Abstract
Aging-induced declines in muscle size and quality are thought to contribute to catabolic alterations in bone, but changes in bone with age also profoundly alter its response to muscle-derived stimuli. This review provides an overview of some of the alterations that occur in muscle and bone with aging, and discusses the cellular and molecular mechanisms that may impact these age-associated changes.
Over the next 10 years, the number of people in the world over the age of 65 is projected to increase on a percentage basis at a rate almost four times faster than that for the younger population (7). As the size of the older population increases, so does the occurence of aging-related morbidities such as osteoporosis and sarcopenia. Bone fractures are directly linked to osteoporosis in aging adults, and in the U.S. the annual incidence of bone fractures in women exceeds the annual incidence of stroke, breast cancer, and heart disease combined (62). Hip fractures in particular are associated with significant morbidity and mortality, and of those suffering a hip fracture, roughly 40% will require nursing home care, and 20% will not walk again (24). Aging is not only associated with a loss of bone density and strength but is also associated with a reduction in muscle mass and strength referred to as sarcopenia. A consensus definition of sarcopenia has not been reached; however, most assessments involve declines in muscle functional capacity (e.g., strength) (11) and/or a morphometric measure (e.g., cross-sectional area, muscle mass). The muscle weakness that occurs with sarcopenia increases the risk for falling (38), which further increases the propensity for bone fracture.
Strong associations between muscle and bone size have been reported across the lifespan (16, 57). Since the late 1800s, it has been assumed that a causal relationship exists between muscle and bone and that this allometric scaling relationship is mechanical in nature (80–82). That is, muscle and bone are proportionally matched in their functional capacity and geometric structure. This relationship does, however, appear to change significantly with age. For example, the capacity for muscle to generate force declines with age, and the anabolic reponse of bone to muscle-derived stimuli also appears to be altered with age. In addition, muscle is now recognized to have paracrine and endocrine effects that may also influence bone independent of a mechanical relationship (26, 27). Here, we review some of the basic mechanisms by which muscle and bone are thought to interact throughout life, and how these may change with age, leading to bone loss and bone fractures. In The Mechanical Relationship Between Muscle and Bone, we discuss the mechanical relationship between muscle and bone, with particular emphasis on the role of muscle in affecting bone size and bone geometry. In Impact of Aging on the Muscle-Bone Relationship, we assess whether the muscle-bone relationship remains relatively constant with aging in both animals and humans. Cellular and Molecular Mechanisms Underlying Age-Related Changes in the Muscle-Bone Relationship addresses the cellular and molecular mechanisms linking bone and muscle that are altered with age and which negatively impact cross talk between the two tissues.
The Mechanical Relationship Between Muscle and Bone
The musculoskeletal system provides the mechanical framework for movement that is powered by contractions of skeletal muscles. To overcome the anatomical disadvantage associated with short lever arms, many muscles are required to produce high forces to generate movement. The muscle-generated loads applied to bone can far exceed external loads resulting from the body interacting with its environment (e.g., ground reaction forces) (19, 35, 46, 47, 54). Specifically, the utilization of instrumented proximal femoral prostheses have demonstrated that the forces produced during muscle contraction can account for >70% of the bending moments applied to the lower limb (46). Bone size and mechanical properties are, therefore, thought to allometrically scale to the magnitude of peak muscle forces (65) in response to mechanical loading (e.g., through increased or decreased use). Julius Wolff (“Wolff's Law”) was the first to describe that bone changes its external shape and internal structure in response to mechanical loads imposed on the skeleton (80–82).
The inclusion of muscle as the primary source of mechanical loading was a central principle of the Utah paradigm of skeletal physiology and the mechanostat hypothesis (21, 22). Specifically, healthy postnatal bone was proposed to adapt its structure and strength to the typical peak mechanical loads it experiences, which are applied via muscular contractions during exercise and/or a person's activities of daily living (13, 21, 41, 45). Bone's detection and transmission of mechanical loading stimuli by its resident cell populations is referred to as mechanotransduction, where the stimulus is the result of bone strain (i.e., the change in length per unit of original length) (4, 12, 15, 25, 42, 79). According to the mechanostat hypothesis, strain magnitudes experienced by mechanosensing cells in the tissue are compared against threshold values to determine whether feedback mechanisms and adaptive responses are necessary. Strains above 3,000 microstrain (με) typically trigger bone formation, whereas strains of <500 με trigger bone resorption. The dense network of osteocytes trapped within the mineralized bone matrix is the primary sensor of changes in bone loading, which occurs primarily through changes in fluid movement within the lacunocanalicular system of bone. These changes in fluid movement in turn stimulate many downstream effects, including Wnt/beta catenin signaling that promotes the downregulation of sclerostin, a potent inhibitor of bone formation (5, 84). Muscle weakness or muscle atrophy is associated with bone loss, even in the absence of changes in load bearing. Specifically, muscle paralysis using botulinum toxin (botox) is observed to decrease bone mass even during hindlimb unloading, where the effects of paralysis on load bearing are removed (75). The mechanical communication between muscle and bone allows for anabolic/catabolic modifications to bone in response to loading that apparently attempt to maintain a constant relationship between the functional and structural capacities of the two tissues.
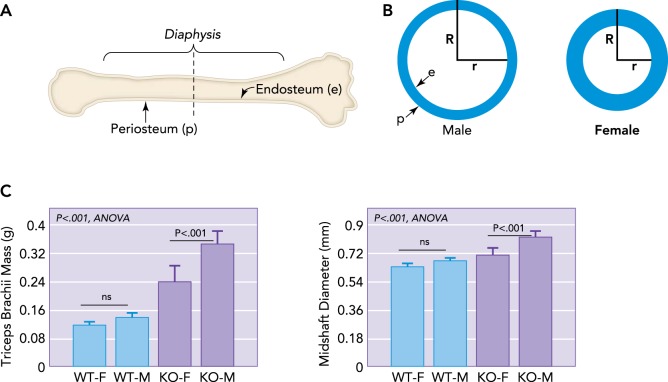
The long bones of the appendicular (limb) skeleton and shorter bones of the axial (spine) skeleton have a dense outer cortex and a spongy network of trabecular bone filling the vertebrae and the articular ends of long bones. Approximately 80% of the total mass of the skeleton is cortical bone, and, with aging, 70% of all bone lost is cortical (69). The nonvertebral (limb) skeleton is primarily cortical bone, and, of all fractures that occur, 80% are nonvertebral (69). Thus a key strategy for preventing bone fractures is to either prevent loss of cortical bone with aging or increase cortical bone formation with aging. The bone cortex has an outer periosteal layer that deposits bone peripherally, which ultimately increases the bone's external diameter, overall cross-sectional area, and resistance to bending loads (FIGURE 1, A AND B). Notably, bone gained from exercise when young can confer improved mechanical properties of bone with aging (76). The bone cortex also has an inner endosteal lining of bone-forming cells that deposits bone, increasing cortical thickness and reducing the cross-sectional area of the medullary cavity (FIGURE 1B). Men typically deposit a greater portion of bone periosteally and thus have relatively wider bones, whereas women deposit more bone endocortically and therefore have bones that are more slender but with a thicker cortex (FIGURE 1B). Bone resorption with aging normally occurs along the endocortical surface so that older, postmenopausal women have bones with a thinning cortex and small outer diameter, predisposing them to risk of fracture (63). Periosteal apposition continues into old age; however, the rate of apposition would need to increase close to 350% to offset the decline in bone strength due to gradual loss of bone on the endosteal surface (39).
FIGURE 1.
Schematic representation of a long bone and dimorphism in bone cross-sectional geometry
A: schematic representation of a long bone indicating the outer periosteal surface (p) and inner endocortical surface lining the medullary cavity (e), with a dotted line showing cross section. B: schematic representations of long bone cross-sectional geometry in males vs. females at the level of the dotted line shown in A. Males typically have a wider bone cross section and outer radius (R), whereas females have a smaller outer radius but thicker bone cortex, leading to a shorter inner radius (r). As women age, bone is resorbed from the endocortical surface so that older women must increase R to compensate for an increasing r. Males have a larger R and so are at lower fracture risk even as r increases. C: increased muscle mass in males is associated with increased bone diameter, even in mice. Male mice (M) lacking myostatin show a greater increase in forelimb triceps brachii mass than female (F) knockout (KO) mice, and forelimb bone (radius) diameter is largest in M mice lacking myostatin (KO) compared with wild-type (WT) mice.
Muscle plays a critical role in the regulation of bone size. An excellent example of this association comes from the human upper limb, which is non-weight bearing and in the case of the non-dominant arm does not normally experience high mechanical loads. In older adults (∼70 yr of age), the periosteal circumference of the radius from the non-dominant upper limb is strongly associated with forearm muscle cross-sectional area, such that greater muscle size is consistently associated with greater periosteal circumference (16). Notably, the association between bone size and muscle cross-sectional area is stronger than the association between bone size and body weight. A similar association is observed in adolescents (57), where cross-sectional area and strength of the non-dominant radius is more strongly associated with forearm muscle cross-sectional area than with fat mass. An interesting comparative example in this regard can be observed in hypermuscular mice lacking myostatin, where larger forelimb muscles in male and female knockout mice are associated with increased midshaft diameter of the radius (FIGURE 1C). In these mice, midshaft diameter of the radius is more highly correlated with triceps brachii mass (r2 = 0.64) than with body weight (r2 = 0.31).
Impact of Aging on the Muscle-Bone Relationship
If the relationship between muscle and bone is indeed mechanical, then the ratio of muscle to bone properties (i.e., functional capacity or size) should remain relatively constant across adulthood. That is, anabolic or catabolic changes within muscle and bone would be expected to occur in parallel. Alternative patterns of change to the muscle-to-bone ratio (i.e., an increase or decrease) with aging could suggest that the functional capacity and/or size of muscle and bone are capable of being mismatched. There have been a few trials in mice to test the mutability of Wolff's Law and the mechanostat model by attempting to disrupt the scaling of mechanical properties in bone to those in muscle. Specifically, our group has investigated how the ratio of muscle function (i.e., peak isometric force assessed in vitro) to bone strength (i.e., ultimate load assessed by three-point bending) differs between young and aged mice with and without a genetic condition mimicking Duchenne muscular dystrophy (i.e., mdx mice) (FIGURE 2; Ref. 52). The muscle-to-bone ratio was not different between young and aged wild-type mice (FIGURE 2A). The ratio was, however, greater in aged compared with young mdx mice, with this being attributed to the active disease state in the young mice and muscle strength subsequently being impaired (52). In the aged mdx mice, muscle strength had normalized, and the muscle-to-bone ratio was increased such that it was not different from that of wild-type mice (52).
FIGURE 2.
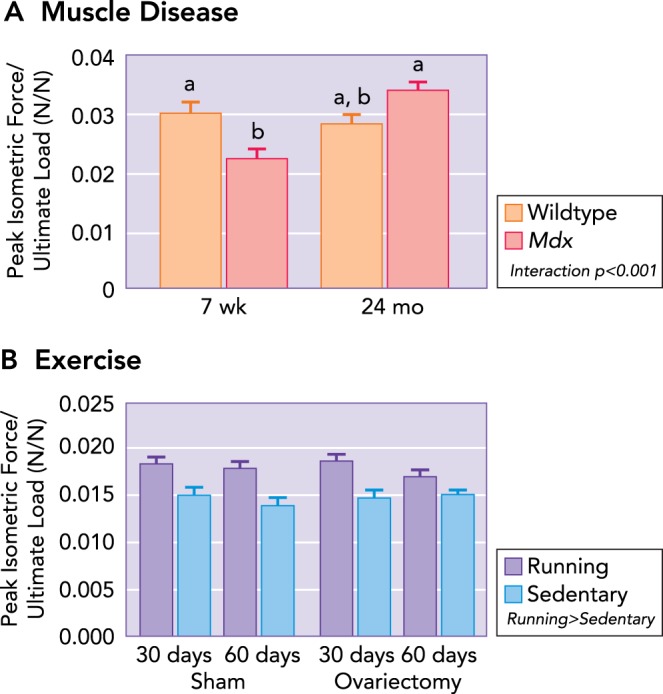
Ratio of muscle maximal isometric tetanic force to ultimate load as a function of muscle disease, physical activity, and estrogen
A: at the peak of muscle-disease onset (i.e., 7 wk of age), a mismatch is present between muscle and bone, such that the bone is stronger (i.e., determined by ultimate load during three-point bending) than the adjacent musculature (i.e., strength determined by in vitro assessment of isolated muscle contractility). With aging, the muscle-to-bone ratio remains constant in healthy wild-type mice. Mdx mice become undistinguishable from 24-mo-old wild-type mice, which is attributed to an improvement in muscle function with age. Data are means (SD) and are unitless. Statistically significant effects are indicated within the figure legend. Significant interactions between genotype and age detected by Holm-Sidak post hoc tests (P < 0.05) are indicated by the lowercase letters above the bars; values with the same letter are not significantly different. B: however, the loss of estrogen does not influence the muscle-to-bone ratio; running increases the ratio between muscle and bone, suggesting a transient mismatch between muscle and bone. Values are means (SD) and are unitless. Figure was adapted with permission from Refs. 52, 78.
We and others have also assessed the effects of physical activity/training (78) and estrogen deficiency (77, 78) on the muscle-bone relationship. In the study by Warren et. al. (78), this was done by assigning female mice to voluntary wheel running or sedentary groups, either with or without ovariectomy surgery. Mice that took part in wheel running as a form of exercise, had higher muscle-to-bone ratios compared with sedentary mice (FIGURE 2B) (78). This suggests that physical activity has the potential to disrupt the muscle-to-bone ratio, which would not be predicted by Wolff's Law. The removal of estrogen by ovariectomy for 30–60 days, however, did not disrupt the muscle-bone relationship (77, 78). Overall, these animal studies suggest that physical activity and muscle disease can create transient mismatches in the muscle-to-bone ratio, and perhaps that the influence of estrogen in mediating this response may be minimal.
To better assess the impact of aging on the muscle-bone relationship in humans, we conducted a systematic revew (Table 1). The bone outcome measure desired for a study's inclusion was bone mechanical strength, but because this is never reported, surrogates for bone strength were permitted. These surrogates included: estimates of bone strength (i.e., strength strain index), bone area (i.e., cortical bone area or cross-sectional area as determined by MRI or computed tomography), and bone mass [i.e., bone mineral content (BMC) or bone mineral density (BMD)] determined by DXA. The preferred muscle outcome measure was contractile strength, but measures of muscle anatomical dimensions (i.e., cross-sectional area or width) or mass were also permitted as a substitute. Studies were excluded if 1) the studied muscles or muscle groups were not in close proximity to bone that was studied or 2) insufficient data were available to calculate the ratio of muscle to bone outcome measures for each age group (i.e., only the correlation between the bone and muscle measures was reported, subjects were grouped based on age plus factors other than age). Fifteen studies met the inclusion and exclusion criteria. Mean muscle-to-bone ratios were calculated for each age group by dividing the reported mean muscle outcome measure by the corresponding mean bone outcome measure; standard deviations for these ratios were calculated using propagation of errors from the muscle and bone outcome standard deviations. Analysis of the ratio data was possible for all studies with the exception of Meema et al. (i.e., sample sizes lacking) (50), and thus for this study, the aging pattern for the muscle-to-bone ratios is only described qualitatively.
Table 1.
Effect of aging on the muscle-to-bone ratio
| Study (Ref.) | Subjects/Age Range (yr)/Sample Size | Muscle Measure | Bone Measure | Effect of Aging on the Muscle-to-Bone Ratio |
|---|---|---|---|---|
| Heikkinen et. al., 1984 (30) | Men/31-75/142 | KE strength (isometric) | Calcaneous BMD | Decreases |
| Hyakutake et al., 1994 (34) | Men/20-89/109 | KE strength (90 deg/s) | Femur BMD | Decreases |
| KF strength (90 deg/s) | Femur BMD | Decreases | ||
| Women/20-79/231 | KE strength (90 deg/s) | Femur BMD | Decreases | |
| KF strength (90 deg/s) | Femur BMD | Decreases | ||
| Calmels et. al., 1995 (9) | Women/44-87/101 | KE strength (30 deg/s) | Femur BMD | Decreases |
| KE strength (180 deg/s) | Femur BMD | No change | ||
| KF strength (30 deg/s) | Femur BMD | Decrease | ||
| KF strength (180 deg/s) | Femur BMD | No change | ||
| Humphries et. al., 1999 (33) | Peri- and post-menopausal women/45-65/96 | KE strength (isometric) | Lumbar spine BMD | No change |
| Ryan et. al., 2004 (66) | Men/25-70/25 | KE strength | Femur BMD | Increase |
| Leg press strength | Femur BMD | No change | ||
| Women/26-68/19 | KE strength | Femur BMD | Trend for decrease | |
| Leg press strength | Femur BMD | No change | ||
| Sherk et. al., 2009 (70) | Men/18-64/68 | DF/PF CSA (pQCT) | Tibia SSI | No change |
| DF/PF CSA (pQCT) | Tibia cortical BMC | No change | ||
| DF/PF CSA (pQCT) | Tibia cortical area | No change | ||
| DF/PF CSA (pQCT) | Tibia cortical density | No change | ||
| Rice et. al. 1989, (61) | Men/25-90/20 | EF CSA (CT) | Humerus CSA | Decreases |
| EE CSA (CT) | Humerus CSA | Decreases | ||
| PF CSA (CT) | Tibia CSA | Decreases | ||
| Overend et. al., 1992 (53) | Men/19-77/24 | KE CSA (CT) | Femur CSA | Decreases |
| KF CSA (CT) | Femur CSA | No change | ||
| Klein et. al., 2002 (43) | Men/20-90/33-44 | EF/EE CSA (MRI) | Humerus cortical area | Decreases |
| FF/FE CSA (MRI) | Radius Cortical Area | No Change | ||
| FF/FE CSA (MRI) | Ulna Cortical Area | Trend for Decrease | ||
| McNeil et. al., 2009 (48) | Men/23-91/39 | Total Leg Muscle CSA (CT) | Tibia CSA | Decreases |
| Horber et.al., 1997 (32) | Men/20-81/60 | Arm lean muscle mass | Arm BMC | No Change |
| Leg lean muscle mass (DXA) | Leg BMC | No Change | ||
| Pre and post-menopausal women/20-79/59 | Arm lean muscle mass (DXA) | Arm BMC | Trend for Increase | |
| Leg lean muscle mass (DXA) | Leg BMC | No change | ||
| Melton et. al., 2006 (51) | Men/20-93/307 | Arm/leg lean muscle mass (DXA) | Femur EI strength | Decreases |
| Women/21-97/345 | Arm/leg lean muscle mass (DXA) | Femur EI strength | No change | |
| Atlantis et. al., 2008 (3) | Men/35-81/1,068 | Arms muscle mass (DXA) | Arms BMC | Decreases |
| Legs muscle mass (DXA) | Leg BMC | No change | ||
| Sanada et. al., 2009 (67) | Women/20-76/138 | Arm lean muscle mass | Arm BMC | Increases |
| Leg lean muscle mass | Leg BMC | Increases | ||
| Meema et. al., 1973 (50) | Men/18-97/305 | Muscle width of adjacent musculature | Radius bone mass | Increases* |
| Women/18-90/308 | Muscle width of adjacent musculature | Radius bone mass | Increases* |
The review involved three steps. In step 1, using the search terms “muscle, bone, and aging” with delimitations to human studies and the English language, 1,097 articles were identified. Article titles and abstracts were reviewed in step 2 to confirm that measures of bone and muscle functional capacity and/or structure were outcome measures in the studies, and that an assessment of aging was apparent; 119 studies met these criteria. In step 3, the following inclusion criteria for aging, bone, and muscle were applied during a full-text examination of the studies. A study's assessment of aging was deemed acceptable if a comparison was made across two or more age groups with an average age difference of at least 20 yr.
KE, knee extensor muscles; BMD, bone mineral density; KF, knee flexor muscles; DF, dorsi flexor muscles; PF, plantar flexor muscles; CSA, cross-sectional area; SSI, strength strain index; EF, elbow flexor muscles; EE, elbow extensor muscles; FF, forearm flexors; FE, forearm extensors; BMC, bone mineral content; EI strength, flexural rigidity.
Statistical analyses were not performed on muscle-to-bone ratio due to missing sample sizes.
Table 1 summarizes the 39 muscle-to-bone ratios from the 15 studies included in the systematic review. The ratio remained unchanged with aging in 19 of the 39 ratios, whereas 15 ratios decreased with age and 5 ratios increased (Table 1). To illustrate the variability among studies in the age-related ratio changes, three studies are discussed here in greater detail. These studies are those with the largest sample sizes, age ranges, and number of age groups compared (3, 50, 51). Atlantis et al. (3) was the largest study included in the analysis, and quantified muscle masses and BMC by DXA in over 1,000 men between the ages of 35 and 81 yr. The ratio of muscle mass to BMC decreased with aging in the arms but remained unchanged in the legs (Table 1). Melton et al. (51) provided raw data for 307 men and 345 women between the ages of 22 and 97 yr, which included lean mass of the arms and legs and a measure of femur bending strength index (i.e., flexural rigidity or EI) for each of the 652 participants (51). The muscle-to-bone ratio significantly decreased in men with aging but not in women (P = 0.14; Table 1). Meema et al. (50) reported bone mass of the radius and the width of the adjacent forearm musculature of the radius for 613 men and women across 11 different groups between the ages of 18–97 yr (FIGURE 3). Because the authors failed to report sample size for each of the 11 groups, this prevented running statistical analyses. However, it appears that the ratio increases with age in both men and women. Overall, these three studies highlight a high variability in how aging affects the muscle-to-bone ratio.
FIGURE 3.
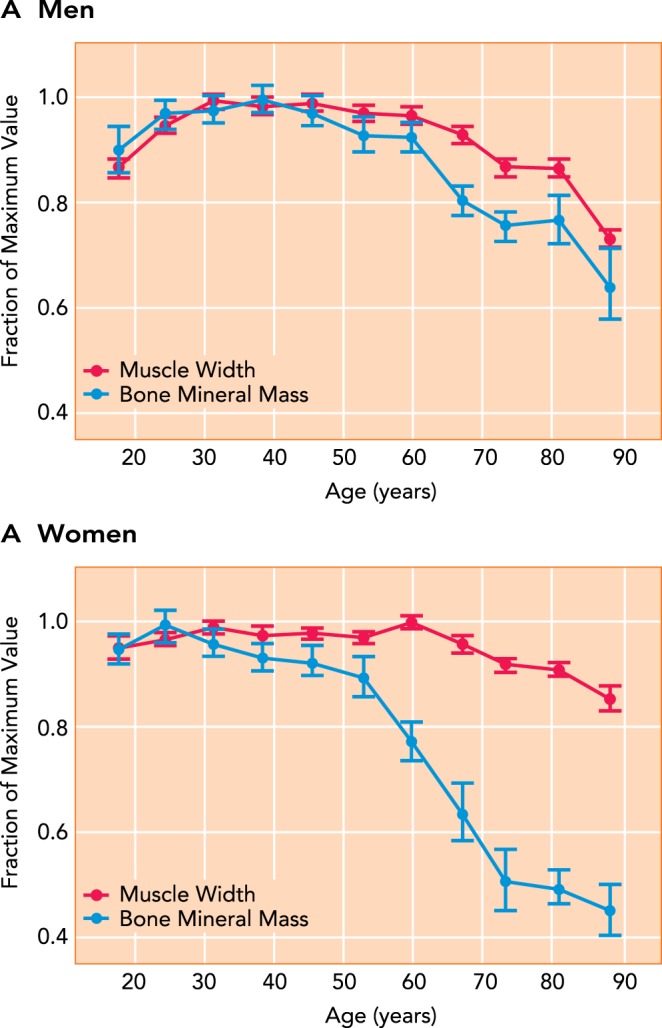
Aging-related changes in bone mineral mass of the radius and muscle width in the forearm in adult men and women
The figure was adapted with permission from Ref. 50, and data were normalized to the peak value for bone and muscle across the lifespan to show relative changes across the lifespan for men (A) and women (B).
To determine whether a study's research design (i.e., the measures used to assess bone and muscle, number of age comparison groups, and age range among groups) affected how the muscle-to-bone ratio changes with aging, a logistic regression and a proportional odds (cumulative logit) model was utilized. The odds of an increase in the muscle-to-bone ratio with aging was dependent on the measures used to assess bone and muscle. For example, an increase in the ratio was >10-fold more likely to occur in studies in which bone was assessed via strength or BMC compared with when it was assessed via cross-sectional area (CSA). Similarly, an increase in the ratio was >50-fold more likely to occur in studies assessing bone via BMD compared with assessing bone via CSA. In contrast, studies that involved “strength” for the muscle strength measurement were more more likely to result in a decrease in the muscle-to-bone strength ratio with aging than the studies that used “area” for the muscle strength measurement. The number of age-comparison groups used in a study was related to the probability of observing an increase in the ratio. For each additional age group, the odds of observing an increase in the muscle-to-bone ratio with aging was reduced by half. These results indicate that study research design features do influence how the muscle-to-bone ratio changes with aging. However, for all of the research design features we considered, we found that they could account for no more than 30% of the variance in whether a muscle-to-bone ratio increased, decreased, or did not change with age. The large unaccounted for proportion of variance highlights the potential for existence of one or more non-mechanical muscle-bone relationships.
Cellular and Molecular Mechanisms Underlying Age-Related Changes in the Muscle-Bone Relationship
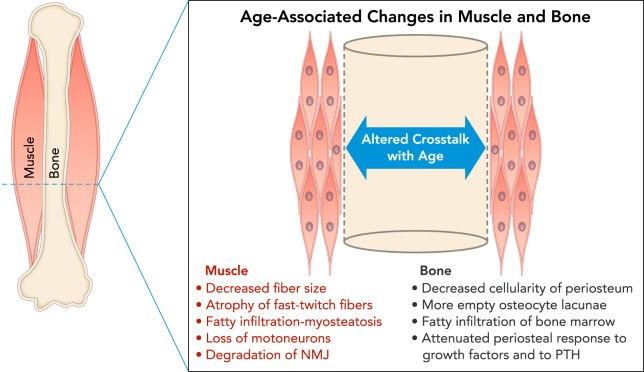
The data reviewed above indicate that mismatches can occur between muscle and bone emanating from age-associated structural and/or functional changes in one tissue vs. another. Such dissociations are also observed with exercise and aging, where it is well known that exercise can significantly enhance bone mass and strength in young animals but not in older, adult animals, even when controlling for the duration and intensity of exercise (18, 55). There are likely a number of mechanisms that underlie this dissociation (FIGURE 4). First, there are changes in bone with aging that likely attenuate its response to muscle-derived stimuli. Osteocytes trapped within the mineralized matrix of bone are the key mechanostransducers in bone tissue, and osteocyte number and density decline with age, resulting in an increased number of empty lacunae in bone (8). These age-associated changes in the lacunocanalicular system of bone ultimately impair the bone signaling network (8, 74) and may deleteriously impact the diffusion of important growth factors and signaling molecules to target cells (FIGURE 4). On the other hand, a greater density of empty osteocyte lacunae has also been observed closest to the periosteal surface (29), and so periosteal expansion with increasing age could be related to an absence of osteocytes secreting the anti-osteogenic factor sclerostin. There are also changes in the periosteum that occur with aging that may limit the potential of muscle to increase bone size. Periosteum has been observed to become thinner and less cellular with age in rodents and other mammals (17), aged rodent periosteal cells show an impaired regenerative capacity and impaired response to parathyroid hormone (83), periosteal osteoblasts demonstrate an impaired capacity to proliferate in response to mechanical loading (49), and human periosteal cells demonstrate a lack of spontaneous cartilage formation in vitro after age 30 (14). Aged osteoprogenitor cells also show an attenuated response to growth factors (FIGURE 4). For example, the dose of IGF-1 required to elicit a mitogenic response in aged osteoprogenitor cells is much greater than that required for younger cells (71).
FIGURE 4.
Schematic cross-section at the midshaft of a long bone diaphysis showing a summary of age-associated changes in muscle and bone
Muscle changes (red) include decreased fiber size, atrophy of fast-twitch fibers, fatty infiltration of muscle tissue, loss of motoneurons, and degradation of the neuromuscular junction (NMJ), all of which can negatively impact force production and potentially the secretion of myokines. Changes in bone (black) include decreased cellularity in the periosteum, loss of osteocytes in bone matrix due to senescence, fatty infiltration of bone marrow, and attenuated periosteal response to growth factors and parathyroid hormone (PTH). All of these changes can impair the capacity of bone to respond to anabolic stimuli.
There are well documented changes in muscle that occur with aging, including an overall decrease in myofiber size, a decline in the number of excitable motor units within muscle, and the gradual infiltration of muscle with fatty tissue (73). There are also pronounced degradative changes in the neuromuscular junction with aging, which is known to contribute to synaptic loss (10) and may be related to oxidative stress and mitochondrial dysfunction (37). All of these changes will alter the contractile behavior of aged muscle, which as noted above could be an important mechanistic link between muscle and bone formation (FIGURE 4). One of the most exciting new developments in the area of muscle-bone interactions is the recognition that skeletal muscle secretes a variety of peptides collectively termed myokines (56), and a number of these myokines are well recognized as having positive effects on bone formation (26–28), as well as inhibitory effects on osteoblast differentiation (40). Importantly, muscle contraction stimulates the release of myokines both in vivo and in vitro (60, 68). IGF-1 expression in skeletal muscle tissue is elevated with muscle contraction (1), and circulating levels of IGF-1 are also increased with resistance exercise (63). Recent experiments also have shown that conditioned medium from whole muscle explants stimulated in vitro can prevent the death of osteocytes when exposed to glucocorticoids (36), suggesting again that muscle contraction leads to the release of myokines that are beneficial for bone. Muscle contraction may also suppress the expression of factors that can inhibit the bone formation. For example, myostatin (GDF-8) expression is downregulated following concentric and eccentric muscle contractions (31), and myostatin suppresses the proliferation of bone marrow-derived stem cells in vitro (6).
Aging has been shown to reduce the anabolic effects of resistance exercise on muscle protein synthesis (23) and thus could potentially affect myokine synthesis and secretion. The effect of myokines on bone may be systemic as well as local, since elevated secretion of muscle-derived IL-15 not only decreases body fat but also increases whole-body bone mineral content (59). Another potential alteration in muscle with aging is that the myokine secretory profile may be altered. Aged human myoblasts, for example, show increased levels of TGF-β1 secretion in vitro compared with younger myoblasts (2), although it is not known how aging alters the secretion of other myokines with muscle contraction. In addition, aging is generally associated with a preferential denervation of fast-twitch fibers along with reinnervation of some of those fibers by α-motoneurons from slow motor units (58). It is not known how this could effect myokine secretion, but single fiber maximal isometric force production, either absolute or normalized to cross-sectional area, is not consistently different between slow- and fast-twitch fibers in older humans (e.g., Refs. 20, 72). It is well recognized that the bone marrow cavity accumulates fat with age (64), and it is certainly possible that increased bone marrow adipogenesis may attenuate the effects circulating myokines on endocortical (endosteal) osteoprogenitor cells (FIGURE 4). It should be noted that many of the associations between myokines and bone formation are more correlative and hypothetical rather than mechanistic. For example, although TGF-β1 and IL-6 are both well established myokines, their size may prevent their diffusion through the periosteum (44). Studies need to be performed to determine how muscle-specific alteration of myokine expression and/or targeted changes in myokine receptors in osteoprogenitor cells impact bone modeling and remodeling.
Summary and Conclusions
Aging is associated with the development of sarcopenia in skeletal muscle and osteoporosis in bone; however, it is not fully understood how the relationship between the two tissues is impacted by aging. Our experiments in mice and our review of human studies demonstrate that the relationship between muscle and bone size and strength changes significantly with age, so that a potential mismatch occurs between these tissues. There are pronounced cellular and molecular changes in bone and muscle cells with age that likely underlie these observations. Such changes include the loss of osteocytes in bone matrix and a decline in the proliferative capacity of osteoprogenitors in the periosteum, which attenuate the response of bone to muscle contraction and normal mechanical stimuli. Loss of motoneurons, reduced fiber size due to decreased muscle protein synthesis and increased degradation from atrogens, and perhaps fatty infiltration in muscle with age all negatively impact the contractile machinery and force-generating capacity of aged muscle. These data indicate that a therapeutic approach for improving bone health will require targeting both muscle and bone; that is, the changes in bone discussed above suggest that improvements in muscle function with age are unlikely to elicit an adequate anabolic stimulus in bone. Rather, strategies to enhance the anabolic capacity of periosteum in conjunction with therapeutics to increase the force- and power-generating capacity of aged muscle could significantly improve musculoskeletal health and function in the elderly.
Footnotes
We are grateful to Dr. Meghan McGee-Lawrence and two anonymous reviewers for comments that helped improve the manuscript.
Funding for this research was provided by the Congressionally Directed Medical Research Programs, Department of the Army (CDMRP093619), and the National Institute on Aging (P01 AG-036675).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.A.N., G.L.W., and M.W.H. conception and design of research; S.A.N., G.L.W., and M.W.H. analyzed data; S.A.N., G.L.W., and M.W.H. interpreted results of experiments; S.A.N., G.L.W., and M.W.H. prepared figures; S.A.N., G.L.W., and M.W.H. drafted manuscript; S.A.N., G.L.W., and M.W.H. edited and revised manuscript; S.A.N., G.L.W., and M.W.H. approved final version of manuscript.
References
- 1.Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J Appl Physiol 103: 1644–1654, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SD. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell 12: 333–344, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Florey Adelaide Male Aging Study. Lifestyle factors associated with age-related differences in body composition: the Florey Adelaide Male Aging Study. Am J Clin Nutr 88: 95–104, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bonewald LF. Mechanosensation and transduction in osteocytes. Bone Key Osteovision 3: 7–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone 42: 606–615, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowser M, Herberg S, Arounleut P, Shi X, Fulzele S, Hill WD, Isales CM, Hamrick MW. Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells. Exp Gerontol 48: 290–297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bureau USC. International programs (Online). Washington, DC: U.S. Census Bureau; (http://www.census.gov/population/international) [Google Scholar]
- 8.Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, Djuric M, Amling M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9: 1065–1075, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Calmels P, Vico L, Alexandre C, Minaire P. Cross-sectional study of muscle strength and bone mineral density in a population of 106 women between the ages of 44 and 87 years: relationship with age and menopause. Eur J Appl Physiol Occup Physiol 70: 180–186, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, Morsch M, Murata Y, Ghazanfari N, Reddel SW, Phillips WD. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLos One 8: e67970, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA. Tools in the assessment of sarcopenia. Calcified Tissue Intl 93: 201–210, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowin S. Mechanosensation and fluid transport in living bone. J Musculoskelet Neuronal Interact 2: 256–260, 2002. [PubMed] [Google Scholar]
- 13.Currey JD. How well are bones designed to resist fracture? J Bone Miner Res 18: 591–598, 2003. [DOI] [PubMed] [Google Scholar]
- 14.De Bari C, Dell'Accio F, Luyten FP. Human periosteum derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum 44: 85–95, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Doty SB. Morphological evidence of gap junctions between bone cells. Calcified Tissue Intl 33: 509–512, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Edwards MH, Gregson CL, Patel HP, Jameson KA, Harvey NC, Sayer AA, Dennison EM, Cooper C. Muscle size, strength, and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res 28: 2295–2304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan W, Crawford R, Xiao Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone 42: 81–89, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Forwood MR, Burr DB. Physical activity and bone mass: exercises in futility? Bone Miner 21: 89–112, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Frankel VH, Burstein AH. Orthopaedic Biomechanics. Philadelphia, PA: Lea and Febiger, 1970. [Google Scholar]
- 20.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A 275: 1081–1101, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Frost HM. Why do bone strength and “mass” in aging adults become unresponsive to vigorous exercise? Insights of the Utah paradigm. J Bone Mineral Metab 17: 90–97, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gawande A. The way we age now. In: The New Yorker. April30, 2007. [Google Scholar]
- 25.Haj AJE, Minter SL, Rawlinson SC, Suswillo R, Lanyon LE. Cellular responses to mechanical loading in vitro. J Bone Miner Res 5: 923–932, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev 39: 43–47, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bone Key Rep 1: 60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact 10: 64–70, 2010. [PMC free article] [PubMed] [Google Scholar]
- 29.Hedgecock NL, Hadi T, Chen AA, Curtiss SB, Martin RB, Hazelwood SJ. Quantitative regional associations between remodeling, modeling, and osteocyte apoptosis and density in rabbit tibial midshafts. Bone 40: 627–637, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Heikkinen E, Arajarvi RL, Era P, Jylha M, Kinnunen V, Leskinen AL, Leskinen E, Masseli E, Pohjolainen P, Rahkila P, et al. Functional capacity of men born in 1906–10, 1926–30 and 1946–50. A basic report Scand J Soc Med Suppl 33: 1–97, 1984. [PubMed] [Google Scholar]
- 31.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102: 573–581, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition 13: 524–534, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Humphries B, Triplett-McBride T, Newton RU, Marshall S, Bronks R, McBride J, Hakkinen K, Kraemer WJ. The relationship between dynamic, isokinetic and isometric strength and bone mineral density in a population of 45 to 65 year old women. J Sci Med Sport 2: 364–374, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Hyakutake S, Goto S, Yamagata M, Moriya H. Relationship between bone mineral density of the proximal femur and lumbar spine and quadriceps and hamstrings torque in healthy Japanese subjects. Calcif Tissue Intl 55: 223–229, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Inman VT. Functional aspects of the abductor muscles of the hip. J Bone Joint Surg Am 29: 607–619, 1947. [PubMed] [Google Scholar]
- 36.Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, Bonewald LF. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cells Materials 24: 197–210, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang YC, Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp Gerontol 46: 193–198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 336: 124–126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jepsen KJ, Andarawis-Puri N. The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J Bone Miner Res 27: 1916–1926, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson RW, White JD, Walker EC, Martin TJ, Sims NA. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone 64: 47–56, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Kannus P, Sievanen H, Vuori I. Physical loading, exercise, bone. Bone 18: 1S–3S, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone 54: 182–190, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Klein CS, Allman BL, Marsh GD, Rice CL. Muscle size, strength, and bone geometry in the upper limbs of young and old men. J Gerontol A Biol Sci Med Sci 57: M455–M459, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Lai X, Price C, Lu XL, Wang L. Imaging and quantifying solute transport across periosteum: implications for muscle-bone crosstalk. Bone 66: 82–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J Bone Miner Res 16: 1937–1947, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Lu TW, Taylor SJ, O'Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: An in vivo study. J Biomech 30: 1101–1106, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Martin RB, Burr DB. Structure, Function and Adaptation of Compact Bone. New York: Raven, 1989. [Google Scholar]
- 48.McNeil CJ, Raymer GH, Doherty TJ, Marsh GD, Rice CL. Geometry of a weight-bearing and non-weight-bearing bone in the legs of young, old, and very old men. Calcif Tissue Intl 85: 22–30, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Meakin LB, Galea GL, Sugiyama T, Lanyon LE, Price JS. Age-related impairment of bones' adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res 29: 1859–1871, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meema S, Reid DB, Meema HE. Age trends of bone mineral mass, muscle width, and subcutaneous fat in normals and osteoporotics. Calcif Tissue Res 12: 101–112, 1973. [DOI] [PubMed] [Google Scholar]
- 51.Melton LJ 3rd, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S. Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res 21: 1847–1855, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Novotny SA, Warren GL, Lin AS, Guldberg RE, Baltgalvis KA, Lowe DA. Bone is functionally impaired in dystrophic mice but less so than skeletal muscle. Neuromusc Disorders 21: 183–193, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS. Thigh composition in young and elderly men determined by computed tomography. Clin Physiol 12: 629–640, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Pauwels F. Atlas zur Biomechanik der Gesunden und Kranken Hufte. Berlin: Springer-Verlag, 1973. [Google Scholar]
- 55.Pearson OM, Lieberman DE. The aging of Wolff's “law”: ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol Suppl 39: 63–99, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen BK. Muscles and their myokines. J Exp Biol 214: 337–346, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr 86: 1530–1538, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev 42: 45–52, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 296: E191–E202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLos One 8: e62008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rice CL, Cunningham DA, Paterson DH, Lefcoe MS. Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol 9: 207–220, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Riggs BL, Melton LJ 3rd. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17: 505S–511S, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Rojas Vega S, Knicker A, Hollmann W, Bloch W, Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm Metab Res 42: 982–986, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nature Clin Practice Rheumatol 2: 35–43, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol 107: 321–327, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Ryan AS, Ivey FM, Hurlbut DE, Martel GF, Lemmer JT, Sorkin JD, Metter EJ, Fleg JL, Hurley BF. Regional bone mineral density after resistive training in young and older men and women. Scand J Med Sci Sports 14: 16–23, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Sanada K, Miyachi M, Tabata I, Miyatani M, Tanimoto M, Oh TW, Yamamoto K, Usui C, Takahashi E, Kawano H, Gando Y, Higuchi M. Muscle mass and bone mineral indices: does the normalized bone mineral content differ with age? Eur J Clin Nutr 63: 465–472, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, de Angelis MH, Haring HU, Weigert C. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol 305: C877–C886, 2013. [DOI] [PubMed] [Google Scholar]
- 69.Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci 68: 1218–1225, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Sherk VD, Karabulut M, Bemben MG, Bemben DA. Age comparisons of bone density and geometry in men. J Musculoskelet Neuronal Interact 9: 256–262, 2009. [PubMed] [Google Scholar]
- 71.Tanaka H, Liang CT. Mitogenic activity but not phenotype expression of rat osteoprogenitor cells in response to IGF-I is impaired in aged rats. Mech Ageing Dev 92: 1–10, 1996. [DOI] [PubMed] [Google Scholar]
- 72.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 25: 17–25, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone 26: 375–380, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Warden SJ, Galley MR, Richard JS, George LA, Dirks RC, Guildenbecher EA, Judd AM, Robling AG, Fuchs RK. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone 54: 98–105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci USA 111: 5337–5342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warren GL, Lowe DA, Inman CL, Orr OM, Hogan HA, Bloomfield SA, Armstrong RB. Estradiol effect on anterior crural muscles-tibial bone relationship and susceptibility to injury. J Appl Physiol 80: 1660–1665, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA. Voluntary run training but not estradiol deficiency alters the tibial bone-soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol 293: R2015–R2026, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomechanics 27: 339–360, 1994. [DOI] [PubMed] [Google Scholar]
- 80.Wolff J. The classic: On the inner architecture of bones and its importance for bone growth. 1870. Clin Orthopaedics Related Res 468: 1056–1065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolff J. The classic: On the significance of the architecture of the spongy substance for the question of bone growth: a preliminary publication. 1869. Clin Orthopaedics Related Res 469: 3077–3078, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolff J. Concerning the interrelationship between form and function of the individual parts of the organism. By Julius Wolff, 1900. Clin Orthopaedics Related Res 2–11, 1988. [PubMed] [Google Scholar]
- 83.Yukata K, Xie C, Li TF, Takahata M, Hoak D, Kondabolu S, Zhang X, Awad HA, Schwarz EM, Beck CA, Jonason JH, O'Keefe RJ. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1–34 treatment. Bone 62: 79–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone 54: 35–43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]