Abstract
Rats raised in an enriched condition (EC) exhibit alterations in the neurobiological and behavioral response to nicotine compared to rats reared in an impoverished condition (IC) or a standard condition (SC). The current study determined whether environmental enrichment differentially regulates extracellular signal-regulated kinase1/2 (ERK1/2) activity in the prefrontal cortex (PFC) in rats following nicotine sensitization or nicotine self-administration. Under the saline control condition, EC rats displayed diminished baseline activity, and greater sensitization to repeated administration of nicotine compared to IC and SC rats. After repeated saline injections, the basal levels of phosphorylated ERK1/2 (pERK1/2) were higher in EC compared to IC and SC rats, which was negatively correlated with their respective baseline activities. Repeated nicotine (0.35 mg/kg) injections induced pERK1/2 to similar levels in SC and IC rats; however, the induction of pERK1/2 in EC rats by nicotine was not significantly different from saline controls, owing to their high baseline. In the self-administration paradigm, EC rats self-administered less nicotine (0.03 mg/kg/infusion) relative to IC or SC rats on a fixed ratio-1 schedule of reinforcement. Accordingly, no differences in pERK1/2 were found between EC and IC rats self-administering saline, whereas nicotine self-administration resulted in an increase in pERK1/2 in IC rats but not in EC rats. Furthermore, the levels of pERK1/2 in EC and IC rats were positively correlated with their respective total number of nicotine infusions. Thus, these findings suggest that environmental enrichment alters the basal and nicotine-mediated pERK1/2, which may contribute to enrichment-induced behavioral alterations in response to nicotine.
Keywords: Environmental enrichment, nicotine, ERK, prefrontal cortex, self-administration
Introduction
Environmental factors contribute to individual vulnerability for drug abuse (Leshner, 2000; Rhee et al., 2003; Stairs & Bardo, 2009). The importance of environmental enrichment to protect against drug addiction is well-acknowledged in research using rodent models (Stairs & Bardo, 2009). In an environmental enrichment model, rats are raised in one of the three different conditions: an enriched condition (EC), an impoverished condition (IC), and a standard condition (SC); which differs in novelty, handling, social cohorts, and physical activity. Exposure within the environmental enrichment paradigm results in neurobiological adaptations, particularly in the prefrontal cortex (PFC) of the mesocorticolimbic dopaminergic system (Bowling et al., 1993; Zhu et al., 2004; Del Arco et al., 2007; Gomez et al., 2012). Environmental enrichment-dependent alterations to this neural circuit are suggested to be protective against maladaptive behaviors associated with drug abuse.
The behavioral effects of nicotine are primarily mediated by its stimulating actions on nicotinic receptors-mediated dopamine (DA) release within the mesocorticolimbic system (Laviolette & van der Kooy, 2004). Extracellular signal-regulated kinase1/2 (ERK1/2), a member of the mitogen-activated protein kinase, is activated by nicotine in vitro and in vivo (Nakayama et al., 2001; Valjent et al., 2004). Nicotine, through DA/D1 receptors, induces activation of DA- and cAMP-regulated phosphoprotein-32 (DARPP-32) and ERK1/2, which triggers their downstream activation of the transcription factor, cAMP-response element-binding protein (CREB) (Nakayama et al., 2001; Brunzell et al., 2003; Hamada et al., 2004; Valjent et al., 2005a; Valjent et al., 2005b), thereby modulating nicotine-mediated behaviors. Nicotine’s potentiating effect on long-term potentiation induction is disrupted by inhibition of ERK1/2 (Raybuck & Gould, 2007), suggesting that nicotine-augmented ERK1/2 activity may facilitate synaptic plasticity and mediate the behavioral effects of nicotine. Furthermore, the ERK signaling pathway is a key integrator of the DA/D1 receptor and glutamate/N-methyl-D-aspartate (NMDA) receptor signaling that induces long-term cellular alterations and adaptive behaviors in response to nicotine exposure. Enrichment-enhanced glutamate-related signaling has been documented (van Praag et al., 2000), which is critical for regulating ERK activation. Our recent study has demonstrated that EC rats display greater nicotine-augmented levels of phosphorylated DARPP-32 at threonine-34 and CREB at serine 133 in the PFC compared to IC and SC rats, which is associated with increased sensitivity to nicotine sensitization in EC rats (Gomez et al., 2012). Emerging evidence demonstrates that the PFC plays a critical role in systemic nicotine-induced excitation of DA neurons in the ventral tegmental area (Mansvelder et al., 2009; Zhang et al., 2012) and enrichment-induced neuroadaptations against drug addiction (Solinas et al., 2010). Therefore, understanding the molecular mechanisms of enrichment-induced changes in the ERK1/2 intracellular signaling cascade within the PFC may provide important insights into how environmental enrichment reduces susceptibility to psychostimulant drug abuse. Considering ERK1/2 activity is regulated by the DA/D1R and glutamate/NMDAR, in this study, we determined the phosphorylation of ERK1/2 in the PFC of rats raised in different conditions after nicotine-induced locomotor sensitization or nicotine self-administration. The results of these studies will provide new mechanistic insights into understanding how, at both the neurobiological and behavioral levels, environmental enrichment may reduce nicotine addiction.
Materials and methods
Materials
Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal antibodies recognizing both rat phosphorylated ERK1/2 (pERK1/2; SC-16982R) and total ERK1/2 (V114A) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Promega (Madison, WI, USA), respectively. Horseradish peroxidase-conjugated anti-rabbit secondary antibody (111–035–144) was purchased from Jackson ImmunoResearch (West Grove, PA, USA).
Animals
Male Sprague-Dawley rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN, USA). Rats arrived at the age of 21 days and were housed with food and water ad libitum in a colony room in the Division of Laboratory Animal Resources at the University of South Carolina. The colony room was maintained at 21 ± 2 °C, 50 ± 10% relative humidity on a 12-h light/dark cycle with lights on at 07:00 AM. All of the experimental procedures using animals were performed according to the National Institute of Health guidelines for AAALAC accredited facilities. The experimental protocol for this study was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina in compliance with animal welfare assurance.
Environmental conditions
Upon arrival at postnatal day 21, rats were randomly assigned to EC, IC, or SC conditions using a previously published method (Wooters et al., 2011). EC rats were group-housed (10–16 per cage) in a metal cage (In cm: 120 length × 60 width × 45 height) and were handled daily. Twelve hard non-chewable plastic objects were placed randomly in the cage. While half of the objects were replaced with new objects every day, the remaining objects were rearranged to increase novelty. It should be noted that EC rats are subjected to multiple components; for discussion please see (Gomez et al., 2012), but this paradigm allows for cross comparisons with our previous reports and other research regarding the neurochemical correlates of the EC paradigm in rats (Zhu et al., 2004; Zhu et al., 2005; Zhu et al., 2007; Wooters et al., 2011; Gomez et al., 2012). IC rats were individually housed in wire mesh hanging cages (In cm: 25 length × 18 width × 17 height) with solid metal sides and wire mesh flooring containing no objects. SC rats were pair-housed in a clear polycarbonate cage (In cm: 43 length × 20 width × 20 height) with a wire rack top. All rats were handled extensively throughout behavioral testing so that novelty and the number of cohorts were the only factors that differed among the groups throughout behavioral paradigms. The SC condition represents the standard housing conditions set in the NIH Guide for the 1996 version of the NIH Guide for the Care and Use of Laboratory Animals. Rats were raised in one of these three housing conditions from 21 to 53 days of age and were maintained in their respective housing condition throughout the experiment.
Nicotine administration and locomotor activity
Nicotine hydrogen tartrate salt was dissolved in sterile saline (0.9% sodium chloride). The nicotine solution (freebase) was prepared immediately prior to injection and neutralized to pH 7.4 with NaOH to reduce irritation. Habituation and nicotine-mediated locomotor activity were performed as described in our previous report (Gomez et al., 2012). Locomotor activity was assessed with 16 square (40 × 40 cm) chambers (Hamilton-Kinder Inc., Poway, CA). Movement was detected by infrared photocell interruptions; each chamber has 32 emitter/detector pairs capable of measuring horizontal and vertical (rearing) activity. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts; photocell emitter/detector pairs were tuned by the manufacturer to handle the extra perspex width. Horizontal activity was measured as all beam breaks in the horizontal plane. All activity monitors were located in an isolated room that is separate from the animal colony.
Beginning at 54 days of age, all rats were habituated to the locomotor activity chambers for two consecutive days. Twenty four hours later, baseline activity was tested following a saline injection. This represents the first time animals received an injection. For a total of 15 days, rats received a daily saline or nicotine (0.35 mg/kg, s.c.) injection. Activity was measured for 30-min session prior to the injection and again for a 60-min post-injection session; however, activity was measured every other day, i.e., on days 1, 3, 5, 7, 9, 11, 13, and 15. On the non-locomotor testing days, rats were transported to the same room that rats were previously injected prior to locomotor testing, and then returned to the home cages after nicotine or saline injection. After completion of the final locomotor session, brains were removed by rapid decapitation 20 min after the last injection. Brain regions were dissected in a chilled matrix for Western blot analyses.
Nicotine self-administration
Operant chambers (ENV-008; Med-Associates, St. Albans, VT), housed within sound-attenuating enclosures, were controlled by Med-PC computer interface software. The front panel of the chamber allowed access to a recessed food dipper (ENV-202M) through a 5 × 5 cm opening. Two retractable metal levers (ENV-112BM) on either side of the opening were located 7.3 cm above a metal grid floor. A dipper equipped with a 0.1 ml cup attached to the end of the arm was raised into the food receptacle, which allowed access to liquid sucrose following the completion of response requirements. A white cue light (28 V; 3 cm in diameter) located above each response lever, was used to signal timeouts. An infrared sensor (ENV-254-CB) was used to detect head entries into the food receptacle. During drug self-administration sessions a syringe pump (PHM-100) was used to deliver intravenous nicotine infusions through a watertight swivel (PHM-115).
At 54 days of age, rats were food restricted in order to maintain 90% of free-feeding weight for three days prior to the beginning of dipper training. Dipper training and autoshaping (Reichel et al., 2008) were conducted according to previous research during which animals learned the operant response for 26% (w/v) sucrose (Harrod et al., 2012; Lacy et al., 2012). During autoshaping, animals responded for 26% sucrose for 2 days on a fixed ratio-1 (FR-1) schedule. A response on the active lever resulted in a 4-sec access to sucrose whereas a response on the inactive lever resulted in no sucrose presentation.
Following 5 days of ab libitum access to food, rats were anesthetized with ketamine (100 mg/kg/ml, IP) and diazepam (5 mg/kg/ml, IP) and implanted with a catheter according to our published method (Harrod et al., 2012). In brief, one end of the catheter was inserted into the jugular vein, and the other was embedded in a dental acrylic head cap mounted to the top of the skull with four stainless steel jeweler’s screws. The stainless steel catheter was capped by an aluminum standoff to prevent catheter damage by rats housed in an enriched condition. Rats were allowed to recover from the surgery for 4 to 5 days. During recovery, the catheter of each rat was flushed twice daily with gentamicin (40 mg/ml) and sterile heparinized saline (0.2 %) to maintain the catheter patency.
Beginning at 66–70 days of age, rats were allowed to self-administer nicotine (0.03 mg/kg/infusion) or saline (as a negative control for neurochemical measurement) on a FR-1 schedule of reinforcement during 3-h daily test sessions for a total of 21 days. Nicotine solution (freebase) was prepared immediately prior to testing and it was neutralized to pH 7.4 with NaOH. Doses of nicotine were calculated based on the body weight of individual rats. Nicotine was infused (60 μl, 3.3 s) following pressing the active lever, while responding on the inactive lever was recorded but produced no consequence. Each drug infusion was followed by a 20-s time out. The time out (20-s) occurred immediately after the active lever response and it was signaled by turning on the cue lights located above the response levers. No drug infusions were delivered if animals responded on either lever during the time-out. Twenty-four hours after last self-administration session (Day 21), brain regions were dissected in a chilled matrix for further analyses.
Western blot analysis
Immunoreactivity of ERK1/2 and pERK1/2 were examined based on our previously published method (Gomez et al., 2012). The PFC was dissected in a chilled matrix and sonicated immediately on ice in a homogenization buffer containing 20 mM HEPES, 0.5 mM EDTA, 0.1 mM EGTA, 0.4 M NaCI, 5 mM MgCI2, 20% glycerol, 1 mM PMSF, phosphatase inhibitor cocktails I (P2850, Sigma-Aldrich, St. Louis, MO, USA) and protease inhibitors (P8340, Sigma-Aldrich, St. Louis, MO, USA). Homogenates were then centrifuged at 12,000 g for 15 min at 4°C. The supernatants were collected and stored at −80°C. Protein concentrations were determined in triplicate using Bio-Rad DC protein detection reagent. In brief, equal amount of protein (30 μg) were loaded, separated by 10% SDS-polyacrylamide gel electrophoresis and then transferred onto PVDF membranes. Samples across all comparisons with treatment and housing conditions were loaded onto two separate gels for pERK and ERK. Membranes were then preincubated with blocking buffer (5% dry milk powder in PBS containing 0.5% Tween-20) and then incubated overnight at 4 °C in blocking buffer with primary antibodies: total ERK1/2 (1:5000) or pERK1/2 (1:1000). The membranes were washed and then incubated in blocking buffer containing secondary affinity-purified, horseradish peroxidase-conjugated anti-rabbit IgG; (1:20,000) from Jackson ImmunoResearch (West Grove, PA, USA) for total ERK1/2 and 1:5000 for pERK1/2. Immunoblots were detected using enhanced chemiluminescence (ECL-plus) and developed on Hyperfilm (Amersham Biosciences UK Ltd., Little Chalfont Buckinghamshire, UK). After detection and quantification of these proteins, each blot was stripped in a Re-blot plus mild antibody stripping solution (Chemicon, Temecula, CA, USA) and reprobed for detection of β-tubulin (1:5000; H-235, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) to monitor equal loading proteins among samples. Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Scion image software (Scion Corp., Frederick, MD, USA).
Statistical analysis
Locomotor activity data from Day 1 and Day 15 are presented as the number of beam breaks and were analyzed by mixed-factor analyses of variance (ANOVA) with housing condition and treatment as between-group factors, and with day and time as within-subject factors. Nicotine self-administration data from the last week of the three-week period were analyzed by mixed-factor ANOVA with housing condition as between-group factor, and testing day (7 days) and lever (active and inactive) as the within-subject factors. One-way ANOVAs were subsequently performed to determine if there was a significant effect for treatment across saline-treated or nicotine-treated groups. Tukey’s post-hoc tests were performed where appropriate. After validating the equal amount of ERK1/2 expression among samples by β-tubulin, the ratio of immunoreactivity of pERK1/2 to ERK1/2 in the PFC was analyzed by separate two-way ANOVAs (housing condition × treatment) with housing condition and treatment as between-group factors. Simple effect comparisons were made for post hoc analyses. To determine potential relationships between behavioral data and immunoreactivity of pERK1/2 and ERK1/2, separate Pearson correlations were conducted. All statistical analyses were performed using IBM SPSS Statistics version 20, and αlevel was set at p< 0.05 for all analyses.
Results
EC rats display increased sensitization to nicotine compared to IC and SC rats
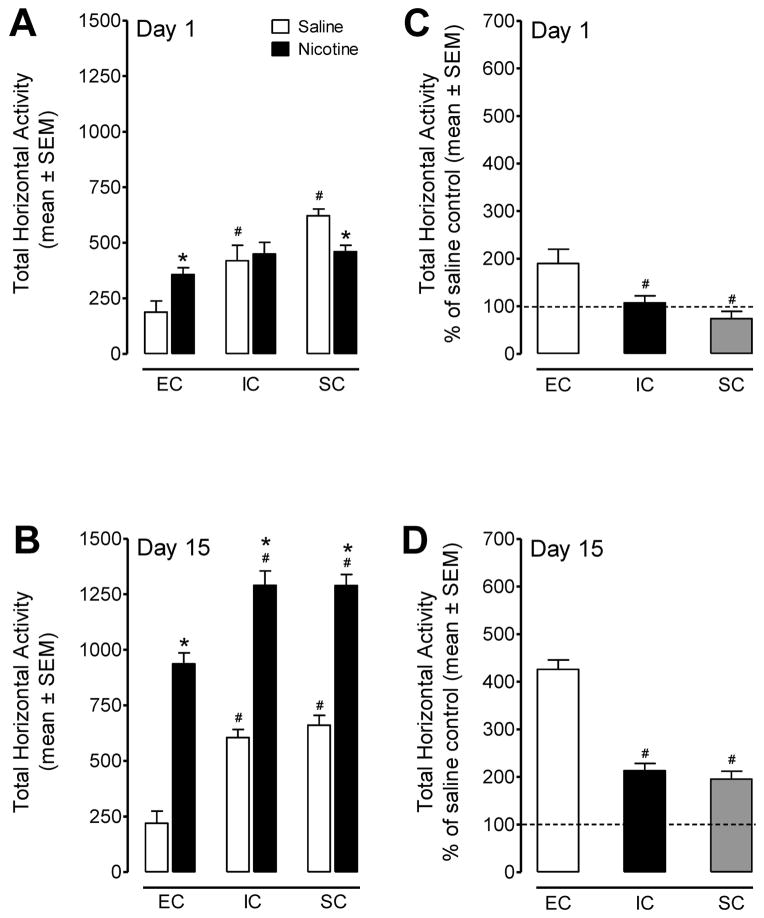
To directly address whether the ERK signaling pathway underlies differential changes in the behavioral effects of nicotine among EC, IC and SC rats, we examined the levels of pERK1/2 in the PFC of these rats after repeated nicotine (0.35 mg/kg) administration. In this procedure, all rats were repeatedly administered an equal amount of nicotine or saline by the experimenter. The horizontal activity for EC, IC and SC rats with repeated nicotine or saline during the 60-min period on Day 1 and Day 15 is shown in Figure 1A and B (% of saline control is shown in 1C and D for comparison). A housing condition × treatment × day × time ANOVA revealed significant main effects of housing condition (F(2,30) = 43.1, p<0.001), treatment (F(1,30) = 124.1, p<0.001), day (F(1,30) = 287.3, p<0.001), and time (F(11,330) = 132.9, p<0.001). In addition, there was a significant housing condition × treatment interaction (F(2,30) = 3.9, p<0.05). First, the saline IC and SC rats showed more activity than the EC animals on day 1 and on day 15. On Day 1, there were no differences in activity among EC, IC and SC rats, following acute nicotine injection. Compared to their respective saline controls, rats treated with acute nicotine exhibited a significant increase in activity in EC (137 %; t(10) = 2.9, p<0.05), but not IC rats. Interestingly, the nicotine-mediated activity was decreased by 27 % in SC rats (t(10) = 3.9, p<0.05) relative to its saline control. In addition, the nicotine-mediated activity in rats across all housing conditions was also expressed as a percent change from their respective saline control on Day 1 and Day 15 (Figure 1C and D, respectively). A housing condition × day ANOVA revealed significant main effects of housing condition (F(2,15) = 32.6, p<0.001), day (F(1,15) = 119.8, p<0.001), and a significant housing condition × day interaction (F(2,15) = 30.2, p<0.001). EC rats displayed greater activity than IC and SC rats in response to either acute or repeated nicotine administration. On Day 15, nicotine administration produced hyperactivity in EC (425 %; t(10) = 9.6, p<0.001), IC (213 %; t(10) = 9.1, p<0.001) and SC (195 %; t(10) = 9.5, p<0.001) rats (Figure 1D). These data were consistent with our previous report showing the EC rats exhibit increased sensitivity to nicotine-mediated locomotor sensitization with a lower baseline locomotor activity is observed in EC rats in relation to IC and SC rats (Gomez et al., 2012).
Figure 1.
The horizontal activity data from Day 1 and Day 15 of the behavioral sensitization experiment. Panels A and B represent the horizontal activity (mean ± SEM) across the 60-min session in the EC, IC, and SC groups following acute (Day 1) and repeated (Day 15) administration of saline or nicotine (0.35 mg/kg, s.c). Panels C and D represent the corresponding horizontal activity (mean ± SEM) across the 60-min session presented as the percentage of saline control (from the respective housing condition). * p< 0.05 compared to the respective saline controls. # p< 0.05 compared to the respective EC group. n=6 rats/group.
Environmental enrichment attenuates nicotine-induced increases in pERK1/2
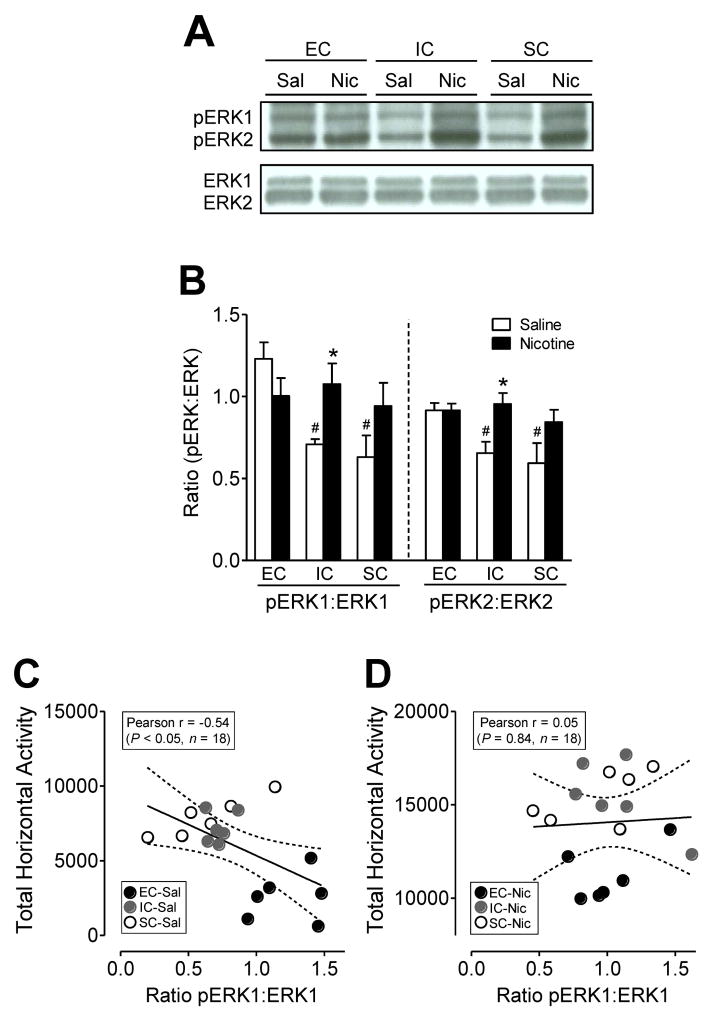
The ERK1/2 pathway is crucial for nicotine-induced neuroadaptation and behavioral changes (Zhai et al., 2008). Therefore, we determined whether the levels of total ERK1/2 and pERK1/2 in the PFC are differentially regulated among EC, IC, and SC rats following repeated nicotine or saline injection. As shown in Figure 2A and B, since no differences in total ERK1/2 were found among the three housing conditions (data not shown), the levels of the phosphorylated ERK1 or ERK2 (pERK1 or pERK2) were presented as the immunoblot densities of the ratios of pERK1 to ERK1 or pERK2 to ERK2, respectively, in the PFC. A two-way ANOVA on pERK1 in the PFC revealed a main effect for housing condition (F(2,30) = 4.5, p<0.05) and a significant interaction of housing condition × treatment (F(2,30) = 4.2, p<0.05). In saline controls, the one-way ANOVA was significant (F(2,17) = 11.3, p<0.05), and the levels of pERK1 in the EC group was higher than that in both the IC (73.5 ± 2.3%; p<0.05) and SC (95 ± 12.5%; p<0.05) groups with no difference between the IC and SC groups. No difference in pERK1 was found among three groups of rats treated with nicotine. When compared to their respective saline control, the level of pERK1 was significantly increased in IC (52 ± 6.5%; t(10) = 3.0, p<0.05) rats and a trend for significance was observed in SC (49 ± 6.9%; t(10) = 1.9, p=0.08) animals. No significant effects were found in EC rats between nicotine-treated and saline control groups.
Figure 2.
Levels of pERK1/2 and ERK1/2 in the prefrontal cortex (PFC) of EC, IC, and SC rats treated repeatedly with nicotine or saline. (A) Representative immunoblots of pERK1/2 and ERK1/2 immunoreactivity in the PFC of EC, IC and SC rats following repeated administration of nicotine (0.35 mg/kg, s.c.) or saline. (B) The ratio of pERK1/2 levels to total ERK1/2 levels in the PFC in corresponding rats. Data are presented as the ratio of pERK1/2 to total ERK1/2 densitometry values of immunoreactivity. Histobars represent means and error bars represent the SEM. The levels of pERK1/2 and total ERK1/2 were measured at the same time with the same loading volume. *p< 0.05 compared to the respective saline controls. # p< 0.05 compared to EC group. n=6 rats/group. Correlation of the phosphorylation level of ERK1 in the PFC with horizontal activity in EC, IC, and SC rats administered repeatedly with saline (C) or nicotine (D). Total horizontal activity counts for each rat within the 60-min session were collected from the last behavioral testing on day 15. Data for the ratio of pERK1 to total ERK1 densitometry values of immunoreactivity in the PFC of saline control rats were collected from panel B. Dashed lines represent the 95% confidence interval of the linear regression fit (solid line). n=6 rats/group.
With regard to the level of pERK2, a two-way ANOVA revealed a main effect of housing condition (F(2,30) = 3.4, p<0.05) and treatment (F(1,30) = 9.1, p<0.05) (Figure 2A and B). In saline controls, one-way ANOVA revealed a significant main effect for basal pERK2 levels (F(2,17) = 4.0, p<0.05) with the EC group having significantly increased pERK2 levels compared to IC (39.7 ± 2.7%; p<0.05) and SC rats (54.2 ± 6.7%; p<0.05). The one-way ANOVA for the nicotine treated rats revealed no differences among the EC, IC, and SC groups. Nicotine on day 15 significantly increased pERK2 levels in IC rats (46 ± 3.1%; t(10) = 3.1, p<0.05) and there was a trend of significance in the SC groups (42 ± 3.1%; t(10) = 1.7, p=0.09). No difference was observed in pERK2 level between EC rats treated with nicotine and saline. Thus, these results suggest that repeated nicotine cannot further potentiate ERK1/2 activity in the PFC of EC rats that is attributed to the higher basal pERK1/2.
Locomotor behavior is associated with pERK1 levels in the PFC
We have recently shown that the basal phosphorylation state of DARPP-32 at threonine-34 site in the PFC from EC, IC, and SC rats under saline control was positively correlated with their respective baseline locomotor activity, and that EC rats displayed greater sensitization to nicotine compared to IC and SC rats (Gomez et al., 2012). To explore whether ERK1/2 activity, a potential downstream signaling protein of DARPP-32, was also associated with locomotor activity, we examined the correlations of immunoblot densities of the ratios of pERK1/2 to total ERK1/2 in the PFC of all rats treated repeatedly with nicotine or saline to their respective locomotor counts collected from the last day (Day 15) of the behavioral test. The ratio of pERK1 to total ERK1 in the PFC of all saline control rats was negatively correlated with total horizontal activity (Figure 2C, p< 0.05, Pearson r = −0.54). However, there were no correlations regarding the ratios of pERK1 to total ERK1 in the PFC of rats administered repeatedly nicotine and the respective horizontal activity (Figure 2D, p> 0.05, Pearson r = 0.84). These results suggest that ERK activity is critical for enriched environment-induced basal changes in locomotor activity.
EC rats exhibit less operant responding for intravenous nicotine compared to IC rats
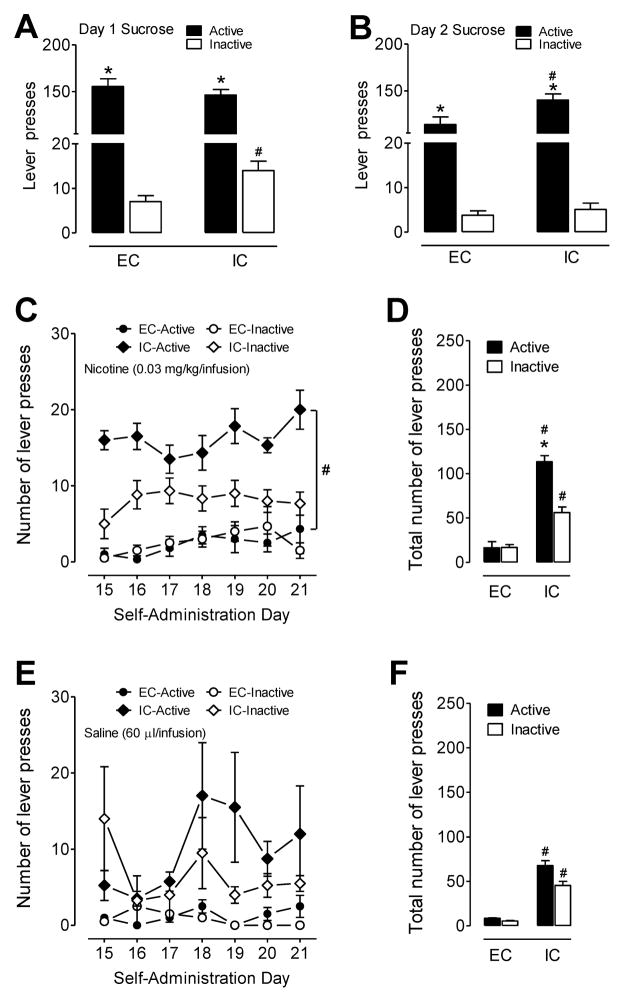
Rats were first trained to respond for sucrose and the last two days of training consisted of 2 consecutive 30-min FR-1 tests. The sucrose training data are shown in Figure 3A and B. A housing condition × lever (active and inactive) × day ANOVA (2 × 2 × 2) revealed main effects for lever (F(1,26) = 785.2, p<0.001) and day (F(1,26) = 37.7, p<0.001), and a significant housing condition × lever × day interaction (F(1,26) = 17.5, p<0.001). Neither the main effect of housing nor the housing condition × lever was significant. Both EC (F(1,26) = 318, p<0.001) and IC (F(1,26) = 516, p<0.001) rats exhibited more responding on the active lever for sucrose than on the inactive lever. Interestingly, it was also found that IC rats significantly pressed the inactive lever more than the EC rats on day 1 (F(1,26) = 6.9, p<0.05). These results indicate that EC and IC rats learned the operant response to an equal level when sucrose was the reinforcer, and that both groups showed good discrimination between the active and inactive levers.
Figure 3.
Sucrose- and nicotine-maintained responding in EC and IC rats. The number of active and inactive lever presses (mean ± SEM) for day 1 (A) and day 2 (B) of the training phase for the sucrose-maintained responding experiments (30 min/session, FR1). * p< 0.05 indicates a difference between active and inactive levers within housing group. # p< 0.05 denotes difference between EC and IC groups. n=14 rats/group. Following sucrose training, rats were then allowed to self-administer nicotine or saline for 21 consecutive days (3 h/day). Nicotine (0.03 mg/kg) or saline was infused (60 μl, 3.3 s) following depression of the active lever, while responding on the inactive lever resulted in no nicotine or saline infusion. Self-administration was conducted under a FR1 schedule of reinforcement. (C) The number of active and inactive responses (mean ± SEM) is plotted for the last week (15–21 days) of nicotine self-administration for EC and IC rats. (D) The total number of active and inactive responses (mean ± SEM) collapsed across the 7 tests (days 15–21). (E) The number of active and inactive responses (mean ± SEM) is plotted for the last week (15–21 days) of saline “self-administration” for EC and IC rats. (F) The total number of active and inactive responses (mean ± SEM) are collapsed across the 7 tests (days 15–21) of “self-administration” with saline. * p< 0.05 denotes difference between active and inactive levers within housing group. # p< 0.05 denotes difference between IC-active and EC-active. n= 6 rats/nicotine group. n = 3–4 rats/saline group.
Rats were then given the opportunity to respond for nicotine (0.03 mg/kg/infusion) or saline daily for 21 consecutive days under FR-1 schedule. Regarding active lever responding (same as nicotine infusions), EC and IC rats exhibited 29 and 43 nicotine infusions, respectively, on day 1 of testing and the number of infusions decreased across the first week (Supplementary Figure 1A). During the second week, average active lever responding began to stabilize: IC rats continued to show greater responding on the active lever, relative to the EC rats, e.g., ~ 14 and 1 infusions/day, respectively (Supplementary Figure 1A). In terms of the inactive lever responding, the pattern differed for the EC and IC rats during the first and second weeks. EC rats exhibited low, but equal responding on the active and inactive levers. In contrast, the IC animals exhibited variable inactive lever responding during the first week, inactive lever responding was approximately half of active lever responding during the second and third weeks (Supplementary Figure 1A). As shown in Figure 3C, the active and inactive lever responding remained stable in EC and IC rats during the third week. The housing × day × lever ANOVA (2 × 7 × 2) revealed a significant main effect of housing (F(1,10) = 92.2, p<0.001), lever (F(1,10) = 18.8, p<0.01), and a housing × lever interaction (F(1,10) = 19.7, p<0.05). Simple effect comparisons between EC and IC rats on active lever responding revealed the total number of nicotine infusions during the last week is about 2.4 and 16.2 infusions in EC and IC rats, respectively (Figure 3D, F(1,10) = 16.2, p<0.01).
Regarding the saline controls, the EC and IC rats exhibited differential amounts of responding on the active and inactive levers across the first and second weeks, with the IC groups showing elevated bouts of responding, relative to the EC groups (Supplementary Figure 1B). The IC active lever responding during the third week was more variable (e.g., days 18–20) relative to the first two-weeks and there were no overall main effects or interactions with lever in the omnibus ANOVA (see figure legend of Supplementary Figure 1B). Figure 3E shows the active and inactive lever responding to saline infusion in EC and IC rats during the third week. The housing × day × lever ANOVA (2 × 7 × 2) for the third week of responding revealed a significant main effect of housing (F(1,5) = 10.8, p<0.05), and there were no other significant main effects or interactions. Simple effect comparisons between EC and IC rats on lever responding revealed that IC rats displayed higher active (t(12) = 4.2, p<0.01) and inactive (t(12) = 3.8, p<0.01) pressing, respectively, compared to EC rats (Figure 3F). These findings suggest that the IC group showed more overall responding on the active and inactive levers compared to EC rats, and this outcome is consistent with data showing that IC rats exhibit more baseline activity than EC rats (see Figure 1 above).
Overall, although the EC rats received substantial nicotine exposure on the first day of the experiment, nicotine did not maintain higher levels of responding on the FR-1 during the remainder of testing. IC rats responded for a greater number of nicotine infusions than EC rats, and conversely, the IC rats exhibited greater active-to-inactive lever responding throughout the second and third weeks of testing. These findings demonstrate that environmental enrichment decreases nicotine self-administration in rats when a FR schedule of reinforcement is used.
Nicotine reinforcement is positively correlated with the nicotine-induced pERK1/2 levels in the PFC
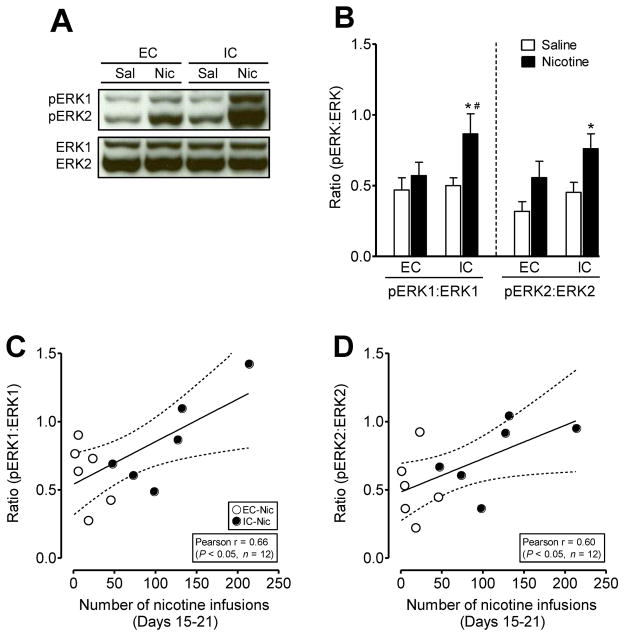
To determine whether the environmental enrichment-induced attenuation of nicotine-augmented pERK1/2 is associated with the reinforcing efficacy of nicotine, twenty-four hours after the last nicotine self-administration session (as described in Figure 3C), the levels of pERK1/2 and total ERK1/2 were correlated in the PFC (Figure 4). Because no differences in total ERK1/2 were found among the two housing conditions (data not shown), the levels of pERK1 or pERK2 were presented as the immunoblot densities of the ratios of pERK1 or pERK2 to total ERK1 or ERK2 in the PFC. In saline control groups, no differences in pERK1/2 were found between EC and IC rats (data not shown). Two-way ANOVAs revealed significant main effects on pERK1 and pERK2 in the PFC (F(1, 20) = 6.9, p<0.05 and F(1, 20) = 11.0, p<0.01, respectively). In the saline control groups, no differences in pERK1/2 were found between EC and IC rats (Figure 4B). Compared to saline controls, IC rats that self-administered nicotine exhibited increased pERK1 (F(1, 10) = 5.7, p<0.05) and pERK2 (F(1, 10) = 5.9, p<0.05). The levels of pERK1 were not altered in EC rats that self-administered nicotine and a non-significant trend of pERK2 induction was observed relative to their saline counterparts (t(9) = 1.9, p = 0.09; Figure 4B). This suggests that an enriched environment produces an attenuation of nicotine-mediated increases in ERK1/2 activity.
Figure 4.
Correlation of the phosphorylation levels of ERK1/2 in the prefrontal cortex and total number of nicotine infusions by EC and IC rats. Twenty-four hours after the last self-administration test, brain regions were collected for determination of pERK1/2 and ERK1/2 immunoreactivity. (A) Representative immunoblots of pERK1/2 and ERK1/2 immunoreactivity and (B) the ratio of pERK1/2 level to total ERK1/2 in the PFC of EC and IC rats that self-administered nicotine (0.03 mg/kg/infusion) or saline. Data are presented as the ratio of pERK1/2 to total ERK1/2 densitometry values of immunoreactivity. Histobars represent means and error bars represent the SEM. Total ERK1/2 and pERK1/2 were measured at the same time with the same loading volume. *p< 0.05 indicates a difference within the same housing condition between the nicotine reinforced and the saline groups. # p< 0.05 denotes difference between EC and IC rats. n=6 rats/group. Correlations of the values of pERK1:ERK1 (C) and pERK2:ERK2 (D) collected from panel B and the total number of nicotine infusions for EC and IC animals during the last week (15–21 days) of the self-administration tests (Fig. 3D). Dashed lines represent the 95% confidence interval of the linear regression fit (solid line). n=6 rats/group.
To explore the contribution of nicotine-mediated ERK activation in the nicotine reinforced behavior of EC and IC rats, we examined the correlations of immunoblot densities of the ratios of pERK1/2 to total ERK1/2 in the PFC of rats that self-administered nicotine with their respective active lever responding collected from operant sessions conducted on days 15–21 of the experiment. The results reveal that the ratios of pERK1/2 to total ERK1/2 were positively correlated with total number of nicotine infusions (Figure 4C and D, p< 0.05, Pearson r = 0.66 and 0.60 for pERK1 and pERK2, respectively). Therefore, these findings suggest that activation of ERK1/2 is associated with enriched environment-induced reductions in nicotine self-administration behavior.
Discussion
The current findings demonstrate that environmental enrichment differentially alters nicotine-induced increases of pERK1/2 in the PFC, which is associated with enriched environment-induced alterations of nicotine-mediated locomotor sensitization and nicotine self-administration, respectively. EC rats displayed greater sensitization to nicotine and self-administered less nicotine when compared to IC or SC rats. In the repeated saline injection groups, EC rats had high basal levels of pERK1/2 in the PFC compared to IC and SC rats, which was negatively correlated with their respective baseline locomotor activity. Specifically, following repeated nicotine administration, pERK1/2 levels were relatively lower in EC than IC and SC rats as compared to their respective basal pERK1/2 levels. After nicotine self-administration, the levels of pERK1/2 in the PFC were increased in IC rats, which were attenuated in EC rats. Further, the pERK1/2 levels in EC and IC rats were positively correlated with their total number of nicotine infusions. Thus, these findings suggest that environmental enrichment mediates changes in the prefrontal ERK1/2 activity, which may contribute to enriched environment-induced alterations in the behavioral effects of nicotine.
The present study and our previous work (Zhu et al., 2004; Wooters et al., 2011; Gomez et al., 2012) demonstrate that EC rats exhibit an inherent decrease in baseline locomotor activity compared to IC and SC rats under basal conditions. Following repeated injection, EC rats displayed lower magnitude nicotine (0.35 mg/kg)-induced behavioral sensitization relative to IC and SC rats when the absolute values were analyzed. The basal difference in activity between EC and IC/SC animals necessitates the use of a percent of control analysis, which indicates that EC rats actually demonstrate higher sensitivity to nicotine administration. Consistent with our current results, a previous study has demonstrated that EC rats were less sensitive to a relatively low dose of nicotine (0.2 mg/kg) than SC rats when data of nicotine-induced activity were expressed as the absolute values (Green et al., 2003); however, when the nicotine-induced locomotor activity was expressed as a percent change from their respective saline controls, EC rats still displayed greater sensitization to nicotine (0.2 mg/kg) than SC rats. Thus, findings from the two of our studies suggest a novel hypothesis: that environmental enrichment produces a leftward shift in the nicotine dose-response curve for locomotor activity. This hypothesis should be directly tested by using a dose-response assessment of nicotine on the measure of behavioral sensitization.
The decreased baseline activity in EC rats is accompanied by parallel decreases in DA transporter function (Zhu et al., 2004; Zhu et al., 2005), the number of D1 receptors (Del Arco et al., 2007) and the basal levels of phosphorylated DARPP-32 at threonine-34 (pDARPP-32 Thr34) and CREB at serine 133 sites (pCREB) (Gomez et al., 2012) within the PFC. In particular, the basal locomotor activity in EC, IC and SC rats under saline control conditions are positively correlated with their respective levels of pDARPP-32 Thr34 (Gomez et al., 2012) but negatively correlated with their basal levels of pERK1/2 within the PFC (current study). ERK is the key protein substrate to integrate both DA/D1R and glutamate/NMDA receptor signaling. Among EC, IC and SC rats, although the immunoreactivity of NMDA glutamate receptor in the PFC or cortex was not altered after repeated saline or nicotine injections (Andin et al., 2007; Gomez et al., 2012), it is unclear whether enrichment functionally changes these receptors. On the other hand, the decrease in pDARPP-32 Thr34 in the PFC of EC rats is companied with a reduction of pCREB (Gomez et al., 2012), suggesting that the increase in the prefrontal pERK may be a compensatory alteration induced by environmental enrichment. Indeed, it has been suggested that the phosphorylation of CREB is more directly associated with cAMP/PKA signaling pathway rather than pERK (Montminy, 1997; Frodin & Gammeltoft, 1999). Thus, an investigation on the effects of environment enrichment on the homeostasis on both signaling pathways is warranted to further decipher its impact on gene transcription. Further, the stimulation of prefrontal D2 receptors could be secondary to enhance ERK activity (Liu et al., 2008). Indeed, our unpublished data using microarrays for gene expression show that mRNA expression levels of the D2 receptor in the PFC is upregulated in EC rats compared to IC rats with repeated saline injection. The most parsimonious interpretation of our data is that enriched environment-induced changes in the balance among D1, D2, and glutamate receptors results in increased basal ERK1/2 phosphorylation levels within the PFC. Interestingly, the basal levels of pERK1/2 in the PFC were not significantly different between EC and IC rats in the saline control groups for nicotine self-administration. It is possible that the behavioral contingencies in the locomotor and self-administration procedures differently affect basal levels of pERK1/2 in EC and IC rats. For instance, compared to the locomotor paradigm, EC and IC rats underwent sucrose dipper training prior to saline self-administration. It has been shown that ERK activity is subject to modification after operant behavior for non-drug reinforcement (Guegan et al., 2013). Furthermore, evidence shows that basal levels of pERK1/2 in the cortex of rodents can vary in an age-dependent manner (Zhen et al., 1999; Spanos et al., 2012), suggesting the extended time frame of our self-administration procedure versus the sensitization paradigm may affect basal levels of pERK1/2. Nevertheless, it is likely that environmental enrichment-dependent prefrontal plasticity may play an important role in the behavioral manifestations of nicotine exposure.
Nicotine has been shown to elevate the phosphorylation levels of ERK1/2 both in vitro and in vivo (Nakayama et al., 2001; Valjent et al., 2004). The present results demonstrate that a significant increase in nicotine-mediated pERK1/2 in the PFC was observed in IC rats, but not in EC rats after nicotine-mediated sensitization or nicotine self-administration as compared to their saline controls. In contrast, our recent findings show that the magnitude of changes in nicotine-induced pDARPP-32 Thr34 and DA clearance rate were greater in EC rats compared to IC animals, based on their basal levels under saline control (Zhu et al., 2007; Gomez et al., 2012). The discrepancy may indicate that ERK1/2 activity is regulated by other upstream activator (e.g. glutamate signaling) in addition to the DA/D1R/cAMP/DARPP-32 pathway. Further investigation in enriched environment-induced changes in glutamate signaling pathway is necessary. Interestingly, repeated nicotine administration eliminated the basal differences in both pDARPP-32 Thr34 and pERK1/2 between EC and IC rats in our current and previous research (Gomez et al., 2012). Thus, the attenuation of nicotine-mediated activation of dopaminergic signaling within the PFC by environmental enrichment may therefore represent compensatory enrichment-dependent neuroadaptations within the PFC in response to nicotine stimulation. In addition, nicotine-mediated pERK1/2 levels in the PFC were not correlated with their respective nicotine-mediated locomotor activity in these animals. It is possible that EC rats have relatively high basal levels of pERK1/2, which may mask the nicotine-induced elevation of ERK1/2 activity, thereby producing a ceiling effect on nicotine-mediated pERK1/2. On the other hand, the levels of pERK1/2 in the PFC of nicotine self-administered EC and IC rats were positively correlated with their respective total number of nicotine infusions during the last week (15–21 days), although no basal differences in pERK1/2 in the PFC between EC and IC rats were observed. One possible explanation for the discrepancy of the correlation of pERK1/2 with the behavioral response to nicotine between the sensitization and self-administration paradigms may be due to the animals being treated with nicotine via different routes of administration. Importantly, systemic nicotine produces profound enhancements of pERK1/2 in the PFC compared to other brain regions (Valjent et al., 2004), indicating that the PFC plays a crucial role in nicotine-mediated ERK1/2 activity and the consequence of its behavioral effects. In accordance, the current findings show that an enriched environment produces neuroadaptations in the prefrontal ERK pathway, which may underlie nicotine-enhanced activation of ERK1/2 and the relevant nicotine-mediated behaviors.
Intravenous nicotine self-administration is the most reliable and direct measure of nicotine reinforcement in animals (Corrigall & Coen, 1994), and is a valuable preclinical tool to assess potential treatment for nicotine addiction. The current study is the first to investigate the effects of an enriched environment on nicotine self-administration and demonstrates that EC rats self-administer less nicotine than IC rats on an FR-1 schedule of reinforcement. This finding is consistent with the previous reports showing that EC rats self-administer less amphetamine (Bardo et al., 2001), methylphenidate (Alvers et al., 2012), and cocaine (Green & Schenk, 2002; Green et al., 2010) on FR schedules compared to IC rats, suggesting a drug-resistance phenotype of an enriched environment. However, nicotine has distinct neuropharmacological regulatory effects on the DA system compared to these psychostimulants. Notably, compared to IC rats, EC rats displayed drastically lower levels of nicotine-maintained responding. This observation, however, is unlikely due to the inability of EC rats to lever press or learn to discriminate between the active and inactive lever because EC and IC rats displayed similar learning and memory capacities for the operant contingencies (i.e., response-reinforce associations) when rats were initially assessed for sucrose-maintained responding. In contrast, when nicotine was the reinforcer, the IC but not the EC animals learned the new operant contingency, suggesting that the EC rats did not learn a relationship between the lever response and the reinforcing effects of nicotine. Consistent with the behavioral findings, the failure to increase pERK in PFC of EC rats following nicotine self-administration could be related to the absence of learning about the relationship between a response and nicotine reinforcement. Alternatively, the lack of nicotine-mediated pERK in PFC of EC animals may be result of a consequence of a lower nicotine exposure in EC rats during nicotine self-administration (e.g., Guegan et al., 2013). Therefore, future studies will be necessary to investigate the implementation of different nicotine doses or higher FR requirements (e.g., FR-5) to obtain the full spectrum of EC-induced differences in nicotine self-administration. Other factors besides learning and memory may also affect nicotine-maintained responding in EC, relative to IC animals. These include differences in sensitivity to the behavioral effects of IV nicotine and it may also reflect differences in other constructs such as environmental-induced thresholds for “impulsive” behavior. Additionally, since the SC rats were not included in our current study, this group should be included in future studies to further determine that EC rats exhibit modulated sensitivity to IV doses of nicotine.
Enriched environment-induced decrease in nicotine self-administration may impact the relationship between drug-taking behavior and impulsivity. The previous studies demonstrate that high impulsive rats show increases in both the acquisition and maintenance of nicotine self-administration compared with low impulsive rats (Diergaarde et al., 2008). EC rats have been shown to be less impulsive compared to IC rats (Wood et al., 2006; Perry et al., 2008), which is consistent with the attenuation of self-administering amphetamine (Perry et al., 2008) and nicotine (current report) in EC compared to IC rats. Considering the important role of the PFC in impulsivity and stimulant self-administration (Perry et al., 2011), differences in ERK1/2 activity in the PFC may contribute to psychostimulant-induced changes in sensitivity and/or in the reinforcing effects of psychostimulant drugs in the differentially reared groups. Thus, the present results, combined with previous results showing low dopaminergic tone (Zhu et al., 2004; Zhu et al., 2005; Del Arco et al., 2007) and diminished basal levels of pDARPP-32 Thr34 and phosphorylated CREB (Gomez et al., 2012) in the PFC of EC rats relative to IC rats, suggest a potential molecular mechanism underlying enriched environment-induced decreases in impulsivity and vulnerability to nicotine dependence. Interestingly, the individual difference in response to acute nicotine may be predictive of subsequent nicotine reward. For example, rodents with low acute nicotine-induced locomotor response displayed significant nicotine conditioned place preference, while those with high locomotor response to acute nicotine failed to demonstrated nicotine conditioned place preference (Pastor et al., 2013; Bernardi & Spanagel, 2014). In terms of behavioral percentage changes, EC rats exhibited higher locomotor response to acute nicotine but self-administered less nicotine, whereas IC rats with low locomotion sensitivity after acute nicotine had higher nicotine intake during self-administration. Considering the baseline of locomotor activity, Bernardi and Spanagel have recently indicated that high basal activity is positively correlated to acute and sensitized response to nicotine which is similar to our IC and SC rats (Bernardi & Spanagel, 2014). The factors underlying effects of environmental enrichment on nicotine-induced behavioral changes are still unknown. However, environmental enrichment resulted in alterations of basal locomotor activity, novelty-seeking or anxiety-like behaviors (Zimmermann et al., 2001; Gomez et al., 2012; Leger et al., 2014; Ravenelle et al., 2014), which may be associated with subsequent nicotine-mediated behavioral responses. Following repeated administration of nicotine or nicotine self-administration, we found that the pERK levels are correlated to basal activity or lever responding (Fig. 2C, Fig. 4C–D), suggesting that ERK activity is associated with enrichment-dependent behavior in response to nicotine. Since the basal and/or acute nicotine-induced locomotor changes potentially predict nicotine sensitization and nicotine rewarding effects, it is worthwhile to evaluate whether the pERK, pDARPP-32 Thr34 or phosphorylated CREB in the PFC of naïve rats and/or after acute nicotine injection serves a biomarker related to subsequent behavioral changes during the development of sensitization and self-administration among groups raised in different environmental conditions.
Direct comparisons between the performance of EC and IC rats in the behavioral sensitization and drug self-administration procedures are not possible because the two procedures measure distinct aspects of behavior. The locomotor activity procedure is a valuable animal model to elucidate the molecular mechanisms underlying the impact of environmental enrichment on the development of behavioral sensitization to nicotine, which is suggested to be an indication of incentive salience, but, not of nicotine reward or reinforcement. In contrast, intravenous nicotine self-administration is the most reliable and direct measure of drug reinforcement in animals. Thus, the increased behavioral sensitization exhibited by EC rats in the current study is not in opposition to the findings of the self-administration experiment, i.e., decreased responding for nicotine, because the two procedures measure different aspects of nicotine’s effects on the mesocorticolimbic DA system. Moreover, less nicotine self-administration, as exhibited by the EC rats, does not necessarily mean those animals have a decreased sensitivity to IV nicotine. Dose-response experiments using fixed ratio schedule of reinforcement, which vary the unit dose of nicotine will be important to determine potential differences in sensitivity to the reinforcing effects of IV nicotine between EC and IC rats (Watkins et al., 1999). It is possible that such studies will reveal that EC animals are more sensitive to detect an IV dose of nicotine, relative to IC and SC control animals. If for example, EC rats are found to be more sensitive to IV nicotine relative to IC rats, then one prediction might be that EC rats will self-administer less nicotine relative to IC rats. Currently, we report that EC rats respond for less nicotine than IC rats when a FR-1 schedule of reinforcement is used, and that exposure to an enriched environment may produce a decreased abuse liability for nicotine addiction. Precise statements about the reinforcing efficacy of nicotine in EC, IC, and SC rats, however, will require the use of progressive-ratio schedules of reinforcement.
In conclusion, the current results suggest that environmental enrichment differentially alters nicotine-mediated pERK1/2 levels in the PFC, which may impact the differences in the behavioral effects of nicotine: nicotine-mediated locomotor sensitization or nicotine self-administration. Future studies involving site-specific blockade of ERK1/2 activity by infusion of U0126, an inhibitor of ERK, into the PFC in EC, IC, and SC rats undergoing nicotine-mediated behaviors will further elucidate the causal relationship of prefrontal pERK1/2 activity with nicotine-associated behaviors. These findings may have important implications for preclinical studies involving the role of enrichment in individual differences in vulnerability to both nicotine abuse and impulsivity. Manipulations of prefrontal cortical ERK activity or the upstream mechanisms responsible for ERK activation may therefore represent an approach in the treatment of drug addiction and other addictive behaviors.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Health to JZ (DA035714, DA024275 and DA026721) and to SBH (DA021287) and by the Award of the South Carolina INBRE Bioinformatics Pilot Project Program to JZ. We thank Ryan Lacy and Ivory Chen for their excellent technical assistance.
Abbreviations
- ANOVA
analysis of variance
- DARPP-32
DA- and cAMP-regulated phosphoprotein-32
- CREB
cAMP-response element-binding protein
- EC
enriched condition
- ERK1/2
extracellular signal-regulated kinase1/2
- pERK1/2
phosphorylated ERK1/2
- IC
impoverished condition
- FR-1
fixed ratio-1
- PFC
prefrontal cortex
- SC
standard condition
References
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andin J, Hallbeck M, Mohammed AH, Marcusson J. Influence of environmental enrichment on steady-state mRNA levels for EAAC1, AMPA1 and NMDA2A receptor subunits in rat hippocampus. Brain Res. 2007;1174:18–27. doi: 10.1016/j.brainres.2007.06.101. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Spanagel R. Basal activity level in mice predicts the initial and sensitized locomotor response to nicotine only in high responders. Behav Brain Res. 2014;264:143–150. doi: 10.1016/j.bbr.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine self-administration and locomotor activity are not modified by the 5-HT3 antagonists ICS 205-930 and MDL 72222. Pharmacol Biochem Behav. 1994;49:67–71. doi: 10.1016/0091-3057(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Molecular and cellular endocrinology. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Midde NM, Mactutus CF, Booze RM, Zhu J. Environmental Enrichment Alters Nicotine-Mediated Locomotor Sensitization and Phosphorylation of DARPP-32 and CREB in Rat Prefrontal Cortex. PLoS One. 2012;7:e44149. doi: 10.1371/journal.pone.0044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Guegan T, Cutando L, Gangarossa G, Santini E, Fisone G, Martinez A, Valjent E, Maldonado R, Martin M. Operant behavior to obtain palatable food modifies ERK activity in the brain reward circuit. Eur Neuropsychopharmacol. 2013;23:240–252. doi: 10.1016/j.euroneuro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–1103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Morgan AJ. Offspring of Prenatal IV Nicotine Exposure Exhibit Increased Sensitivity to the Reinforcing Effects of Methamphetamine. Frontiers in pharmacology. 2012;3:116. doi: 10.3389/fphar.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Hord LL, Morgan AJ, Harrod SB. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299–306. doi: 10.1016/j.drugalcdep.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Leger M, Paizanis E, Dzahini K, Quiedeville A, Bouet V, Cassel JC, Freret T, Schumann-Bard P, Boulouard M. Environmental Enrichment Duration Differentially Affects Behavior and Neuroplasticity in Adult Mice. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu119. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Vulnerability to addiction: new research opportunities. Am J Med Genet. 2000;96:590–591. doi: 10.1002/1096-8628(20001009)96:5<590::aid-ajmg2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buck DC, Neve KA. Novel interaction of the dopamine D2 receptor and the Ca2+ binding protein S100B: role in D2 receptor function. Mol Pharmacol. 2008;74:371–378. doi: 10.1124/mol.108.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Pastor V, Andres ME, Bernabeu RO. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology (Berl) 2013;226:551–560. doi: 10.1007/s00213-012-2928-1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenelle R, Santolucito HB, Byrnes EM, Byrnes JJ, Donaldson ST. Housing environment modulates physiological and behavioral responses to anxiogenic stimuli in trait anxiety male rats. Neuroscience. 2014;270:76–87. doi: 10.1016/j.neuroscience.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci. 2007;121:1119–1124. doi: 10.1037/0735-7044.121.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Spanos M, Besheer J, Hodge CW. Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behavioural brain research. 2012;230:158–166. doi: 10.1016/j.bbr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Herve D, Girault JA. Drugs of abuse, protein phosphatases, and ERK pathway. Med Sci (Paris) 2005a;21:453–454. doi: 10.1051/medsci/2005215453. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005b;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res. 2011;219:98–107. doi: 10.1016/j.bbr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cellular and molecular neurobiology. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Gao M, Xu D, Shi WX, Gutkin BS, Steffensen SC, Lukas RJ, Wu J. Impact of prefrontal cortex in nicotine-induced excitation of ventral tegmental area dopamine neurons in anesthetized rats. J Neurosci. 2012;32:12366–12375. doi: 10.1523/JNEUROSCI.5411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Uryu K, Cai G, Johnson GP, Friedman E. Age-associated impairment in brain MAPK signal pathways and the effect of caloric restriction in Fischer 344 rats. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54:B539–548. doi: 10.1093/gerona/54.12.b539. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Green TA, Wedlund PJ, Dwoskin LP. Nicotine increases dopamine clearance in medial prefrontal cortex in rats raised in an enriched environment. J Neurochem. 2007;103:2575–2588. doi: 10.1111/j.1471-4159.2007.04951.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Stauffacher M, Langhans W, Wurbel H. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behav Brain Res. 2001;121:11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.