGCG and Wnt3a-CM did not affect the activity of FOPFlash, a negative control reporter with mutated β-catenin/Tcf binding elements, in HEK293 control cells (Fig. 1B). The inhibitory effect of EGCG on the Wnt/β-catenin pathway was confirmed using HEK293-SEAP reporter cells that stably harbored a synthetic β-catenin/Tcf-dependent secreted alkaline phosphatase (SEAP) reporter and hFz-1 expression plasmid. Consistent with the results from the FL reporter assay, EGCG attenuated Wnt3a- induced SEAP activity in a concentration-dependent manner (Fig. 1C). Taken together, these results indicate that EGCG is an antagonist of the Wnt/β-catenin pathway.
3.2. EGCG Promotes a β-TrCP-dependent Proteasomal Degradation of β-Catenin
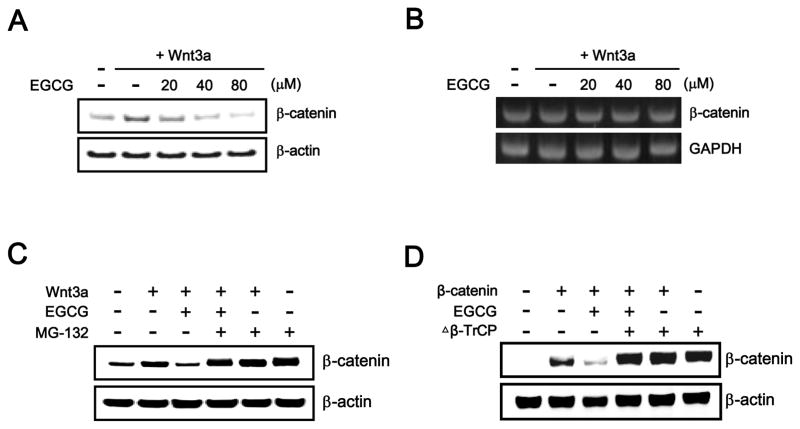
The level of intracellular β-catenin, which is regulated by the ubiquitin-dependent proteasomal degradation pathway, is important for controlling the Wnt/β-catenin pathway. Because EGCG suppressed the Wnt/β-catenin pathway, we examined whether EGCG decreases the intracellular level of β-catenin by Western blot analysis with an anti-β-catenin antibody. As shown in Fig. 2A, incubation of HEK293-FL reporter cells with different concentrations of EGCG produced a concentration-dependent decrease in the amount of cytosolic β-catenin that accumulated in response to Wnt3a-CM. In contrast, the mRNA level of β-catenin was not altered by any concentrations of EGCG used in HEK293-FL reporter cells (Fig. 2B), suggesting that EGCG attenuates Wnt/β-catenin signaling by decreasing the β-catenin protein level rather than by repressing β-catenin gene expression. We next examined the involvement of the proteasome in EGCG-induced down-regulation of cytosolic β-catenin. Treatment with EGCG consistently resulted in a decrease in cytosolic β-catenin levels in HEK293-FL reporter cells (Fig. 2C); however, the addition of MG-132, which is a proteasome inhibitor, abrogated EGCG-mediated β-catenin down-regulation (Fig. 2C). These results suggest that EGCG inhibits the Wnt/β-catenin pathway via proteasome-dependent β-catenin degradation.
Fig. 2.
EGCG promotes the degradation of β-catenin through a β-TrCP-dependent proteasomal degradation pathway. A: Cytosolic proteins were prepared from HEK293-FL reporter cells treated with vehicle (DMSO) or indicated concentrations of EGCG in the presence of Wnt3a-CM for 15 h and then subjected to Western blotting with an anit-β-catenin antibody. B: Semi-quantitative RT-PCR for β-catenin and GAPDH was performed using total RNA prepared from HEK293-FL reporter cells treated with vehicle (DMSO) or the indicated concentrations of EGCG in the presence of Wnt3a-CM for 15 h. C: Cytosolic proteins prepared from HEK293-FL reporter cells that were incubated with vehicle (DMSO) or EGCG (40 μM) in the presence of Wnt3a-CM and exposed to MG-132 (10 μM) for 8 h were subjected to Western blotting with anti-β-catenin antibody. D: HEK293 cells were co-transfected with the Δβ-TrCP expression plasmid and then incubated with the vehicle (DMSO) or EGCG (40 μM) in the presence of Wnt3a-CM for 15 h. Cytosolic proteins were subjected to Western blotting with anti-β-catenin or anti-myc antibodies. In A, C and D, to confirm equal loading, the blot was re-probed with an anti-actin antibody.
Next, we investigated the mechanism underlying EGCG-mediated β-catenin degradation. It has been reported that the association of β-catenin with β-TrCP results in its subsequent proteasomal degradation. To test whether β-TrCP is necessary for β-catenin degradation induced by EGCG, a dominant negative form of β-TrCP (Δβ-TrCP) that had been observed to interact with β-catenin but was unable to form a SCFβ-TrCP ubiquitin ligase complex [29] was ectopically expressed in the presence of EGCG. As shown in Fig. 2D, the over-expression of Δβ-TrCP abrogated the EGCG-induced degradation of β-catenin, suggesting that EGCG promotes the degradation of β-catenin via a β-TrCP-dependent mechanism.
3.3. N-terminal β-Catenin Phosphorylation Is Required for Its Degradation
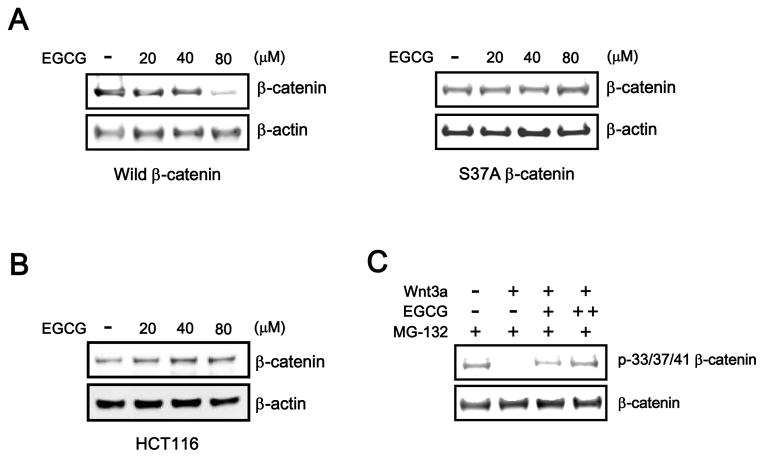
The N-terminal phosphorylation of β-catenin at Ser45 and Ser33/37 catalyzed by CK1 and GSK-3b is a prerequisite event for β-TrCP-dependent β-catenin degradation. To elucidate the mechanism underlying EGCG-mediated β-catenin degradation, we examined the effect of EGCG on the stability of wild-type β-catenin or S37A mutant β-catenin. Wild-type β-catenin or S37A mutant β-catenin was transfected into HEK293 cells, followed by treatment with increasing amounts of EGCG. As shown in Fig. 3A, wild-type β-catenin was efficiently down-regulated in response to EGCG, whereas the level of S37A mutant β-catenin was largely unaffected by EGCG treatment. In addition, EGCG did not decrease the intracellular β-catenin level in HCT116 colon cancer cells that contain a Ser45 deletion mutation in β-catenin (Fig. 3B), suggesting that the N-terminal residues of β-catenin are required for EGCG-meidiated β-catenin degradation. We next determined whether EGCG induces Ser33/37/Thr41 phosphorylation of β-catenin by Western blot analysis with a phosphor-specific β-catenin antibody. When HEK293-FL reporter cells were incubated with Wnt3a-CM, the phosphorylation of β-catenin residues Ser33/37/Thr41 decreased (Fig. 3C); this observation is in agreement with previous reports [11, 12]. However, the addition of EGCG resulted in phosphorylation at these residues in the presence of Wnt3a-CM (Fig. 3C). These results suggest that EGCG promotes β-catenin degradation via N-terminal phosphorylation of β-catenin.
Fig. 3.
N-terminal β-catenin phosphorylation is required for its degradation. A: HEK293 cells were transfected with wild-type β-catenin or β-catenin S37A plasmids and incubated with EGCG (20, 40, and 80 μM) for 15 h. Subsequently, cytosolic proteins were immunoblotted with an anti-β-catenin antibody. B: Cytosolic proteins were prepared from HCT116 cells treated with vehicle (DMSO) or the indicated concentrations of EGCG for 15 h and then subjected to western blotting with an anti-β-catenin antibody. C: EGCG induces the N-terminal phosphorylation of β-catenin. HEK293 reporter cells were incubated with EGCG (+, 40 μM; ++, 80 μM) and MG-132 (10 μM) for 15 h in the absence or presence of Wnt3a-CM. Cytosolic fractions were prepared and subjected to western blot analysis with anti-phospho-p33/37-β-catenin and anti-β-catenin antibodies.
3.4. EGCG-mediated β-Catenin Phosphorylation/degradation Is Independent of GSK-3β and PP2A
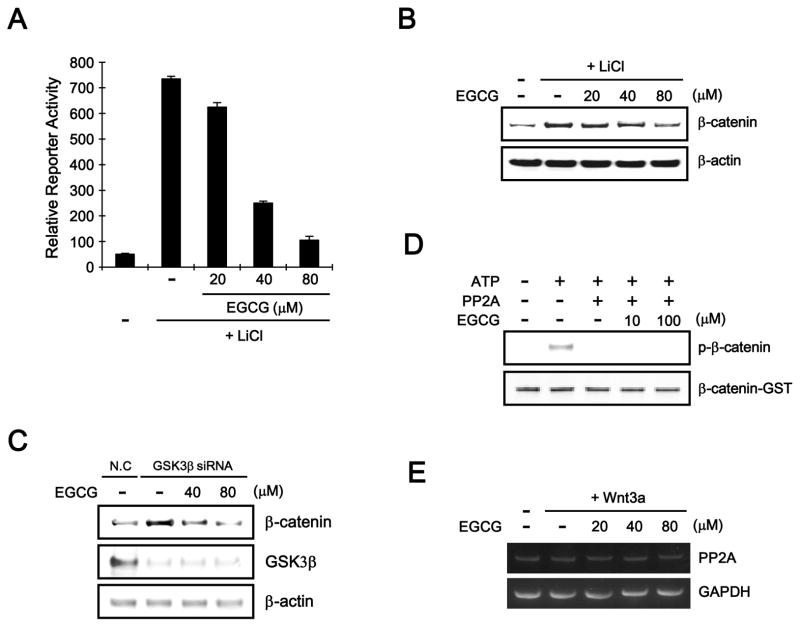
In the Wnt/β-catenin pathway, the N-terminal phosphorylation of β-catenin at Ser33/37/Thr41 is catalyzed by GSK-3β [14, 15]. Given that EGCG induces this phosphorylation, we examined the involvement of GSK-3β activity in EGCG-mediated β-catenin phosphorylation. In the presence of LiCl, which is an inhibitor of GSK-3β, EGCG still suppressed CRT in HEK293-FL cells (Fig. 4A). Consistently, Western blot analysis showed that EGCG reduced the cytosolic β-catenin accumulation induced by LiCl treatment (Fig. 4B). In addition, when we depleted endogenous GSK-3β using siRNA, EGCG decreased the level of intracellular β-catenin in HEK293-FL cells (Fig. 4C). These results indicate that β-catenin degradation by EGCG is independent of GSK-3β.
Fig. 4.
EGCG induces β-catenin degradation by a mechanism independent of GSK-3β and PP2A. A: HEK293-FL reporter cells were incubated with EGCG (20, 40 and 80 μM) in the presence of 20 mM LiCl. After 15 h, luciferase activity was determined. The results represent the average of 3 experiments, and the bars indicate the standard deviation. B: Cytosolic proteins were prepared from HEK293-FL reporter cells treated with vehicle (DMSO) or EGCG (40 and 80 μM) in the presence of 20 mM LiCl for 15 h and then subjected to western blotting with an anti-β-catenin anti-body. C: HEK293-reporter cells were transfected with negative control siRNA (NC, 40 nM) and GSK3-β siRNA (40 nM) for 36 h and then incubated with EGCG (40 and 80 μM) for 12 h. The cell lysates were subjected to western blot analysis with anti-GSK-3β and anti-β-catenin antibodies. D: EGCG does not affect PP2A activity. GST-β-catenin (100 ng) was incubated with purified CK1, GSK-3β, PP2Ac, and EGCG (10 and 100 μM). The samples were analyzed by western blotting with an anti-phospho-p33/37-β-catenin antibody. E: Semi-quantitative RT-PCR for PP2Ac and GAPDH was performed with total RNA prepared from HEK293-FL reporter cells treated with vehicle (DMSO) or the indicated concentrations of EGCG in the presence of Wnt3a-CM for 15 h.
It has been reported that protein phosphatase 2A (PP2A) dephosphorylates Ser33/37/Thr41 phosporylation of β-catenin, thereby positively regulating the Wnt/β-catenin pathway [30]. To test whether EGCG down-regulates PP2A activity, we performed an in vitro phosphatase assay using purified β-catenin, CK1, GSK-3β, and the catalytic subunit of PP2A (PP2Ac). Consistent with previous reports, CK1/GSK-3β readily catalyzed the N-terminal phosphorylation of β-catenin and PP2Ac dephosphorylated this phosphorylated N-terminal in vitro (Fig. 4D). Interestingly, EGCG did not affect PP2Ac-mediated β-catenin dephosphorylation (Fig. 4D). In addition, the expression of PP2Ac was unchanged in response to various concentrations of EGCG and/or Wnt3a-CM in HEK293-FL reporter cells (Fig. 4E). These results suggest that PP2A is not involved in EGCG-mediated β-catenin phosphorylation and degradation.
3.5. EGCG Promotes β-Catenin Phosphorylaion/degradation in SW480 Colon Cancer Cells
As aberrant activation of CRT frequently occur in colon cancer cells, we next examined whether EGCG is able to attenuate CRT in SW480 colon cancer cells, which exhibit elevated CRT due to inactivating mutation of APC [18]. SW480 cells were transfected with TOPFlash and FOPFlash reporter plasmids followed by treatment with EGCG. As shown in Fig. 5A, TOPFlash reporter activity was decreased by EGCG in a concentration-dependent manner, and the activity of FOPflash was not affected. In addition, Western blot analysis consistently showed that EGCG reduced the levels of cytosolic β-catenin in SW480 cells (Fig. 5B). In contrast to the protein level of β-catenin, the mRNA expression of β-catenin was not altered by EGCG treatment (Fig. 5C). Furthermore, EGCG-mediated β-catenin down-regulation was abolished by incubation with MG-132 in SW480 cells (Fig. 5D). Notably, Western blot analysis using a phospho-specific β-catenin antibody showed that phosphorylation of β-catenin at Ser33/37/Thr41 residues was induced by treatment with EGCG (Fig. 5E). These results indicate that EGCG, in agreement with the results in HEK293-FL reporter cells, suppresses CRT through the promotion of β-catenin phosphorylaion and degradation in SW480 colon cancer cells.
Fig. 5.
APC is not involved in EGCG-mediated β-catenin phosphorylation and degradation. A: SW480 cells were co-transfected with TOPFlash or FOPFlash and pCMV-RL plasmids and incubated with EGCG for 15 h. Luciferase activity was measured 39 h after transfection. TOPFlash activity is reported as relative light units (RLUs) normalized to Renilla luciferase activity. The results represent the average of 3 experiments, and the bars indicate the standard deviation. B: Cytosolic proteins were prepared from SW480 cells treated with the vehicle (DMSO) or EGCG for 15 h and then subjected to western blotting with the anti-β-catenin antibody. The blots were reprobed with an anti-actin antibody as a loading control. C: Semi-quantitative RT-PCR for β-catenin and GAPDH was performed with total RNA prepared from SW480 cells treated with vehicle (DMSO) or the indicated concentrations of EGCG for 15 h. D: Cytosolic proteins prepared from SW480 cells, which were incubated with vehicle (DMSO) or EGCG (40 μM) in the presence of Wnt3a-CM and exposed to MG-132 (10 μM) for 8 h, were subjected to western blotting with the anti-β-catenin antibody. E: SW480 cells were incubated with EGCG (40 μM) and MG-132 (10 μM) for 15 h. Cytosolic fractions were prepared and subjected to western blot analysis with anti-phospho-p33/37-β-catenin and anti-β-catenin antibody.
3.6. EGCG Represses the Expression of β-Catenin-dependent Gene and Inhibits the Proliferation of Colon Cancer Cells
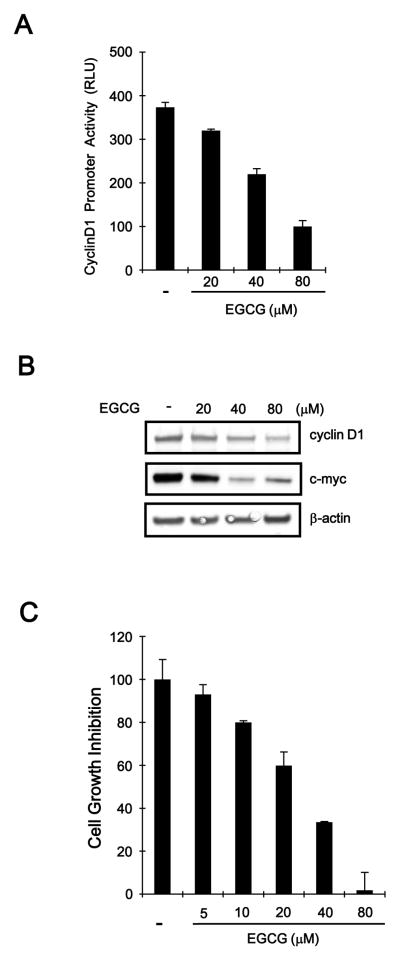
Given that EGCG promotes β-catenin degradation, we investigated the effect of EGCG on the expression of β-catenin downstream genes in SW480 colon cancer cells. SW480 cells were transfected with a reporter construct that contains a β-catenin/TCF-4 responsive region and subsequently incubated with increasing amounts of EGCG. As shown in Fig. 6A, cyclin D1 promoter activity was repressed by EGCG in SW480 cells. We also measured the protein expression of cyclin D1 in EGCG-treated SW480 cells. Consistent with results from the reporter assay, a concentration-dependent decrease in cyclin D1 protein level was observed in response to EGCG (Fig. 6B). In addition, the expression of c-myc, which is an established downstream target of β-catenin, was reduced in SW480 cells after incubation with EGCG (Fig. 6B). The specific reduction of β-catenin by antisense oligonucleotides or small interference RNA has been shown to inhibit the proliferation of cancer cells in vitro as well as tumor growth in a xenograft mouse model [31–33]. Therefore, we evaluated whether EGCG inhibits the growth of colon cancer cells. SW480 cells were incubated with varying concentrations of EGCG, and cell growth was monitored. As shown in Fig. 6C, EGCG suppressed the growth of SW480 cells in a concentration-dependent manner.
Fig. 6.
EGCG inhibits the expression of β-catenin-dependent genes. A: SW480 cells were co-transfected with cyclin D1-RL and pSV40-FL and then incubated with the indicated amounts of EGCG for 15 h. Luciferase activity was measured 39 h after transfection. Cyclin D1 promoter activity is reported as relative light units (RLU) normalized against firefly luciferase activity. The results represent the average of 3 experiments, and the bars indicate the standard deviation. B: SW480 cells were incubated with vehicle (DMSO) or EGCG for 15 h, and the cell extracts were prepared for western blotting with anti-cyclin D1 and anti-c-myc antibodies. To confirm equal loading, the blots were re-probed with the anti-actin antibody. C: The effect of EGCG on cell growth. SW480 cells were incubated in the indicated concentrations of EGCG for 48 h in 96-well plates. Cell viability was examined using the CellTiter-Glo assay (Promega). To calculate the inhibition of growth, the value at time 0 was subtracted. The results represent the average of 3 experiments, and the bars indicate the standard deviation.
4. Discussion
EGCG exhibits chemopreventive properties against cancer by modulating a wide array of signal transduction pathways, including JAK/STAK, MAPK, PISK/AKT, and Notch [4–7]. EGCG is especially reported to regulate the Wnt/β-catenin pathway but the underlying mechanism of action is less clear because of contradictory results in the published studies. EGCG blocks the Wnt/β-catenin pathway by inducing HBP1 transcriptional repressor without affecting β-catenin levels in breast cancer cells [34]. Singh et al. [35] found that EGCG increases phosphorylation of β-catenin on Ser33/37 residues through activation of CK1/GSK-3β, thereby reducing accumulation of nuclear β-catenin, in skin cancer cells. Conversely, it has been reported that EGCG inhibits GSK-3β activity, resulting in decrease of β-catenin phosphorylation, in HT29 colon cancer cells [36]. In this study, we used genetically engineered HEK293 cells, in which the Wnt/β-catenin pathway is robustly activated by Wnt3a-CM, to demonstrate that EGCG down-regulates β-catenin through a GSK-3β-independent proteasomal degradation pathway.
The stability of intracellular β-catenin protein is predominantly regulated by two APC-dependent pathways; a destruction complex (APC/Axin/CK1/GSK-3β)-dependent pathway and an APC/Siah-1-dependent pathway. In the destruction complex-dependent pathway, the N-terminal phosphorylation of β-catenin is catalyzed by CK1/GSK-3β in a complex with APC and axin, which results in the degradation of β-catenin through an ubiquitin-dependent mechanism [12–15]. In the APC/Siah-1-dependent pathway, the carboxyl terminus of APC interacts with Siah-1, which recruits the ubiquitination complex, and then promotes the degradation of β-catenin [37]. Several findings of the present study suggest that EGCG-mediated β-catenin degradation is distinct from the above-described APC-dependent pathways. We found that oncogenic β-catenin proteins, which have mutations at the CK1 phsophorylation site (Ser45) or GSK-3β phosphorylation site (Ser37), were not down-regulated in response to EGCG, implying that phosphorylation of β-catenin at N-terminal residues is essential for EGCG-mediated β-catenin degradation. In addition, when GSK-3β activity was inhibited or GSK-3β was depleted, EGCG still destabilized intracellular β-catenin protein, suggesting that EGCG-mediated β-catenin degradation is GSK-3β-independent. Moreover, EGCG still induced the degradation of β-catenin in SW480 cells, which have mutation in APC, suggesting that APC is not required for β-catenin degradation by EGCG. The dominant negative β-TrCP abrogated the effect of EGCG on the level of β-catenin, indicating that EGCG promotes β-catenin degradation through a β-TrCP-dependent proteasome pathway. Similarly, activation of PKCα has been reported to induce β-catenin degradation by a mechanism dependent of β-TrCP and independent of the destruction complex [38].
In the Wnt/β-catenin pathway, the N-terminal phosphorylation status of β-catenin is predominantly regulated by CK1/GSK-3β protein kinases and PP2A protein phosphatase [12, 13, 30]. In the dual kinase mechanism, Ser45 phosphorylation of β-catenin by CK1 is a prerequisite for subsequent GSK-3β-mediated Ser33/37 phosphorylation. PP2A counters the activity of CK1/GSK-3β but catalyzes dephosphorylation of Ser33/37 phospho-β-catenin. This dephosphorylation instantly eliminates the β-TrCP binding site and consequently prevents β-catenin degradation [30]. In this study, we found that EGCG induces β-catenin phosphorylation at Ser33/37 residues in the presence of Wnt3a-CM, which inhibits the function of the destruction complex, and in SW480 cells, which contains dysfunctional destruction complex. Although a previous report [39] suggested that PP2A expression was decreased by 24 h of EGCG treatment in JB6 mouse cells, we found no evidence for this mechanism in HEK293 cells, which are known to display normal Wnt/β-catenin signaling. In addition, an in vitro phosphatase assay showed that EGCG did not inhibit PP2A activity when we used β-catenin phophorylated by CK1/GSK-3β as a substrate. These data suggest that CK1/GSK-3β and PP2A are not involved in EGCG-induced β-catenin phosphorylaion. The mechanism underlying EGCG-induced β-catenin phosphorylation/degradation needs to be investigated further.
Aberrant up-regulation of the Wnt/β-catenin pathway is a major pathological event in the progression of human colon cancer at an early stage, during the formation of the primary lesion, and in the advanced stage [19]. Several natural dietary compounds have been identified for preventing the activation of the Wnt/β-catenin pathway. Isoflavone, which is found in soybeans, inactivates Wnt signaling to inhibit the growth of prostate cancer cells by up-regulation of GSK-3β expression [40]. In addition, curcumin, which is isolated from turmeric, was shown to stimulate the caspase-3-mediated cleavage of β-catenin [41] and its natural derivatives inhibited the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300 [25]. Quercetin is a representative flavonol that suppresses Wnt/β-catenin signaling by decreasing the nuclear level of β-catenin and Tcf4 [42]. In the present study, EGCG inhibited the Wnt/β-catenin pathway by induction of β-catenin N-terminal phosphorylation at the Ser33/37 residues, marking it for proteasomal degradation, and as a result represses the expression of cyclin D1 and c-myc, which play important roles in tumorigenesis and cell cycle progression.
In this study, EGCG exhibited anti-proliferative activity against SW480 colon cancer cells at 40 to 80 μM. However, these concentrations are higher than the concentrations of EGCG that is found in the blood and tissues after oral ingestion of tea. It is commonly found that the effective concentrations of EGCG observed in cell culture system are higher than the physiological concentrations measured in animal [43]. The reasons for these differences still remain unclear. One possible reason is the short-term exposure to EGCG in cell culture study versus the long-term treatment of animals. It has been reported that prolonging the treatment period can reduce the effective concentration of EGCG in cell culture [44]. The generation of more active metabolites of EGCG in vivo is also a possibility, even though our previous results indicate that metabolites of tea catechins have lower biological activities than their parent compounds [45]. The environment for cells in culture is also very different from that in xenograft tumors.
In conclusion, we investigated the mechanism underlying the EGCG-mediated inhibition of the Wnt/β-catenin pathway using a refined cell-based system. EGCG induced the phophorylation of β-catenin at Ser33/37 residues through a mechanism independent of GSK-3β and PP2A, and subsequently promoted the degradation of β-catenin, thereby inhibiting the proliferation of colon cancer cells. Whether the phosphorylation of β-catenin is a key cancer preventive or anticancer mechanism of EGCG in animals remains to be investigated.
Acknowledgments
We thank Dr. N. Ha for providing CK1, GSK-3β, and PP2Ac recombinant proteins. We also thank Dr. M. Davis for dominant negative β-TrCP expression plasmid. This work was supported by the Basic Science Research Program (2012R1A2A2A01002941, 2013R1A1A1009085, and 2009-0093822) and the Fundamental Technology Program (2012M3A9B2028335) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology as well as by US NIH grants CA120915 and CA133021.
References
- 1.Yang CS, Lambert JD. Research on tea and health. Pharmacol Res. 2011;64:85–86. doi: 10.1016/j.phrs.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 3.Crespy V, Williamson G. Review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 4.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea (Invited Review) Mol Nutr Food Res. 2011;55:819–831. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 5.Yang CS, Wang H, Li GX, Yang Z, Guan F, et al. Cancer prevention by tea: Evidence from laboratory studies (Invited Review) Pharmacol Res. 2011;64:113–122. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou Z, Lambert JD, Chin KV, Yang CS. Effect of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Li Y, Semenov M, Han C, Baeg GH, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 11.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latres E, Chiaur DS, Pagano M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 14.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, et al. Axin-mediated CKI phosphorylation of β-catenin at Ser 45, a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 17.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 18.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 19.Morin PJ. β-Catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 21.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the β-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- 23.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, et al. Natural derivatives of curcumin attenuate the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun. 2008;377:1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 25.Hou Z, Sang S, You H, Lee MJ, Hong J, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: Auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 26.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwak J, Hwang SG, Park HS, Choi SR, Park SH, et al. Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012;22:237–247. doi: 10.1038/cr.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, et al. The SCF β-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Yang J, Liu Y, Chen X, Yu T, et al. PR55, a regulatory subunit of PP2A, specifically regulates PP2A-mediated β-catenin dephosphorylation. J Biol Chem. 2009;284:22649–22656. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roh H, Green DW, Boswell CB, Pippin JA, Drebin JA. Suppression of β-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res. 2001;61:6563–6568. [PubMed] [Google Scholar]
- 32.Verma UM, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against β-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- 33.Zi X, Guo Y, Simoneau AR, Hope C, Xie J, et al. Expression of Frzb/Secreted Frizzeled-related protein 3, a secreted Wnt antagonis, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 2005;65:9762–9770. doi: 10.1158/0008-5472.CAN-05-0103. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, et al. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 35.Dashwood WM, Carter O, Al-Fageeh M, Li Q, Dashwood RH. Lysosomal trafficking of β-catenin induced by the tea polyphenol epigallocatechin-3-gallate. Mutat Res. 2005;591:161–172. doi: 10.1016/j.mrfmmm.2005.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Z, Xu Z, Hung MS, Lin YC, Wang T, et al. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res. 2009;29:2025–2030. [PubMed] [Google Scholar]
- 37.Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, et al. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 38.Gwak J, Jung SJ, Kang DI, Kim EY, Kim DE, et al. Stimulation of protein kinase C-α suppresses colon cancer cell proliferation by down-regulation of β-catenin. J Cell Mol Med. 2009;13:2171–2180. doi: 10.1111/j.1582-4934.2008.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin J, Chen HG, Yan Q, Deng M, Liu J, et al. Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res. 2008;28:4150–4162. doi: 10.1158/0008-5472.CAN-08-0839. [DOI] [PubMed] [Google Scholar]
- 40.Liss MA, Schlicht M, Kahler A, Fitzgerald R, Thomassi T, et al. Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics. 2010;7:245–252. [PubMed] [Google Scholar]
- 41.Jaiswal AS, Marlow BP, Gupta N, Narayan S. β-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 42.Park CH, Chang JY, Hahm ER, Park S, Kim HK, et al. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 43.Moiseeva EP, Almeida GM, Jones GD, Manson MM. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007;6:3071–3019. doi: 10.1158/1535-7163.MCT-07-0117. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu M1, Sakai H, Shirakami Y, Yasuda Y, Kubota M, et al. Preventive effects of (−)-epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db Mice. Cancer Prev Res (Phila) 2011;4:396–403. doi: 10.1158/1940-6207.CAPR-10-0331. [DOI] [PubMed] [Google Scholar]
- 45.Lu H, Meng X, Li C, Sang S, Patten C, et al. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]