Abstract
Previous studies have identified over 3,000 genes that are differentially expressed in male and female skeletal muscle. Here, we review the sex-based differences in skeletal muscle fiber composition, myosin heavy chain expression, contractile function, and the regulation of these physiological differences by thyroid hormone, estrogen, and testosterone. The findings presented lay the basis for the continued work needed to fully understand the skeletal muscle differences between males and females.
Cardiac, smooth, and skeletal are the three muscle types in mammals, with skeletal muscle being the most abundant tissue in the human body. Skeletal muscles are composed of different types of fibers which diverge morphologically, biochemically, and functionally. Early studies describing muscle fiber-type composition and development did not address the potential for differences between species and sex. This is often the case in experimental models where sex is thought to confound results and therefore usually only males are studied. Although there is a significant appreciation of sex-based differences in cardiovascular health and disease, much less is known about the effects of sex on the physiology and pathophysiology of skeletal muscle. More than 3,000 genes have been identified as being differentially expressed between male and female skeletal muscle (96). Thus many of the key differences that exist between the sexes that will be presented here are likely to be the result, at least in part, of these differences in gene expression. In this review, we discuss differences in skeletal muscle fiber-type composition and contractility between sexes and how these differences may be hormonally regulated.
The Mammalian Myosin Heavy Chain Gene Family and Muscle Fiber Types
Because the myosin motor protein is so closely linked to muscle function, it is important to consider the effects of sex on myosin gene expression. There are 11 myosin heavy chain (MyHC) genes that encode different myosin isoforms in mammals (77). Table 1 lists these genes and the muscle fibers in which they are expressed. Four of these genes constitute the majority of MyHCs expressed in adult mammalian skeletal muscle: MYH1 (MyHC-IIx), MYH2 (MyHC-IIa), MYH4 (MyHC-IIb), and MYH7 (MyHC-β). Although there are varying degrees of MyHC isoform co-expression in single skeletal muscle fibers, individual fibers often contain a predominant MyHC isoform. Thus individual fibers may be “typed” or classified by MyHC isoform expression, with four main classifications in mammalian skeletal muscle: type-I (MyHC-I/β), type-IIA (MyHC-IIa), type-IIX (MyHC-IIx), and type-IIB (MyHC-IIb).
Table 1.
Mammalian myosin genes, the proteins they encode, and where they are expressed
| Gene | Protein | Expression | Fiber Type |
|---|---|---|---|
| MYH1 | MyHC-IIx | Fast IIX fibers | Type-IIX |
| MYH2 | MyHC-IIa | Fast IIA fibers | Type-IIA |
| MYH3 | MyHC-embryonic | Developing muscle | |
| MYH4 | MyHC-IIb | Fast IIB fibers | Type-IIB |
| MYH6 | MyHC-α | Masseter, extraocular muscle and heart | |
| MYH7 | MyHC-β/slow | Slow muscle (Type-I/β) and heart | Type-I/β |
| MYH7B | MyHC-slow/tonic | Extraocular muscle | |
| MYH8 | MyHC-neonatal | Developing muscle | |
| MYH13 | MyHC-EO | Extra-ocular muscle, pharyngeal muscle | |
| MYH15 | MyHC-15 | Extra-ocular muscle | |
| MYH16 | MyHC-M | Jaw muscle |
Genes, protein, expression locations, and fiber types in bold represent those prevalent in mammalian skeletal muscle.
Functionally, MyHC isoform expression directly affects muscle fiber-type contractile velocity via myosin ATPase activity (74), with relative velocities of I < IIA < IIX < IIB (73, 74). Importantly, MyHC isoform expression closely correlates with fiber-type morphology and enzymatic make-up. In general terms, muscle fibers expressing MyHC-IIb tend to be larger fibers, rich in glycolytic enzymes, whereas fibers expressing MyHC-I/β tend to be smaller with a higher oxidative capacity. In addition to these functional differences across fiber types, there are species differences in MyHC isoform expression and, therefore, differences in skeletal muscle fiber-type composition. For example, rodent skeletal muscle (especially mouse) is predominantly comprised of muscle fibers expressing MyHC-IIb, with an overall MyHC abundance across murine muscles of IIb > IIx > IIa > I/β. In contrast, human skeletal muscle does not express MyHC-IIb protein, a phenomenon that appears to be, at least in part, due to a reduction in the activity of the human MyHC-IIb promoter region (37). In contrast to murine skeletal muscle, the overall MyHC isoform abundance across human skeletal muscles is I/β > IIa > IIx, although there are certainly regional and muscle-specific differences in MyHC isoform expression, as described below.
Sex-Based Differences in Fiber-Type Composition
Overall, evidence to date suggests that skeletal muscle fiber-type composition is dependent on species, anatomical location/function, and sex. In general terms, MyHC isoform expression tends to be “slower” (i.e., higher relative expression of MyHC-I/β and MyHC-IIa) in larger mammals compared with smaller mammals. As mentioned above, these species differences in MyHC isoform expression are most pronounced with respect to the MyHC-IIb gene. Across individual muscle groups (at least in rodents), deeper postural muscles such as the soleus tend to have higher relative expression of MyHC isoforms associated with a more oxidative phenotype (MyHC-I/β and MyHC-IIa), whereas superficial muscle groups, such as the gastrocnemius, tend to be comprised almost entirely of muscle fibers expressing MyHC-IIx and MyHC-IIb. Although studies investigating sex-based differences in muscle fiber type are somewhat limited, there is also evidence of sexual dimorphisms with respect to muscle fiber-type composition (Table 2).
Table 2.
Fiber-type expression and area
| Muscle | Male | Female | Reference |
|---|---|---|---|
| Mouse Masseter (average) | IIX > IIB > IIA | IIX > IIA > IIB | 28 |
| Rabbit Masseter (average) | IIA > I | I > IIA | 27 |
| Human vastus lateralis (CSA) | IIA > IIX > I | I > IIA > IIX | 90 |
| Human VL MHC isoform % | IIa > I > IIx | I > IIa > IIx | 90 |
Differences in fiber-type size and composition of listed muscle bodies. Data are presented as fiber-type contribution in decreasing order. CSA, cross-sectional area; VL, vastus lateralis.
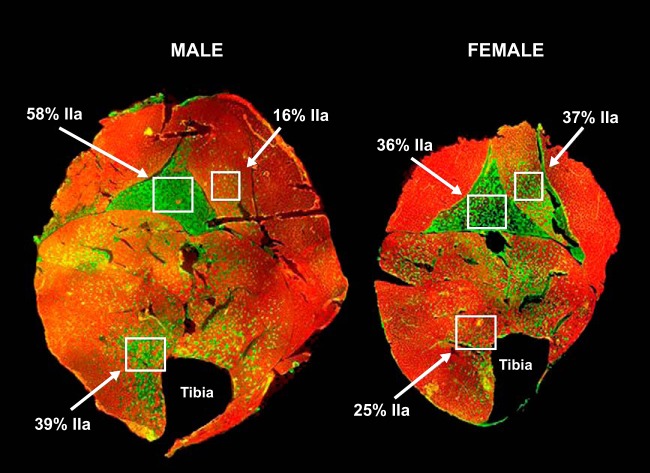
The mouse masseter is composed of high numbers of IIX fibers in both sexes; however, there are threefold fewer IIB fibers and more IIA fibers in females compared with males (27). Another study of male and female mouse masseter also finds threefold fewer fibers expressing MyHC-IIb in females compared with males (22). Male rabbit masseter is comprised of nearly 80% type-IIA fibers, whereas the female masseter contains only ∼50% (28). Separating the rabbit masseter into 10 different muscle compartments reveals that two of the compartments consist of more type-IIA fibers in young males vs. more type-I fibers in young females (27). For illustrative purposes, FIGURE 1 is an immunohistochemical analysis that highlights potential sexually dimorphic regional differences in MyHC isoform expression in the mouse hindlimb. For example, MyHC-IIa expression is higher in males vs. females in the soleus (58% in males vs. 36% in females) and tibialis anterior (TA) (39% in males vs. 25% in females), whereas in the plantaris, IIa expression is higher in females (37%) than in males (16%).
FIGURE 1.
Type-IIa fibers are differentially expressed in male vs. female hindlimb muscle sections and in different muscle bodies
In the soleus, IIa expression is 58% in the male 36% in the female. In the plantaris, IIa expression is16% in the male and 37% in the female. In the tibialis anterior muscle, IIa expression is 39% in the male and 25% in the female. Green staining identifies IIa fibers, whereas red staining identifies IIb fibers. Figure was generously provided by Brooke Harrison, PhD.
Just as in experimental animal models, myosin isoform mRNA and protein composition can be very different between men and women. Many human studies are conducted on the vastus lateralis muscle due to the relative ease of sample collection. Analysis of mRNA from both male and female vastus lateralis biopsies by microarray shows (when normalized to α-actinin) that the female muscle has 35% more MyHC-I/β, 30% less MyHC-IIa, and 15% less MyHC-IIx mRNA than the corresponding male muscle (96). Type-I fibers account for 36% of the total biopsy area in men and 44% in women, whereas type-IIA fibers account for 41% in men and only 34% in women (89). Overall, in this analysis, the average MyHC isoform percentages in the male vastus lateralis are IIx (20%), IIa (46%), and I (34%), whereas the females samples are IIx (23%), IIa (36%), and I (41%) (89). Additionally, all of the fibers measured in men have significantly larger cross-sectional areas (CSA) compared with women as normalized to biopsy section area (89). The difference in CSA seems likely to be due to the overall greater mass in males compared with females since the increase in CSA is nearly proportional to the differences in mass.
Contractility
Studies on chemically skinned human fibers reveal that MyHC isoform composition is the key determinant of muscle fiber contractile velocity and rate of force development (11, 72). Although evidence suggests that maximum unloaded shortening velocity is not different between young male and female fibers (25), sex-based differences are seen during fatigue recovery and endurance testing (Table 3). Analysis of tetanic force in the mouse masseter shows no significant differences between the sexes on force production (22). However, the same study reveals that, during a tetanic force protocol, the maximal rate of force generation is significantly higher in males than in females (516 vs. 382 g/s). Additionally, the rate of relaxation is significantly faster in the male masseter during a tetanus compared with the female masseter (41 vs. 48 ms) (22).
Table 3.
Baseline contractility measurements in males and females
| Measurement | Muscle | Result | Reference |
|---|---|---|---|
| Rate of force development | Mouse masseter | Higher in males | 22 |
| Rate of relaxation | Mouse masseter | Lower in males | 22 |
| Force | Human elbow flexor | Equal in males and females | 1 |
| Endurance | Human elbow flexor | Lower in males | 41 |
| Endurance | Human adductor pollicis | Lower in males | 32 |
The capacity for fatigue of a muscle is an indicator of recovery capabilities and can differ between species and sex. Generally, male muscles are more fatigable than female muscles. In vivo studies on fatigue focus on the ability to maintain the contractile strength of a muscle. This maintained contractile force is measured as exerted force or maximal voluntary contraction from single or multiple muscle fibers. At the start of a fatigue experiment, the maximal voluntary contraction is determined for each subject and used to quantify the relative force decline during the protocol. Although there are reports of sex-based differences in fatigability, the reasoning for these differences in fatigability could be associated with differences in muscle substrate utilization, neuromuscular activation, or muscle morphology (38). Additionally, fatigue can be induced through impairment of central drive or motivation, neuromuscular propagation, or peripherally through the impairment of excitation-contraction coupling (30, 32).
Studies on elbow flexor and knee extensor muscles show a significant loss of motor unit activation in males vs. females following a standard fatigue protocol (1). Although forces exerted by both young men and women are not significantly different, endurance is lower for men than for women (11 vs. 18 min) (39). In the elbow flexor muscle, endurance time to fatigue is 1,806 s in females and 829 s in males (40). After intermittent bouts of isometric contraction (5 s on and 5 s off) in the adductor pollicis muscle, women fatigue more slowly and to a smaller degree than men (31). Specifically, force falls to ∼93% of the maximum in women vs. 80% in men after <1 min of exercise (31). Endurance is longer in women (15 min) compared with men (8 min), and after reaching exhaustion, recovery occurs faster in women than in men (31). Fatigue also appears to be muscle-specific, as evidenced by analysis of both the biceps femoris and lumbar extensors for fatigability. More specifically, women fatigue similarly in both muscles, whereas men fatigue more in the lumbar musculature compared with the biceps femoris (19). These studies suggest that force generation and relaxation are faster during fatigue in men compared with women, whereas endurance is higher and recovery is quicker in women than in men. These differences in muscle performance between men and women may be due to the larger numbers of type-I fibers in women that are characterized by slow oxidative metabolism and thus higher endurance. Overall, these studies lay the groundwork for more extensive basic research on sex differences in skeletal muscle composition and function.
Hormonal Regulation of Myosin Isoforms
It seems logical that hormones contribute to sexual dimorphisms in fiber-type composition and contractility. In fact, hormonal regulation of skeletal muscle development and contractility are well documented (29, 70). Skeletal muscle is a major target of thyroid hormone (42); estrogen exerts its effects on the cardiovascular and musculoskeletal systems (45); and testosterone is heavily studied for its pro-hypertrophic/anabolic effects on skeletal muscle. Fiber-type composition and contractile function can be altered by the presence or removal of specific hormones. In the following section, we present findings on the effects of thyroid hormone, estrogen, and testosterone on contractility, fiber type, and the differences that occur between the sexes.
Thyroid Hormone
Thyroid hormone (T3) can regulate MyHC gene expression (42). T3 affects muscle protein expression at the posttranscriptional, translational, and posttranslational levels (for review, see Ref. 15). Individuals with hypothyroidism, or an underactive thyroid, typically display symptoms such as low heart rate, fatigue, weight gain, muscle weakness, a conversion from fast to slow fiber types (41), and a more efficient energy metabolism (5). Hypothyroidism produces a lower type-II fiber percentage in male and female patients compared with healthy patients (63). Hypothyroid females appear to have a higher proportion of type-II fibers compared with hypothyroid males; however, type-II fiber atrophy occurs in hypothyroid female patients but not in males (63). Graves' disease, a common immune disorder, is characterized by an overproduction of T3 or hyperthyroidism. According to the Mayo Clinic, individuals with Graves' disease typically experience symptoms such as difficulty sleeping, fatigue, sensitivity to heat, and a rapid or irregular heartbeat (www.mayoclinic.org). Treatment of euthyroid animals with T3 induces hyperthyroidism, producing a reversible slow-to-fast MyHC isoform transition from I → IIa → IIx → IIb (for review, see Ref. 78). Before treatment with T3, almost all soleus muscle myofibers in male and female rats express type-I fibers (101). Four weeks of T3 treatment induces an increase in type-IIA fibers and a downregulation of type-I fibers in male and female rat soleus muscle (57, 101). Specifically, after treatment with T3, male rat soleus muscle expresses 73% type-I/IIA and 26% type-I/IIAX of the total fibers, whereas females express 37% type-I, 49% type-I/IIA, and only 6% type-I/IIAX fibers (101). Overall, the increase in IIX content from the type-I/IIAX fibers is greater in young T3-treated males (21%) than females (10%), and the upregulation of IIA is greater in young females (43%) than in young males (27%) (101). In another study of euthyroid animals, treatment with T3 induces expression of IIX in the soleus of aged male rats (4–15%), whereas there is no detectable IIX in females at any age (52).
The rat extensor digitorum longus (EDL) contains predominately fast MyHC isoforms (IIa, IIx, and IIb). Hypothyroidism leads to elevated levels of MyHC-IIa mRNA compared with MyHC-IIb mRNA in rat EDL (47). Long-term treatment with T3 in euthyroid rats (for 24 wk) results in a decrease in mRNA levels and the percentage of protein isoforms for MyHC-IIb and MyHC-IIa in the EDL muscle (93). Other studies in rat EDL and TA show that chronic hyperthyroidism enhances MyHC-IIb mRNA, downregulates MyHC-IIa mRNA, and leads to a decrease in IIa protein levels with no change in IIb protein levels compared with euthyroid control animals (47). T3 treatment in euthyroid animals leads to a conversion from IIA to IIB fibers in the EDL of young and old female rats vs. no transition in males (52). Table 4 summarizes the fiber-type changes in distribution as it relates to sex and thyroid hormone levels. These studies highlight the differences in fiber-type conversion as it relates to sex and pinpoints the differences in IIx expression in males and females. These fine differences in fiber-type contribution could effectively change contractile function, endurance, and the response to fatigue.
Table 4.
Hormonal regulation of fiber-type expression by thyroid hormone
| Insult | Male | Female | Reference |
|---|---|---|---|
| Hypothyroidism in humans | ↑ Type-II | ↑↑ Type-II | 64 |
| T3 treatment of rat soleus | I/IIA > I/IIAX | I/IIA > I > I/IIAX | 102 |
| T3 treatment on rat soleus | ↑IIX | ↑ IIA | 53, 102 |
| T3 treatment in rat EDL | No change | IIA → IIB | 53 |
T3 treatment, hyperthyroidism.
Contractile regulation by thyroid hormone.
T3 is typically implicated in cardiac contractility, but there are also studies showing T3 effects on skeletal muscle contractility (16, 43). In hyperthyroid individuals, ATP turnover rate is faster than in normal individuals, whereas in hypothyroid individuals, ATP turnover is slower (99). Analysis of hyperthyroid canine quadriceps femoris muscle reveals a T3-induced increase in maximal contractile rate and in rate of relaxation over euthyroid levels (65). Given the T3-induced sex-based differences in fiber-type conversion, it is logical to hypothesize that T3 may produce differences in contractility between the sexes. Contractility of rat soleus and EDL is not different in maximal unloaded shortening velocity in isolated fibers (type I, I/IIA, and I/IIAX) between hyperthyroid males and females. However, a faster shortening velocity is observed in soleus fibers from hyperthyroid females (old and young) compared with euthyroid controls (25). On the other hand, other reports have not seen T3-dependent differences in shortening velocity of EDL muscle and only slight variances in soleus (57). These studies suggest a potential role for T3 in skeletal muscle contractility and in sex-based differences that may exist in the response to changing T3 levels but point to the need for further investigations.
Thyroid hormone receptors and estrogen receptors are suggested to interact in a way that modulates estrogen-sensitive gene expression (26, 102). Thus the effects of estrogen on skeletal muscle fiber-type composition may correlate with the previously described findings on the effects of T3 on skeletal muscle.
Estrogen
The relationship between estrogen and skeletal muscle function and recovery has been analyzed for decades. In the following section, we present the current understanding of the effects of estrogen and its changing levels on skeletal muscle physiology. In humans, a decline in estrogen can be due to conditions such as hypogonadism, hypopituitarism, anorexia nervosa, perimenopause, or menopause. During menopause, the decline in estrogen levels is paralleled with a slight increase in injury risk and a decline in lean body mass (9). Animal models of low estrogen levels, which occur after ovariectomy (OVX), reveal an increase in overall body mass and in the mass of individual muscles (68). Skeletal muscle is an estrogen-responsive tissue such that estrogen receptor (ER) mRNA and protein levels change with circulating estrogen levels (7). Skeletal muscle expresses two forms of ERs (α and β), and the effects of estrogen are mediated, in part, through these receptors (14, 21, 56, 98). In male and female vastus lateralis muscle, expression of ERα mRNA is 180-fold higher than ERβ, with no significant difference in expression levels between males and females (98). Additionally, immunohistochemical analysis highlights ERα and ERβ localization to the nuclei of skeletal muscle equally in males and females (97, 98). ERα-null mice have a decreased muscle regenerative capacity following injury, as evidenced by small myofibers and centrally located nuclei (50). This decline in regenerative capacity is thought to be directly related to findings that estrogen is associated with maintenance of muscle strength in pre- and postmenopausal women (69) and suggests a role for ERs in skeletal muscle maintenance (64, 84, 85, 88).
Mechanism of action.
Estrogen is thought to induce cellular effects, such as transcription, through the activation of ERK1/2 and p38 followed by phosphorylation of both cAMP response element-binding protein and elk-1, which play a role in regulating the early c-Fos gene (75). Estrogen has anti-apoptotic effects through the PI3K/Akt pathways (10). Additionally, the IGF-1 pathway, which plays a positive role in skeletal muscle growth and regeneration, is implicated in estrogen signal transduction (36, 53, 54, 94). Studies on muscle repair following exercise-induced injury highlight a sex-based difference in which higher levels of muscle creatine kinase (indicative of injury) are observed in male than in female rats following injury. Following OVX, levels of muscle creatine kinase in females are similar to levels in injured males (3, 4, 8).
Recent studies suggest a beneficial role for hormone replacement therapy (HRT), supplementation with estrogen and progesterone, and the induction of pathways by which estrogen can aide in skeletal muscle maintenance and repair (71). In a test of physical performance, HRT leads to a significant increase in vertical jumping height in women compared with exercise alone and no treatment (85). Ronkainen et al. (76) found that HRT is associated with better mobility, greater muscle power, and favorable body and muscle composition in women ages 54–62 (76). A role for estrogen in the prevention of the inflammatory response, which can exacerbate muscle injury and inhibit recovery, is also suggested (3, 92).
Influence of estrogen on muscle fibers.
Estrogen has been shown to influence fiber size, overall muscle weight, muscle regeneration, and contractility, and to induce minimal changes in fiber-type distribution. OVX leads to an increase in overall body weight and muscle weight (68), and a 70% increase in ERα mRNA with no change in ERβ mRNA (7). Soleus muscle weight increases after OVX in 10-wk-old female rats (68, 95). Supplementation with estrogen following OVX leads to a reduction in body weight (62) and attenuates the increase in ERα mRNA (7). In rat soleus and EDL muscles, OVX leads to a slight increase in the mean fiber diameter of type-I, -IIA, and -IIB fibers. Estrogen supplementation decreases fiber diameter in all fiber types in both the soleus and EDL significantly from OVX levels to below baseline levels (90). There is no difference in fiber-type composition of the soleus of OVX mice (67). However, in the mouse plantaris muscle, OVX does lead to a reduction in the relative amount of type-IIX fibers from 38% in sham animals to 33% (70). Supplementation with estrogen increases the type-IIX percentage composition in the plantaris back to 42% (70).
Contractile regulation by estrogen.
OVX rats supplemented with estrogen have also been analyzed for changes in contractile function (among other parameters). OVX induces a reduction in time to peak tension and increases time to 50% relaxation in rats, whereas estrogen replacement reverses the OVX-induced reduction in peak tension (62). Analysis of contractile function in OVX mice reveals a significant decrease in maximal isometric tetanic force from sham levels (180 mN) to 168 mN (67, 68). This decline is attenuated to 187 mN with estrogen supplementation (67). In the gonadally intact female rat EDL, twitch tension is 41 g, which increases to 51 g following OVX but then decreases to 31 g in OVX animals treated with estrogen (90). Since a strong actin-myosin bond will produce a greater force, this has been measured in muscles with and without estrogen manipulation. The fraction of strong binding myosin in OVX muscle is 0.263 or ∼15% lower than baseline (67, 68). Following estrogen treatment, the fraction of strongly bound myosin increases to 0.311, which is indistinguishable from sham levels (0.300) (67). The relative strength of the bond between actin and myosin is proportional to the energy available for the production of force (44). The differences in actin-myosin bond strength suggest that estrogen may play a role in increasing force production at the myofilament level.
In the studies referenced in the previous sections, there is no change in MyHC isoform expression or overall muscle fiber-type composition, but there are estrogen-related changes in fiber-type diameter, muscle size, contractility, and the actin-myosin interaction. Previous studies highlight the calcium-induced increase in force production in skeletal muscle and the subsequent decline in force following the removal of calcium (58, 91). Because of the OVX-induced decrease in tetanic force, which requires rapid replenishment and utilization of calcium stores, and the increase in fiber diameter, which could compensate for a decline in calcium sensitivity, calcium handling and sensitivity may be playing a role in the regulation of contractile differences in samples with and without estrogen. OVX induces a decline in twitch tension and slows twitch kinetics in the soleus (95). Additionally, there is a decline in maximal tension produced in the soleus of 10-wk-old OVX rats (∼19%) and 14-wk-old OVX rats (∼20%) compared with sham (95). These changes are not paralleled with changes in calcium sensitivity in OVX animals; however, the decline in the strength of the actin-myosin bond seen by Moran et al. (67) or a decline in calcium storage capacity following OVX remains uninvestigated.
Sex-based differences in fatigue.
As stated previously, there is no sexually dimorphic expression in either ERα or ERβ in skeletal muscle (98). Estrogen is present in both males and females (albeit at different levels), and it is suggested to play a role in male sexual behavior and cognition (80). ERα-null mice have decreased tetanic tension of gastrocnemius and TA muscles in females compared with wild-type controls (14). In an estrogen-elimination animal model (aromatase null), tetanic tension produced by the gastrocnemius muscle and TA is lower than in controls (14). The role of ERβ has been investigated through the use of ERβ-null mice (ERβ −/−) (49). These data are summarized in Table 5. Force production in soleus muscle declines slightly in ERβ−/− but is not significantly different (33). Contraction duration is significantly longer in the female soleus in both wild-type (91 ms) and null (100 ms) compared with male wild-type (57 ms) and null (63 ms) mice. Relaxation time during a basic contraction in the soleus is significantly shorter in wild-type males (104 ms) than in females (158 ms) and in null males (104 ms) compared with null females (191 ms). In the EDL, half contraction time during a tetanic twitch protocol lasts 40 ms in male wild-type mice and 46 ms in male ERβ−/−, whereas in female wild-type, half contraction time lasts 50 ms compared with 61 ms in ERβ−/− females (33). Following a fatiguing protocol on soleus and EDL muscle, force decreases less in ERβ−/− males than in wild-type males, whereas the reverse is true for females, suggesting a role for ERβ knockout in enhancing endurance in males and reducing endurance in females (33). Overall, ERβ deletion increases the contractile time and decreases the speed of contractile kinetics in both males and females. However, because baseline contractile duration is already longer in females than in males, ERβ deletion simply exacerbates the wild-type phenotypes. During fatigue, where females typically have greater endurance, ERβ elimination decreases the endurance in the female and enhances endurance in the male. This could be due to minute changes in fiber-type contribution, CSA, or changes in calcium-handling proteins. Taken together, these findings on estrogen and the ER-mediated regulation of the contractile response and fiber-type distribution suggest a role for estrogen and its receptors in contractile maintenance and function, with a differential effect of estrogen in males vs. females.
Table 5.
Effects of ERβ-null mice
| Measurement | Male WT | Female WT | Male ERβ−/− | Female ERβ−/− |
|---|---|---|---|---|
| Force production (soleus) | 19.4 mN | 35.0 mN | 16.9 mN | 29.7 mN |
| Contraction time (soleus) | 57.4 ms | 90.8 ms | 62.6 ms | 99.6 ms |
| Relaxation time (soleus) | 100.4 ms | 158.2 ms | 104.0 ms | 191.4 ms |
| Relaxation time during tetanus (EDL) | 39.8 ms | 49.6 ms | 46.0 ms | 60.6 ms |
Data are republished from Ref. 34.
Testosterone
Testosterone is a highly studied androgen that is associated with an increase in muscle mass (66, 83). With advancing age, testosterone levels drop, and testosterone deficiency is associated with early death, primarily from cardiovascular disease, a decrease in muscle mass and/or strength, and sexual dysfunction (46). Testosterone has been shown to induce its effects through binding to intracellular androgen receptors (ARs) (34), which then regulate gene transcription (18, 81). Myoblasts treated with testosterone have enhanced hypertrophic responses via the PI3K pathway (23). Testosterone supplementation leads to an increase in muscle mass and a decrease in fat content in hypogonadal men (13). This response may be through a testosterone-induced increase in the number of satellite cells and thus hypertrophy (82, 83). The testosterone-mediated proliferation and differentiation of satellite cells is thought to be due to an upregulation of follistatin and an inhibition of transforming growth factor-β (12). ARs are widely expressed in myoblasts, myofibers, and motoneurons in both males and females (17, 100), and testosterone injected into the masseter of female guinea pigs leads to an increase in fiber size (35). Additionally, testosterone supplementation in postmenopausal women leads to a 50% increase in protein synthesis rate compared with no effect with estrogen treatment (87). These anabolic effects of testosterone are well characterized and provide the catalysts for further investigation of the response of muscle fiber-type composition to testosterone deficiency and supplementation in males and females.
Influence of testosterone on muscle fiber type and morphology.
As mentioned earlier, studies of vastus lateralis biopsies show fiber-type CSA to be larger in men than in women. Specifically, type-I fibers are 19% larger, type-IIA fibers are 59% larger, and type-IIX fibers are 66% larger in men than in women (normalized to total biopsy section analyzed) (89). In the adult guinea pig, castration produces atrophy of several muscles, including the latissimus dorsi, sternomastoid, and spinotrapezius (48). In mice, castration produces a decrease in body weight, which is attenuated with testosterone supplementation, and although overall muscle weight increases, the fiber CSA of the soleus and TA do not significantly change (6). However, there is a correlation between increases in fiber CSA and overall muscle mass. The role of testosterone on muscle fiber-type distribution has been, in part, determined through the analysis of the hypogonadal male and female mouse models. Analysis of fiber-type distribution in hypogonadal males vs. females reveals no significant changes in type-I, -IIA, -IIX, or -IIB fibers in gastrocnemius muscle or in type-I or -IIA fibers in the soleus (79). Additionally fiber-type distribution of the gastrocnemius is relatively unchanged from wild-type mice, with I < IIX < IIA < IIB being the relative contribution of each fiber-type in males and females (79). However, in other instances, IIB fiber-type diameter in male hypogonadal mice significantly declines compared with wild-type males. Surprisingly, in females, the hypogonadal phenotype leads to an increase in IIB fiber diameter compared with wild-type females (79).
Sex-based differences in contractility.
Testosterone is not typically associated with enhanced contractile function in that testosterone replacement is not associated with increased endurance. For instance, hypogondal and eugonadal men exhibit similar limb muscle strength and endurance during exercise (51). The increase in strength associated with testosterone supplementation is thought to be due to its anabolic effects. Male AR-null mice have a decrease in muscle mass that is not seen in AR-null females (59). Additionally, in AR-null males, force production decreases in fast-twitch fibers, whereas fatigue resistance increases in slow-twitch fibers to levels similar to wild-type females (59). Unfortunately, the contractile function in AR-null females is not analyzed in this study. Analysis of mRNA reveals an upregulation of genes encoding slow-twitch muscle contractile proteins in AR-null mice (2, 59). Because aromatase converts testosterone to estrogen (60), testosterone can induce its effects via either ERs or ARs, thus this contractile effect is thought to be mediated by the activation of ERs. Overall, the effects of testosterone deficiency are exacerbated in males compared with females. Testosterone deficiency leads to a decline in body mass, a decrease in fast-twitch fiber diameter, a conversion to slow-twitch fibers, and enhanced fatigue resistance in males but not females.
Conclusions
Sex-based differences in skeletal muscle fiber-type composition and function are apparent in numerous species and are present in specific anatomical locations. Here, we present findings on sexual dimorphisms present in the mammalian musculoskeletal system. There are four main MyHC isoforms present in adult mammalian muscle (MyHC-I, -IIa, -IIx, and -IIb), which increase in contractile speed in the presented order. There is a prevalence of slower type-I and -IIA fibers in females compared with males that parallels the lower contractile velocity in females compared with males. The prevalence of the slower-twitch fibers is also a benefit to female performance in that the slower oxidative fibers and higher oxidative capacity allow for increased endurance and recovery, highlighting the sex-based differences in response to fatigue or muscle tetanus. To explain the potential cause of differences in skeletal muscle performance and fiber-type composition, we also present the differential effects of increases and decreases in levels of thyroid hormone, estrogen, and testosterone. Although thyroid hormone induces a conversion from slow to fast fibers and increases contractile velocity, sex-specific hormones estrogen and testosterone are implicated in skeletal muscle growth, fiber size, and minimally in contractile function. Some reports highlight enhanced contractile function and increased β-oxidative gene expression in men supplemented with estrogen and enhanced muscle growth in females treated with testosterone (61).
The identification of over 3,000 genes differentially regulated in male and female muscle highlights the complex differences that occur in skeletal muscle from both sexes (96). In this study, the authors focus on two particular genes that are upregulated in women compared with men and that are known to code for proteins that are in signaling pathways of growth factors known to regulate muscle mass: growth factor receptor-bound protein 10 (GRB10) and activin receptor type-2A (ACVR2A). GRB10 codes for a protein that suppresses IGF-1, which has an anabolic effect, whereas ACVR2A codes for a myostatin receptor, which has a role in muscle size determination. GRB10 knockout in male and female mice produces an increase in muscle weight and a decrease in body fat percentage and thus enhances muscularity (86). Another study reveals that knockout of ACVR2A causes muscle hypertrophy in female mice (55). Further analysis must be done to validate the role of these newly identified targets in skeletal muscle sex differences. In this review, we present changes in fiber-type composition, size, and contractile function in males and females; however, one or two genes cannot be responsible for altering all of these factors. To fully validate a gene as regulating the sex differences, for instance, in fiber-type composition, a thorough in vitro and in vivo analysis must be completed to first understand how the gene is regulated, the proteins' function and interacting partners, and then how these interactions might lead to sexually dimorphic differences in muscle fiber-type composition and function. The complexity of skeletal muscle and the role of sex adding to that complexity cannot be overlooked.
Future Directions
The lack of studying both males and females in the laboratory has recently attracted the attention of the public and the NIH (20). One recommendation made is that investigators report the sex of the animals or cell lines being studied. For instance, previous studies have identified sex as a determinant for the ability of muscle-derived stem cells to regenerate. Specifically, female muscle-derived stem cells regenerate more efficiently when transplanted into dystrophic mice (24). Sex differences should be accounted for in studies of skeletal muscle composition, function, and adaptive responses to different forms of exercise training and regression. For example, FIGURE 1 depicts differences in fiber-type composition in male and female mouse hindlimb muscles. Further studies of these fiber differences in the context of skeletal muscle adaption should provide insight into the regulatory differences between the sexes. Another neglected area is epigenetic differences in males and females in skeletal muscle, and studies should be aimed at determining the role of hormonal interventions in males and females given their clinical relevance. Numerous skeletal muscle therapies are built based on results from studies in men alone or with only a small subset of women. Having an appreciation for the differences that exist between the sexes is the first step to understanding the mechanisms underlying these sex differences. This review summarizes key findings in skeletal muscle physiology in the hopes of bringing to the forefront areas of future research and sexual disparities in current investigations.
Footnotes
This research was supported in part by National Institutes of Health grants to K.H. (GM-029090-30A1S1), B.H. (5K01 AR-55676), and L.L. (GM-29090-31).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: K.M.H. and B.C.H. prepared figures; K.M.H. drafted manuscript; K.M.H., B.C.H., and L.A.L. edited and revised manuscript; K.M.H. and L.A.L. approved final version of manuscript.
References
- 1.Albert WJ, Wrigley AT, McLean RB, Sleivert GG. Sex differences in the rate of fatigue development and recovery. Dyn Med DM 5: 2, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altuwaijri S, Lee DK, Chuang KH, Ting HJ, Yang Z, Xu Q, Tsai MY, Yeh S, Hanchett LA, Chang HC, Chang C. Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine 25: 27–32, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Amelink GJ, Bar PR. Exercise-induced muscle protein leakage in the rat. Effects of hormonal manipulation. J Neurol Sci 76: 61–68, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Amelink GJ, Koot RW, Erich WB, Van Gijn J, Bar PR. Sex-linked variation in creatine kinase release, and its dependence on oestradiol, can be demonstrated in an in-vitro rat skeletal muscle preparation. Acta Physiol Scand 138: 115–124, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Argov Z, Renshaw PF, Boden B, Winokur A, Bank WJ. Effects of thyroid hormones on skeletal muscle bioenergetics. In vivo phosphorus-31 magnetic resonance spectroscopy study of humans and rats. J Clin Invest 81: 1695–1701, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axell AM, MacLean HE, Plant DR, Harcourt LJ, Davis JA, Jimenez M, Handelsman DJ, Lynch GS, Zajac JD. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab 291: E506–E516, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLos One 5: e10164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci 42: 2677–2681, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Bea JW, Zhao Q, Cauley JA, LaCroix AZ, Bassford T, Lewis CE, Jackson RD, Tylavsky FA, Chen Z. Effect of hormone therapy on lean body mass, falls, and fractures: 6-year results from the Women's Health Initiative hormone trials. Menopause 18: 44–52, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17beta-Estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids 73: 859–863, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor-beta signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol 350: 39–52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 81: 3469–3475, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab 296: E854–E861, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caiozzo VJ, Haddad F. Thyroid hormone: modulation of muscle structure, function, and adaptive responses to mechanical loading. Exerc Sport Sci Rev 24: 321–361, 1996. [PubMed] [Google Scholar]
- 16.Carr AN, Kranias EG. Thyroid hormone regulation of calcium cycling proteins. Thyroid 12: 453–457, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol 186: 21–31, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem 282: 29584–29593, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Clark BC, Manini TM, The DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol 94: 2263–2272, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138: 4613–4621, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Daniels DW, Tian Z, Barton ER. Sexual dimorphism of murine masticatory muscle function. Arch Oral Biol 53: 187–192, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deane CS, Hughes DC, Sculthorpe N, Lewis MP, Stewart CE, Sharples AP. Impaired hypertrophy in myoblasts is improved with testosterone administration. J Steroid Biochem Mol Biol 138: 152–161, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177: 73–86, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degens H, Yu F, Li X, Larsson L. Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol Scand 163: 33–40, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Dellovade TL, Zhu YS, Krey L, Pfaff DW. Thyroid hormone and estrogen interact to regulate behavior. Proc Natl Acad Sci USA 93: 12581–12586, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eason JM, Schwartz GA, Pavlath GK, English AW. Sexually dimorphic expression of myosin heavy chains in the adult mouse masseter. J Appl Physiol 89: 251–258, 2000. [DOI] [PubMed] [Google Scholar]
- 28.English AW, Eason J, Schwartz G, Shirley A, Carrasco DI. Sexual dimorphism in the rabbit masseter muscle: myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs 164: 179–191, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med 40: 41–58, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve 25: 763–764, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Fulco CS, Rock PB, Muza SR, Lammi E, Cymerman A, Butterfield G, Moore LG, Braun B, Lewis SF. Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand 167: 233–239, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab 287: E1125–E1131, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Gustafasson J, Pousette K. Demonstration and partial characterization of cytosol receptors for testosterone. Biochemistry 14: 3094–3101, 1975. [DOI] [PubMed] [Google Scholar]
- 35.Gutmann E, Hanzlikova V, Lojda Z. Effect of androgens on histochemical fibre type. Differentiation in the temporal muscle of the guinea pig. Histochemistry 24: 287–291, 1970. [PubMed] [Google Scholar]
- 36.Hamelers IH, Steenbergh PH. Interactions between estrogen and insulin-like growth factor signaling pathways in human breast tumor cells. Endocr Related Cancer 10: 331–345, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Harrison BC, Allen DL, Leinwand LA. IIb or not IIb? Regulation of myosin heavy chain gene expression in mice and men. Skelet Muscle 1: 5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev 29: 109–112, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol 97: 1723–1732, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91: 2686–2694, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Ianuzzo D, Patel P, Chen V, O'Brien P, Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature 270: 74–76, 1977. [DOI] [PubMed] [Google Scholar]
- 42.Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science 231: 597–600, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Kaasik A, Minajeva A, Paju K, Eimre M, Seppet EK. Thyroid hormones differentially affect sarcoplasmic reticulum function in rat atria and ventricles. Mol Cell Biochem 176: 119–126, 1997. [PubMed] [Google Scholar]
- 44.Karatzaferi C, Chinn MK, Cooke R. The force exerted by a muscle cross-bridge depends directly on the strength of the actomyosin bond. Biophys J 87: 2532–2544, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzenellenbogen BS, Montano MM, Le Goff P, Schodin DJ, Kraus WL, Bhardwaj B, Fujimoto N. Antiestrogens: mechanisms and actions in target cells. J Steroid Biochem Mol Biol 53: 387–393, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116: 2694–2701, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Kirschbaum BJ, Kucher HB, Termin A, Kelly AM, Pette D. Antagonistic effects of chronic low frequency stimulation and thyroid hormone on myosin expression in rat fast-twitch muscle. J Biol Chem 265: 13974–13980, 1990. [PubMed] [Google Scholar]
- 48.Kochakian CD, Tillotson C. Influence of several C19 steroids on the growth of individual muscles of the guinea pig. Endocrinology 60: 607–618, 1957. [DOI] [PubMed] [Google Scholar]
- 49.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95: 15677–15682, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labarge S, McDonald M, Smith-Powell L, Auwerx J, Huss JM. Estrogen-related receptor-alpha (ERRalpha) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J 28: 1082–1097, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laghi F, Langbein WE, Antonescu-Turcu A, Jubran A, Bammert C, Tobin MJ. Respiratory and skeletal muscles in hypogonadal men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 598–605, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Larsson L, Yu F. Gender-related differences in the regulatory influence of thyroid hormone on the expression of myosin isoforms in young and old rats. Acta Physiol Scand 159: 81–89, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 13: 787–796, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Lee AV, Weng CN, Jackson JG, Yee D. Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J Endocrinol 152: 39–47, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102: 18117–18122, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc 35: 439–443, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Larsson L. Contractility and myosin isoform compositions of skeletal muscles and muscle cells from rats treated with thyroid hormone for 0, 4 and 8 weeks. J Muscle Res Cell Motil 18: 335–344, 1997. [DOI] [PubMed] [Google Scholar]
- 58.MacIntosh BR. Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18: 222–225, 2003. [DOI] [PubMed] [Google Scholar]
- 59.MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 22: 2676–2689, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Maggiolini M, Donze O, Jeannin E, Ando S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res 59: 4864–4869, 1999. [PubMed] [Google Scholar]
- 61.Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genom 42: 342–347, 2010. [DOI] [PubMed] [Google Scholar]
- 62.McCormick KM, Burns KL, Piccone CM, Gosselin LE, Brazeau GA. Effects of ovariectomy and estrogen on skeletal muscle function in growing rats. J Muscle Res Cell Motil 25: 21–27, 2004. [DOI] [PubMed] [Google Scholar]
- 63.McKeran RO, Slavin G, Andrews TM, Ward P, Mair WG. Muscle fibre type changes in hypothyroid myopathy. J Clin Pathol 28: 659–663, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meeuwsen IB, Samson MM, Verhaar HJ. Evaluation of the applicability of HRT as a preservative of muscle strength in women. Maturitas 36: 49–61, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Miyashita A, Suzuki S, Suzuki M, Numata H, Suzuki J, Akahori T, Okubo T. Effect of thyroid hormone on in vivo contractility of the canine diaphragm. Am Rev Respir Dis 145: 1452–1462, 1992. [PubMed] [Google Scholar]
- 66.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev 8: 1–28, 1987. [DOI] [PubMed] [Google Scholar]
- 67.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 102: 1387–1393, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol 100: 548–559, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Petrofsky JS, Burse RL, Lind AR. Comparison of physiological responses of women and men to isometric exercise. J Appl Physiol 38: 863–868, 1975. [DOI] [PubMed] [Google Scholar]
- 70.Piccone CM, Brazeau GA, McCormick KM. Effect of oestrogen on myofibre size and myosin expression in growing rats. Exp Physiol 90: 87–93, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Pollanen E, Ronkainen PH, Horttanainen M, Takala T, Puolakka J, Suominen H, Sipila S, Kovanen V. Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Hormone IGF Res 20: 372–379, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Ranatunga KW, Thomas PE. Correlation between shortening velocity, force-velocity relation and histochemical fibre-type composition in rat muscles. J Muscle Res Cell Motil 11: 240–250, 1990. [DOI] [PubMed] [Google Scholar]
- 73.Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem 260: 9077–9080, 1985. [PubMed] [Google Scholar]
- 74.Resnicow DI, Deacon JC, Warrick HM, Spudich JA, Leinwand LA. Functional diversity among a family of human skeletal muscle myosin motors. Proc Natl Acad Sci USA 107: 1053–1058, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronda AC, Buitrago C, Colicheo A, de Boland AR, Roldan E, Boland R. Activation of MAPKs by 1alpha,25(OH)2-Vitamin D3 and 17beta-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J Steroid Biochem Mol Biol 103: 462–466, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Ronkainen PH, Kovanen V, Alen M, Pollanen E, Palonen EM, Ankarberg-Lindgren C, Hamalainen E, Turpeinen U, Kujala UM, Puolakka J, Kaprio J, Sipila S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol 107: 25–33, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. [DOI] [PubMed] [Google Scholar]
- 78.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996. [DOI] [PubMed] [Google Scholar]
- 79.Sciote JJ, Horton MJ, Zyman Y, Pascoe G. Differential effects of diminished oestrogen and androgen levels on development of skeletal muscle fibres in hypogonadal mice. Acta Physiol Scand 172: 179–187, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab 15: 110–115, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem 266: 510–518, 1991. [PubMed] [Google Scholar]
- 82.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144: 5081–5088, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91: 3024–3033, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Sipila S, Poutamo J. Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand J Med Sci Sports 13: 19–25, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 101: 147–157, 2001. [PubMed] [Google Scholar]
- 86.Smith FM, Holt LJ, Garfield AS, Charalambous M, Koumanov F, Perry M, Bazzani R, Sheardown SA, Hegarty BD, Lyons RJ, Cooney GJ, Daly RJ, Ward A. Mice with a disruption of the imprinted Grb10 gene exhibit altered body composition, glucose homeostasis, and insulin signaling during postnatal life. Mol Cell Biol 27: 5871–5886, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, Moseley AC, Mittendorfer B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab 99: 256–265, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spangenburg EE, Geiger PC, Leinwand LA, Lowe DA. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc 44: 1653–1662, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48: 623–629, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki S, Yamamuro T. Long-term effects of estrogen on rat skeletal muscle. Exp Neurol 87: 291–299, 1985. [DOI] [PubMed] [Google Scholar]
- 91.Thornton AM, Zhao X, Weisleder N, Brotto LS, Bougoin S, Nosek TM, Reid M, Hardin B, Pan Z, Ma J, Parness J, Brotto M. Store-operated Ca2+ entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Milano) 3: 621–634, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tiidus PM. Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev 31: 40–44, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Vadaszova A, Hudecova S, Krizanova O, Soukup T. Levels of myosin heavy chain mRNA transcripts and protein isoforms in the fast extensor digitorum longus muscle of 7-month-old rats with chronic thyroid status alterations. Physiol Res 55: 707–710, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J 26: 1909–1920, 2012. [DOI] [PubMed] [Google Scholar]
- 95.Wattanapermpool J, Reiser PJ. Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. Am J Physiol Heart Circ Physiol 277: H467–H473, 1999. [DOI] [PubMed] [Google Scholar]
- 96.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLos One 3: e1385, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiik A, Ekman M, Johansson O, Jansson E, Esbjornsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol 131: 181–189, 2009. [DOI] [PubMed] [Google Scholar]
- 98.Wiik A, Glenmark B, Ekman M, Esbjornsson-Liljedahl M, Johansson O, Bodin K, Enmark E, Jansson E. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand 179: 381–387, 2003. [DOI] [PubMed] [Google Scholar]
- 99.Wiles CM, Young A, Jones DA, Edwards RH. Muscle relaxation rate, fibre-type composition and energy turnover in hyper- and hypo-thyroid patients. Clin Sci (Lond) 57: 375–384, 1979. [DOI] [PubMed] [Google Scholar]
- 100.Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol 44: 308–319, 2000. [DOI] [PubMed] [Google Scholar]
- 101.Yu F, Degens H, Li X, Larsson L. Gender- and age-related differences in the regulatory influence of thyroid hormone on the contractility and myosin composition of single rat soleus muscle fibres. Pflügers Arch 437: 21–30, 1998. [DOI] [PubMed] [Google Scholar]
- 102.Zhu YS, Yen PM, Chin WW, Pfaff DW. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc Natl Acad Sci USA 93: 12587–12592, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]