Abstract
Langerhans cells (LCs) are a distinct subset of DCs that resides in the epidermis and other epithelia. They are potent antigen-presenting cells and strong inducers of T-cell responses. Like other DC types, LCs express C-type lectins that serve as antigen/pathogen uptake receptors, with Langerin/CD207 being the characteristic LC C-type lectin. In this issue of the European Journal of Immunology, Geijtenbeek and colleagues [Eur. J. Immunol. 2011. 41: 2619–2631] assign a role to Langerin on human LCs for binding and capturing measles virus. Interestingly, however, this function does not correlate with productive infection or with cross-presentation of measles virus. These authors show that measles virus does not infect the LCs via Langerin, and that LCs cannot cross-present the virus to CD8+ T cells; however, presentation of this virus to CD4+ T cells occurs and is dependent on virus capture by Langerin. Thus, cross-presentation of measles virus may be left to skin DCs other than LCs. This highlights the complexity of anti-viral T-cell responses that originate in the skin and also emphasizes the need for intensified investigations into human skin DCs in order to be able to ultimately harness their potential for immunotherapy.
Keywords: DCs, Dermatology, Host/pathogens interactions, Innate immunity, Langerhans cells
The function of Langerhans cells
The precise function of Langerhans cells (LCs) in vivo is still not entirely clear [1]. Without doubt, LCs make first contact with many microbes entering the body through the surface of the skin and mucosae. From this, it was inferred that LCs would invariably induce immunity against these pathogens. This assumption was supported by ample in vitro evidence for an immunogenic, T-cell stimulatory function of LCs [2]; however, about 8 years ago, this concept began to be repeatedly challenged with regard to certain viral and parasitic infections (and in other models). For example, LCs in skin-draining lymph nodes cannot present antigen derived from cutaneous herpes simplex virus-1 [2]; and vaginal submucosal DCs, but not LCs, induce protective Th1 responses to herpes simplex virus-2 [3]. Likewise, during infection with leishmania, parasites are presented by DCs other than LCs in the draining lymph nodes [4]. Complementing these studies, other groups demonstrated that LCs are involved in immune responses in a downregulatory, rather than a protective, manner against pathogens invading the skin. For example, Kautz-Neu et al. [5] recently reported that LCs are negative regulators of immune responses against Leishmania by inducing regulatory T cells which downmodulate excessive T-cell responses. The notion that LCs may predominantly play an immune-dampening, perhaps tolerogenic role, thus appears to be gaining ground.
The function of Birbeck granules and the Langerin receptor
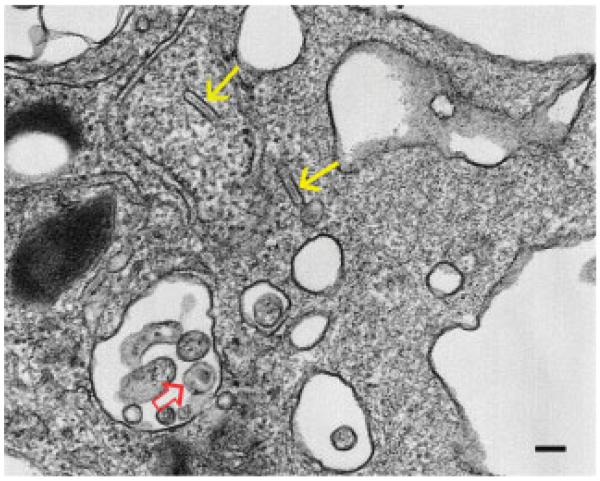
Langerin/CD207 was first identified by Saeland and Valladeau [6] as a main component of Birbeck granules (Fig. 1). For many years, this organelle remained functionally “enigmatic” but it was a helpful ultrastructural hallmark for LCs by its conspicuous tennis racket or rod shape. Thorough cell biological studies by Daniel Hanau’s group suggest that Birbeck granules may not be primarily involved in endocytosis but rather are part of the endosomal recycling network [7, 8]. Although the Langerin receptor was initially regarded as a highly specific marker for LCs, it soon became clear that other cell types also express it, namely dermal/interstitial CD103+ DCs and lymph node resident CD8+ DCs [1, 9]. Nonetheless, Langerin remains a highly useful and reliable marker for LCs.
Figure 1.
Transmission electron microscopy of a human LC, as identified by the rod-shaped Birbeck granules (yellow arrows), that was exposed to HIV-1 in vitro. The cell contains one clearly discernible HIV-1 particle (open red arrow). This is the “stage” where the article by Geijtenbeek and colleagues “plays.” Diameter of the HIV-1 particle and the scale bar is about 100 nm.
Langerin binds mannose- and fucose-containing carbohydrates as are present in the glycoproteins of HIV and HSV envelopes. Additionally, it binds mannose and β-glucans from fungal cell walls, e.g. Candida, Saccharomyces, or Malassezia furfur. For all these pathogens, Langerin is probably important for their incorporation into cells and it influences the ensuing immune response. “Probably” because virtually no studies exist to date that directly investigate the interactions of defined pathogens with Langerin in binding, uptake, and intracellular routing in LCs, nor the magnitude and quality of the resulting immune responses – except the group of Theo Geijtenbeek, whose article in this issue of the European Journal of Immunology [10] we discuss here.
These authors have been working on the concept of LCs as a first line of defense against virus penetrating the skin and mucosa for several years (reviewed in [11]). They pinpointed Langerin as a major, broadly specific pathogen receptor on LCs that can capture HIV-1 [12], herpes simplex virus (HSV)-2 [13], or fungi [14]. For HIV, Langerin acts as a neutralizing molecule in that it captures low numbers of HIV and causes degradation of the viral particles within the Birbeck granules. This leads to a block in virus transmission to T cells. Thus, it functions as a fast innate defense mechanism [12] and the group of Geijtenbeek was the first to highlight such a hitherto unexpected innate effector function of LCs.
In this issue of the European Journal of Immunology, Geijtenbeek’s group extend their studies to another virus, namely measles virus, and show that this virus also binds to Langerin [10]. Tracing endocytosed anti-Langerin antibody by immunoelectron microscopy, they reveal that Langerin moves from the plasma membrane to Birbeck granules and, at least in part, into lysosomes and MHC-class II-containing vesicles, i.e. those organelles where antigen processing for the MHC II pathway occurs. This suggests that the pathway for Langerin-captured measles virus may be the same as for the anti-Langerin antibody. The authors’ observation that measles virus is presented to CD4+ T cells may be taken as confirmation and ultrastructural tracing studies of the virus particles themselves will certainly follow soon.
Physiological context of Langerin-mediated capture of measles virus
LCs in lung epithelial tissue, where the measles virus typically enters the body [15], are immature and express only Langerin but not CD150, the other measles virus receptor. Geijtenbeek and colleagues [10] show that these immature LCs – at least their skin equivalents – do not become productively infected but rather present measles antigen by MHC class II to CD4+ T cells. In contrast, mature LCs (migratory LCs from skin explants in this case) are infected and are able to stimulate CD8+ T-cell responses via the classical MHC class I pathway. Importantly, neither immature nor mature LCs were able to cross-present UV-inactivated virus nor apoptotic measles virus-infected cells. This leads to a hypothetical scenario whereby immature LCs lining the lung tissue in the upper respiratory tract can incorporate invading measles virus and induce CD4+ T-cell responses to fight the infection, whereas mature LCs in the mediastinal lymph nodes are able to catch virus there and stimulate an additional CD8+ T-cell response.
DCs in the subepithelial lung tissue might also be involved in immune responses against measles virus but they were not tested for T-cell stimulation in the current paper by Geijtenbeek and colleagues [10]. Since it is logistically extremely difficult to retrieve sufficient numbers of these cells from human lung tissue, the authors used monocyte-derived DCs instead. These DCs, following culture with measles virus, were able to stimulate CD4+ T cells to a similar extent as virus-cultured LCs but induced stronger CD8+ T cell responses. Furthermore, the monocyte-derived DCs potently cross-presented UV-inactivated and cell-associated measles virus on MHC class I molecules indicating that interstitial DCs, for which monocyte-derived DCs sometimes serve as a model, could – in addition to LCs – be involved in anti-measles virus responses and be in charge of the induction of CD8+ T-cell responses. This is an interesting open question to be addressed in subsequent studies. These data also support the notion that different subsets of DCs may not only differ in the types of pathogens that they handle, but may perhaps also divide the labour when fighting one type of pathogen such as the measles virus discussed here.
“Real” LCs versus “model” LCs
One point of the paper by Geijtenbeek and colleagues [10] merits further emphasis. It goes without saying that “real” LCs, as obtained from the skin by various techniques, are not necessarily identical in all features with “model” LCs, such as the frequently used CD34-derived LCs [16, 17]. Yet, quite often these two categories of cells are used in one and the same study but are not semantically separated in a clear way. The study by Geijtenbeek and colleagues [10] is very clear in this regard as only “real” LCs were used. These cells were isolated from human skin by standard trypsinization techniques (immature LCs) or by migration from epidermal explant (sheet) cultures (mature LCs). It is plausible indeed, as discussed by the authors, that this may account for an important discrepancy in the cross-presentation data. Although “real” human LCs in the Geijtenbeek and colleagues study [10] cannot cross-present, the Banchereau-Palucka group have reported cross-presentation by in vitro-generated “LCs” [18, 19]; however, only when the antigen was conjugated to an antibody against an antigen uptake receptor on LCs (human DCIR) could CD8+ T-cell responses be measured by pentamer staining and interferon-γ release, i.e. was cross-presentation by “real” LCs truly determined [20]. Incidentally, we have recently pointed out another area where profound differences in LC preparations occur, namely LC responses to thymic stromal lymphopoietin (TSLP). The Th2-skewing capacity of thymic stromal lymphopoietin can only be observed with “real” LCs from skin [21] but not with CD34-derived LCs [22].
These considerations regarding LC preparation become particularly relevant when LCs are used as targets for immunotherapy [23], be it when in vitro-generated LC-like cells are loaded with tumor antigens and administered to cancer patients as recently shown [24, 25] or when LCs are directly manipulated in vivo by means of C-type lectin-specific antibodies as envisaged for the future [26, 27].
Mouse versus human
In a similar way, caution is warranted when comparing data from mouse and human models. Obviously, it is logistically much easier to work with mouse LCs, and therefore more is known about the functions of these cells. In the mouse, it has been unequivocally shown that “real” LCs can cross-present protein antigens in vitro. These experiments were performed in a way that a “contaminating” contribution of Langerin+ dermal DCs could definitively be ruled out [28]. At first glance, this contrasts with the data from human LCs reported by Geijtenbeek and colleagues [10]. However, different antigens were used: ovalbumin protein in the mouse and virus particles in human. Clearly, much more work is needed and premature generalizations should be avoided.
Outlook
Further questions are stimulated by this report. The authors have shown previously that HIV-1 is captured by Langerin on LCs and subsequently degraded in the Birbeck granules [12]. Are measles virus particles also destroyed in the Birbeck granules? The authors have not directly addressed this. Can this novel innate function of LCs (reviewed in [29]) possibly be generalized to other viruses beyond HIV and measles? Conversely, one wonders whether HIV-1 is also not cross-presented to CD8+ T cells by human LCs? Since HIV-1 and measles virus seem to use the same Langerin uptake receptor in LCs, it is reasonable to assume that similar intracellular pathways of processing are used. Is the degradation of HIV-1 in Birbeck granules complete such that there is nothing left for routing into the cross-processing/cross-presentation pathway? The authors speculate that this may be a reason for the lack of cross-presentation of measles virus by LCs. Thus, for HIV-1 we now know its intracellular fate but not whether it is cross-presented [12], with measles virus the situation is reversed: we do not know its intracellular fate but it seems clear that it is not cross-presented [10]. All in all, this new study by Geijtenbeek and colleagues is another important and stimulating step toward understanding the in vivo role of LCs as defenders against pathogens invading the body through epithelia, or – paradigmatically – as “catchers in the rye” [30].
Acknowledgements
P. Stoitzner was supported by a grant from the Austrian Science Fund (FWF P21487).
Abbreviation
- LC
Langerhans cell
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Romani N, Clausen BE, Stoitzner P. Langerhans cells & more: Langerin-expressing dendritic cell subsets in the skin. Immunol. Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan RS, Smith CM, Belz GT, Van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8a+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 3.Zhao XY, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective th1 responses to herpes simplex virus-2. J. Exp. Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritter U, Meissner A, Scheidig C, Körner H. CD8a- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 5.Kautz-Neu K, Noordegraaf M, Dinges S, Bennett CL, John D, Clausen BE, Von Stebut E. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 2011;208:885–891. doi: 10.1084/jem.20102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 7.Uzan-Gafsou S, Bausinger H, Proamer F, Monier S, Lipsker D, Cazenave JP, Goud B, et al. Rab11A controls the biogenesis of Birbeck granules by regulating Langerin recycling and stability. Mol. Biol. Cell. 2007;18:3169–3179. doi: 10.1091/mbc.E06-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mc Dermott R, Ziylan U, Spehner D, Bausinger H, Lipsker D, Mommaas M, Cazenave JP, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+dendritic cells to the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Vlist M, De Witte L, De Vries RD, Litjens M, de Jong MAWP, Fluitsma D, de Swart RL, Geijtenbeek TBH. Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4+T cells but are incapable of crosspresentation. Eur. J. Immunol. 2011;41:2619–2631. doi: 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham AL, Carbone F, Geijtenbeek TB. Langerhans cells and viral immunity. Eur. J. Immunol. 2008;38:2377–2385. doi: 10.1002/eji.200838521. [DOI] [PubMed] [Google Scholar]
- 12.De Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 13.de Jong MA, De Witte L, Taylor ME, Geijtenbeek TB. Herpes simplex virus Type 2 enhances HIV-1 susceptibility by affecting Langerhans cell function. J. Immunol. 2010;185:1633–1641. doi: 10.4049/jimmunol.0904137. [DOI] [PubMed] [Google Scholar]
- 14.de Jong MA, Vriend LE, Theelen B, Taylor ME, Fluitsma D, Boekhout T, Geijtenbeek TB. C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol. Immunol. 2010;47:1216–1225. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur. J. Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 16.Strobl H, Bello-Fernandez C, Riedl E, Pickl WF, Majdic O, Lyman SD, Knapp W. flt3 ligand in cooperation with transforming growth factor-b1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–1434. [PubMed] [Google Scholar]
- 17.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, De Saint-Vis B, Jacquet C, Yoneda K, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFα. J. Exp. Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, et al. Functional specializations of human epidermal Langerhans cells and CD14+dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao T, Ueno H, Glaser C, Fay JW, Palucka AK, Banchereau J. Both Langerhans cells and interstitial DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur. J. Immunol. 2007;37:2657–2667. doi: 10.1002/eji.200636499. [DOI] [PubMed] [Google Scholar]
- 20.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, et al. Cross-priming CD8+T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu Y-J, Romani N. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen presenting cells that induce pro-allergic T cells. J. Allergy Clin. Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen VA, Dubrac S, Forstner M, Huter O, Del Frari B, Romani N, Ebner S. CD34+-derived Langerhans cell-like cells are different from epidermal Langerhans cells in their response to thymic stromal lymphopoietin (TSLP) J. Cell. Mol. Med. 2010 doi: 10.1111/j.1582-4934.2010.01206.x. DOI: 10.1111/j.1582-4934. 2010.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoitzner P, Sparber F, Tripp CH. Langerhans cells as targets for immunotherapy against skin cancer. Immunol. Cell Biol. 2010;88:431–437. doi: 10.1038/icb.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano E, Rossi M, Ratzinger G, de Cos MA, Chung DJ, Panageas KS, Wolchock JD, et al. Peptide-loaded Langerhans cells, despite increased IL15 secretion and T-cell activation in vitro, elicit antitumor T-cell responses comparable to peptide-loaded monocyte-derived dendritic cells in vivo. Clin. Cancer Res. 2011;17:1984–1997. doi: 10.1158/1078-0432.CCR-10-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fay JW, Palucka AK, Paczesny S, Dhodapkar M, Johnston DA, Burkeholder S, Ueno H, Banchereau J. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol. Immunother. 2006;55:1209–1218. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol. Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+and CD4+T cell responses with broad antigen specificity. J. Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- 28.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch LS, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jong MA, Geijtenbeek TB. Langerhans cells in innate defense against pathogens. Trends Immunol. 2010;31:452–459. doi: 10.1016/j.it.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Salinger JD. The Catcher in the Rye. Little, Brown and Company; New York: 1951. [Google Scholar]