Abstract
BACKGROUND AND PURPOSE
For symptomatic patients with carotid artery stenosis the risk-benefit for surgical intervention may vary among patient groups. Various modalities of plaque imaging have been promoted as potential tools for additional risk stratification, particularly in patients with moderate stenosis. However, it remains uncertain to what extent carotid plaque components predict risk of future ipsilateral ischaemic stroke.
METHODS
In two large atherosclerotic carotid plaque biobank studies, we related histological characteristics of 1640 carotid plaques with a validated risk model for the prediction of individual 1- and 5-year stroke risk.
RESULTS
No significant heterogeneity between the studies was found. Predicted 5-year stroke risk (top versus bottom quartile) was related to plaque thrombus (OR=1.42, 95%CI 1.11-1.89, p=0.02), fibrous content (0.65, 0.49-0.87, p=0.004), macrophage infiltration (1.41, 1.05-1.90, p=0.02), high micro-vessel density (1.49, 1.05-2.11, p=0.03), and overall plaque instability (1.40, 1.05-1.87,p=0.02). This association was not observed for cap thickness, calcification, intra-plaque haemorrhage, or lymphocyte infiltration. Plaques removed within 30-days of most recent symptomatic event were most strongly correlated with predicted stroke risk.
CONCLUSIONS
Features of ‘the vulnerable carotid plaque’ including plaque thrombus, low fibrous content, macrophage infiltration and microvessel density correlate with predicted stroke risk. This study provides a basis for plaque imaging studies focused on stroke risk stratification.
Keywords: Acute Stroke, Carotid atherosclerotic plaque, Risk Prediction
Introduction
Approximately 15% of strokes are thought to be secondary to carotid artery atherosclerosis.1 Over the last 20 years, the degree of carotid artery stenosis has been the determining factor in selecting symptomatic patients for carotid endarterectomy (CEA).2 In symptomatic patients with carotid artery stenosis of 70% or more, CEA reduces ipsilateral stroke risk and has proven to be superior to best medical treatment.2 In symptomatic patients with lower degrees of stenosis, the benefit of CEA is less clear and is dependent on timing of surgery, the type of presenting event, gender, patient age, and co-morbidity.3 Recent improvements in intensive medical treatment and the slightly higher peri-operative risks in routine clinical practice compared to the large trials need also to be taken into account.4 This has led to a debate on risk-benefit ratios, correct patient selection, and the management of (moderate) symptomatic carotid stenosis. 5
To assess stroke risk in patients with symptomatic carotid stenosis more precisely, a risk prediction model has been developed using data of patients on best medical treatment in European Carotid Surgery Trial (ECST) that has been subsequently validated in the North American Symptomatic Carotid Endarterectomy Trial (NASCET).6,7 The model calculates individual ipsilateral stroke risk for recently symptomatic patients incorporating known significant risk predictors. Apart from the presence of an irregular/ulcerated plaque surface on conventional arterial angiography,8 no other carotid plaque features are included in the risk model as, to-date, their influence on future stroke risk are unknown. However, histopathological studies have revealed correlations between carotid plaque composition and several clinical key risk predictors. Male gender has been associated with lipid rich and inflammatory plaques,9 age has been linked with presence of large lipid cores,10,11 and plaques removed soon after last symptomatic event tend to be more inflammatory and show greater instability.12,13 Despite these associations, it remains uncertain whether any plaque feature can predict future stroke and risk-stratify patients independently of age, sex and more easily measured traditional vascular risk factors.
The concept of the “vulnerable plaque” was first introduced for coronary arteries following coronary plaque studies confirming that ruptured, inflammatory plaques with thin fibrous caps are often associated with acute coronary syndromes, whereas fibrous plaques tend to be associated with stable syndromes.14-16 This concept is less certain in the cerebral circulation, as only 50-60% of carotid plaques removed from symptomatic patients are ruptured17,18 and the pathophysiological mechanisms determining stroke secondary to large-vessel atherosclerosis (i.e embolism) differ greatly from those determining myocardial infarction (i.e. thrombosis). Despite this, there has been growing interest in the evaluation of carotid plaque morphology and functional characteristics, via the interpretation of various imaging modalities, biochemical markers, and embolic signal detection.19,20,21 Several plaque imaging studies have shown that development of intraplaque hemorrhage (IPH) is associated with carotid plaque progression, and recently it has also been associated with risk factors for future ipsilateral events.22-24 Among asymptomatic patients with moderate carotid artery stenosis (50-79%), several plaque characteristics including thinned or ruptured fibrous caps, intraplaque hemorrhage, and larger lipid and necrotic cores, and were associated with the occurrence of subsequent cerebrovascular events.25 However at present, the clinical utility of these techniques is still uncertain and current studies have lacked power to convincingly identify any influence of individual plaque features on future stroke risk.25-27 These studies have also been unable to follow-up patients with severe symptomatic carotid artery stenosis, as many undergo early intervention. The present study assesses the extent to which histological features in carotid plaques from symptomatic patients are related to individual predicted stroke risk.
Methods
We studied data from two large carotid plaque biobanks in the UK (Oxford Plaque Study) and the Netherlands (Athero-Express) in which plaques from consecutive patients undergoing carotid endarterectomy underwent detailed histological assessment with reproducible semi-quantitative scales. The Oxford Plaque Study included 481 plaques from symptomatic patients collected between 1975 - 2002 and the Athero-Express study included 1159 plaques from symptomatic patients collected between 2002 - 2012 (total n=1640). For each individual patient in both studies the predicted stroke risk was calculated and correlated to the carotid plaque histological features. Patients were excluded if they were undergoing surgery for asymptomatic stenosis, restenosis or radiotherapy-induced carotid stenosis.
Ipsilateral stroke risk
The stroke risk was calculated using a carotid stenosis risk prediction model, which has been described in detail previously and was validated against the NASCET patient database with a c-statistic of 0.67, 95% CI 0.63-0.72 (p<0.0001).6-7 The model can be accessed at www.stroke.ox.ac.uk/model/form1.html, and calculates 1-year and 5-year risk for ischaemic ipsilateral stroke based on clinical characteristics including patient age, sex, degree of stenosis, and relevant co-morbidities, together with type of primary symptomatic event and time from last symptomatic event. The characteristics at the time of CEA were used to calculate predicted stroke risks and the degree of stenosis was defined according to NASCET criteria.4 All patients in both studies had at least 50% carotid artery stenosis and were reviewed by a neurologist before consideration for CEA. The primary symptomatic event was defined as the most severe ipsilateral vascular event during the previous 6 months before CEA. The time since the last event refers to the most recent ipsilateral event. Diabetes was restricted to those cases receiving medical treatment, including insulin and oral medications. Previous myocardial infarction and peripheral vascular disease refer to previous confirmed clinical diagnoses. Treated hypertension referred to patients previously diagnosed with hypertension and actively treated with anti-hypertensives at the time of event. Monocular symptoms included amaurosis fugax or retinal artery occlusion. Transient ischemic attack (TIA) was defined as a symptomatic event lasting up to 24 hours, whereas minor strokes included symptoms lasting 24 hours to 7 days, and major strokes were non-disabling with residual symptoms present after 7 days.
Carotid plaque histopathology and phenotyping
The processing and assessment of carotid plaques has been described in detail for both studies previously.8,18,28,29 Briefly, carotid plaques were formalin fixed after endarterectomy and either the whole plaque (Oxford Plaque Study), or the portion of the plaque showing the highest plaque burden (Athero-Express), was paraffin embedded. After creating transverse sections, plaques were immunohistochemically stained with (1) hematoxylin and eosin (H&E) for assessment of overall plaque stability, lipid core, thrombus, and intraplaque haemorrhage, (2) elastin von Gieson (EVG) for fibrous plaque content, and (3) CD68 for plaque macrophage content. Both studies applied comparable 3-, or 4-point semi-quantitative scales for the assessment of overall plaque stability, lipid core, fibrous plaque content, micro vessel density (neovascularisation), macrophage infiltration, and calcifications, as defined previously (Supplemental Table I).8,18,28,29 However, to maximize comparability and overlap of definitions between studies, the scales were transformed into binominal variables (Supplemental Table I). In addition to the above, the Oxford Plaque Study scored the presence of plaque rupture, cap thickness and cap infiltration with macrophages and lymphocytes, whereas Athero-Express assessed for the presence of smooth muscle cells (alpha-actin). These histological characteristics that were recorded in only one of the two studies, were separately correlated with stroke risk for each study individually.
Analysis
SPSS version 18.0 (Chicago, IL, United States of America) was used for all statistical analyses. Baseline characteristics were compared between the two studies with students t-test and Mann-Whitney U test for parametric, and non-parametric continuous variables respectively. Baseline differences in nominal variables were examined with Pearson’s Chi-square test. The calculated 1-year and 5-year stroke risks were divided into quartiles. Associations between plaque characteristics and 1-year and 5-year stroke risks were assessed using odds ratios produced from binary logistic regression analysis. Regression analysis was also used to evaluate the strength of association between stroke risk and the presence of multiple plaque features. The key plaque features chosen for this analysis are those commonly thought to represent plaque vulnerability; presence of thrombus, large lipid core, low fibrous content, intraplaque haemorrhage, and inflammation (macrophage infiltration). As plaque stability is dependent on several characteristics, and a result of biological interaction of inflammation, plaque haemorrhage, and lipid metabolism; an additional analysis was performed to analyze the total number of vulnerable plaque characteristics per plaque in relation to predicted stroke risk. Heterogeneity between study cohorts for individual plaque features was assessed for using interaction terms in binary logistic regression. As no significant heterogeneity was found, binominal data from both studies were combined for pooled analysis. All such analyses were stratified by study cohort.
Results
The Oxford Plaque Study comprised 481 plaques and Athero-Express 1159 plaques, resulting is a pooled sample of 1640 symptomatic carotid plaques. Baseline characteristics for both studies are provided in table 1. In the Oxford Plaque study patients were younger, more frequently male, less frequently diabetic, less often on prior statin treatment, and had a longer time period between last event and CEA as compared with Athero-express patients. In addition, the Oxford Plaque Study included more patients with monocular events and major stroke, whereas Athero-Express included more patients with TIA and minor stroke. The 1-year predicted stroke risk was comparable between the two studies, but the 5-year stroke risk was marginally higher in Athero-Express (table 1).
Table 1. Patient characteristics in Oxford Plaque Study and Athero-Express.
| Characteristic | Oxford Plaque (n=481) | Athero-Express (n=1159) | P value | ≤ 30-days (n=553) | >30-days (n=1087) | P value |
|---|---|---|---|---|---|---|
| Median 1year stroke risk [IQ range] | 7.6 [5.1-12.0] | 8.3 [5.4-11.8] | 0.360 | 11.0 [8.2-15.2] | 6.77 [5.0-9.9] | <0.001 |
| Median 5 year stroke risk [IQ range] | 19.0 [12.2-29.4] | 21.0 [14.0-29.0] | 0.014 | 27.22 [20.8-36.1] | 17.3 [11.7-24.8] | <0.001 |
| Age, years (±SD) | 67.0 (±8.7) | 69.0 (±9.4) | <0.001 | 68.6 (±9.6) | 68.3 (±9.0) | 0.42 |
| Male gender | 71.9% (346/481) | 65.9% (762/1158) | 0.017 | 65.6 (363/553) | 68.7 (746/1086) | 0.21 |
| Median time since last event, days [IQ range] | 87 [34-150] | 45 [18-95] | <0.001 | NA | NA | |
| Presenting event | ||||||
| Monocular | 27.0% (130/481) | 16.7% (193/1159) | <0.001 | 17.7 (98/553) | 20.7 (225/1087) | 0.15 |
| TIA , single | 10.8% (52/481) | 36.8% (426/1159) | <0.001 | 26.6 (147/553) | 30.5 (331/1087) | 0.10 |
| TIA, multiple | 28.9% (139/481) | 15.4% (179/1159) | <0.001 | 28.8 (159/553) | 14.6 (159/1087) | <0.001 |
| Minor stroke | 7.3% (35/481) | 17.6% (203/1159) | <0.001 | 13.4 (74/553) | 15.1(164/1087) | 0.35 |
| Major stroke | 26.0% (125/481) | 13.5% (157/1159) | <0.001 | 13.6 (75/553) | 19.1 (208/1087) | 0.005 |
| Diabetes | 10.8% (52/481) | 17.9% (207/1159) | <0.001 | 17.9 (99/553) | 14.7 (160/1087) | 0.10 |
| Treated hypertension | 58.2% (280/481) | 59.2% (680/1149) | 0.717 | 56.5 (310/549) | 60.1 (650/1081) | 0.16 |
| Treated hypercholesterolaemia* | 17.9% (86/481) | 74.5% (828/1111) | <0.001 | 62.9 (329/523) | 54.7 (584/1068) | 0.002 |
| Previous myocardial infarction | 12.1% (58/481) | 9.6% (108/1121) | 0.145 | 12.2 (65/531) | 9.4 (101/1070) | 0.08 |
| Peripheral vascular disease | 17.5% (84/481) | 17.2% (194/1128) | 0.898 | 19.7 (105/534) | 16.1 (173/1074) | 0.08 |
Abbreviations: IQ, Interquartile; SD, Standard deviation; TIA, Transient ischemic attack.
As measured by prior statin use
Table 2 shows the odd ratios for the presence of individual plaque features in relation to 1-year and 5-year stroke risk analysed separately in each study. These were similar in the two studies with no significant heterogeneity found for any plaque characteristic. In the pooled analysis several statistically significant associations between plaque characteristics and stroke risk were observed. The presence of luminal thrombus, heavy macrophage staining, high micro-vessel density, overall plaque instability and a lower prevalence of fibrous content were significantly correlated with predicted stroke risk. The presence of large lipid-core was weakly correlated with stroke risk, whereas calcification and intra-plaque haemorrhage were not. Out of all non-overlapping histological plaque features, phenotyped separately in each study, none were significantly correlated with predicted stroke risk (table 3).
Table 2. Odds-ratios for the presence of individual plaque characteristics in the highest versus lowest quartile of stroke risk.
| Plaque characteristic | Oxford Plaque Study (n=481) | Athero-Express (n=1159) | Pooled Data (n=1640) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| 1 YEAR STROKE RISK | |||||||||
| Overall plaque instability | 1.24 | 0.74-2.06 | 0.41 | 1.52 | 1.07-2.15 | 0.02 | 1.42 | 1.07-1.90 | 0.02 |
| Thrombus | 1.47 | 0.85-2.52 | 0.17 | 1.37 | 0.98-1.92 | 0.06 | 1.40 | 1.05-1.86 | 0.02 |
| Heavy macrophage staining | 1.78 | 1.05-3.00 | 0.03 | 1.19 | 0.85-1.67 | 0.30 | 1.39 | 1.04-1.84 | 0.03 |
| High micro-vessel density | 1.43 | 0.86-2.38 | 0.17 | 1.49 | 0.92-2.41 | 0.11 | 1.46 | 1.03-2.07 | 0.03 |
| Large lipid core | 1.22 | 0.74-2.02 | 0.44 | 1.34 | 0.93-1.97 | 0.11 | 1.31 | 0.97-1.76 | 0.08 |
| Plaque haemorrhage | 1.39 | 0.83-2.33 | 0.22 | 1.06 | 0.71-1.59 | 0.78 | 1.18 | 0.85-1.62 | 0.32 |
| Fibrous plaque | 0.63 | 0.37-1.07 | 0.09 | 0.66 | 0.47-0.93 | 0.02 | 0.65 | 0.49-0.87 | 0.004 |
| Heavy calcification | 0.91 | 0.56-1.49 | 0.71 | 0.82 | 0.59-1.14 | 0.24 | 0.84 | 0.64-1.11 | 0.23 |
| 5 YEAR STROKE RISK | |||||||||
| Overall plaque instability | 1.29 | 0.78-2.12 | 0.32 | 1.47 | 1.03-2.09 | 0.03 | 1.40 | 1.05-1.87 | 0.02 |
| Thrombus | 1.44 | 0.85-2.45 | 0.17 | 1.41 | 1.00-1.98 | 0.05 | 1.42 | 1.11-1.89 | 0.02 |
| Heavy macrophage staining | 1.83 | 1.10-3.05 | 0.02 | 1.26 | 0.89-1.76 | 0.19 | 1.41 | 1.05-1.90 | 0.02 |
| High micro-vessel density | 1.42 | 0.87-2.33 | 0.17 | 1.57 | 0.96-2.56 | 0.07 | 1.49 | 1.05-2.11 | 0.03 |
| Large lipid core | 1.26 | 0.77-2.07 | 0.35 | 1.31 | 0.90-1.92 | 0.16 | 1.29 | 0.96-1.75 | 0.09 |
| Plaque haemorrhage | 1.40 | 0.84-2.32 | 0.19 | 1.02 | 0.67-1.53 | 0.94 | 1.15 | 0.84-1.59 | 0.38 |
| Fibrous plaque | 0.58 | 0.35-0.98 | 0.04 | 0.68 | 0.48-0.97 | 0.03 | 0.65 | 0.49-0.87 | 0.004 |
| Heavy calcification | 1.03 | 0.64-1.67 | 0.90 | 0.81 | 0.58-1.13 | 0.22 | 0.88 | 0.67-1.16 | 0.35 |
Abbreviations: OR, odds ratio; CI, confidence interval. Pooled data stratified by study cohort.
No significant heterogeneity was found between the study groups for any individual plaque features
Table 3. Odds-ratios for the presence of non-overlapping individual plaque characteristics in the highest versus lowest quartile of stroke risk.
| Plaque characteristic | Oxford Plaque Study (n=481) | Athero-Express (n=1159) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| 1 YEAR STROKE RISK | ||||||
| Plaque rupture | 1.33 | 0.81-2.20 | 0.26 | NA | NA | NA |
| Foam cells | 0.82 | 0.49-1.36 | 0.43 | NA | NA | NA |
| Plaque lymphocytes | 1.18 | 0.71-1.95 | 0.53 | NA | NA | NA |
| Thin Cap | 1.28 | 0.73-2.24 | 0.39 | NA | NA | NA |
| Cap inflammation | 0.82 | 0.44-1.52 | 0.52 | NA | NA | NA |
| Smooth muscle cells | NA | NA | NA | 0.70 | 0.48-1.02 | 0.06 |
| 5 YEAR STROKE RISK | ||||||
| Plaque rupture | 1.39 | 0.85-2.27 | 0.19 | NA | NA | NA |
| Foam cells | 0.81 | 0.49-1.33 | 0.40 | NA | NA | NA |
| Plaque lymphocytes | 1.21 | 0.74-1.99 | 0.44 | NA | NA | NA |
| Thin Cap | 1.17 | 0.68-2.02 | 0.57 | NA | NA | NA |
| Cap inflammation | 0.95 | 0.52-1.73 | 0.87 | NA | NA | NA |
| Smooth muscle cells | NA | NA | NA | 0.72 | 0.50-1.05 | 0.09 |
Abbreviation: NA, not available.
As there is current uncertainty as to whether statin therapy influences carotid plaque morphology30-32 and an affect could potentially bias our results, we performed the same analyses for patients on prior statin therapy (n=913) and those not so (n=678) (supplemental table II). Odd ratios for individual plaque features in these subgroups (in relation to stroke risk) were similar, except for plaque inflammation (heavy macrophage staining), which was less associated with predicted stroke risk when compared to non-users. The Oxford Plaque Study patients were largely non-statin users (18%), whereas Athero-express were mainly users (75%). However, for all other features analysed, prior statin therapy appeared to have minimal impact on their correlation with predicted stroke risk.
To investigate whether plaque stabilisation over time post event may also influence the association between plaque features and future stroke risk, we performed the same analyses on all patients who underwent CEA within 30 days of event (n=552) compared to those operated greater than 30 days from most recent event (n=1088). The odd ratios for the presence of most plaque characteristics in relation to stroke risk were substantially greater for the ≤ 30-day sub-group when compared to the > 30-day subgroup (table 4). In particular, the presence of plaque inflammation and large lipid core were highly correlated with predicted stroke risk in ≤ 30-day sub-group, but these relationships were lost in the > 30-day subgroup. In the > 30-day subgroup, plaques from patients with no prior statin use did reveal significant correlation between plaque inflammation and predicted stroke risk (OR 1.81, 95% CI 1.03-3.18, p=0.04) whereas those with prior statin use did not (0.91, 0.54-1.55, p=0.74). Patient characteristics, including age, gender, vascular risk factor profiles, and event types did not differ significantly between these two subgroups (table 1). Analyses were also performed for sub-groups separated by event type (monocular, TIA, and stroke events) with no significant differences found (data not shown.)
Table 4. Odds-ratios for the presence of individual plaque characteristics in the highest versus lowest quartile of 1-year stroke risk for the subgroups of patients operated ≤ and > 30 days from the last event.
| Plaque characteristic | Operated ≤ 30 Days from Event (n=553) | Operated > 30 Days from Event (n=1087) | P-Value for Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N (%) | OR | 95% CI | P-value | Total N (%) | OR | 95% CI | P-value | ||
|
|
|
|
|||||||
| Overall plaque instability | 366 (66.9) | 2.55 | 1.23-5.28 | 0.01 | 727 (67.2) | 1.18 | 0.81-1.72 | 0.39 | 0.08 |
| Large lipid core | 398 (72.2) | 2.80 | 1.32-5.94 | 0.007 | 776 (71.7) | 0.90 | 0.62-1.33 | 0.61 | 0.02 |
| High micro-vessel density | 101 (33.8) | 4.52 | 0.98-20.87 | 0.05 | 268 (32.0) | 1.47 | 0.95-2.29 | 0.09 | 0.05 |
| Fibrous plaque | 180 (32.6) | 0.43 | 0.21-0.89 | 0.02 | 347 (31.9) | 0.84 | 0.57-1.22 | 0.35 | 0.07 |
| Heavy macrophage staining | 316 (57.6) | 2.51 | 1.20-5.28 | 0.02 | 667 (61.9) | 1.31 | 0.90-1.91 | 0.16 | 0.09 |
| Heavy calcification | 259 (47.0) | 0.57 | 0.27-1.20 | 0.14 | 577 (53.1) | 1.03 | 0.71-1.49 | 0.87 | 0.15 |
| Plaque haemorrhage | 123 (22.3) | 0.99 | 0.40-2.43 | 0.98 | 279 (25.7) | 1.40 | 0.93-2.10 | 0.11 | 0.75 |
| Thrombus | 219 (39.7) | 1.50 | 0.71-3.17 | 0.30 | 433 (39.9) | 1.41 | 0.97-2.05 | 0.07 | 0.96 |
Abbreviations: N, number of cases; OR, odds ratio; CI, confidence interval. All data stratified by study cohort.
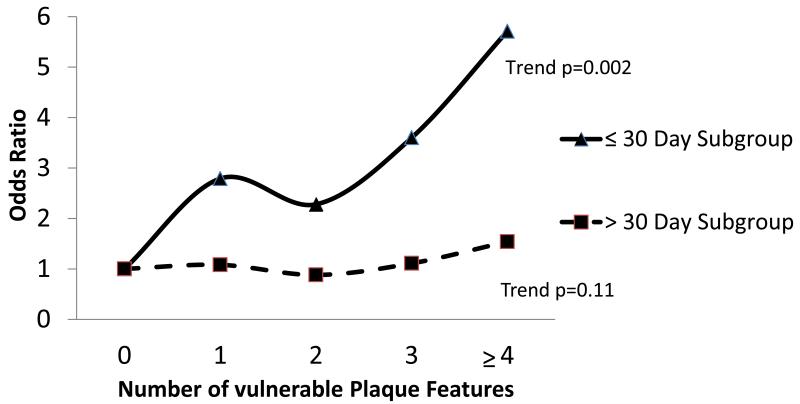
To evaluate the strength of association between overall carotid plaque vulnerability and predicted stroke risk, we performed regression analysis for the presence of multiple plaque features currently thought to represent plaque “vulnerability”. Odd ratios for the presence of 0 up to ≥4 plaque features increased from 1.0 to 5.7 in relation to 1-year predicted stroke risk for patients operated within 30 days of event (trend p=0.001) (table 5, figure 1). For the > 30 day subgroup, however, these ranged from 0.9 – 1.5 (trend: non-significant).
Table 5. Odd-ratios for the presence of multiple vulnerable plaque characteristics in relation to stroke risk (upper versus lower quartile) for patients operated ≤ 30 days and > 30 days of last event.
| CEA ≤ 30 DAYS FROM EVENT | 1 year stroke risk | 5 year stroke risk | ||||
|---|---|---|---|---|---|---|
| (n=553) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| 0 vulnerable plaque features | 1.00 | 1.00 | ||||
| 1 vulnerable plaque feature | 2.79 | 0.74-10.60 | 0.13 | 3.06 | 0.71-13.21 | 0.13 |
| 2 vulnerable plaque features | 2.28 | 0.75-6.89 | 0.15 | 2.27 | 0.72-7.18 | 0.16 |
| 3 vulnerable plaque features | 3.60 | 1.21-10.69 | 0.02 | 3.13 | 1.04-9.45 | 0.04 |
| ≥4 vulnerable plaque features | 5.71 | 1.80-18.13 | 0.003 | 4.31 | 1.39-13.35 | 0.01 |
| CEA > 30 DAYS FROM EVENT | 1 year stroke risk | 5 year stroke risk | ||||
|---|---|---|---|---|---|---|
| (n=1087) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| 0 vulnerable plaque features | 1.00 | 1.00 | ||||
| 1 vulnerable plaque feature | 1.08 | 0.53-2.21 | 0.84 | 1.16 | 0.57-2.36 | 0.69 |
| 2 vulnerable plaque features | 0.88 | 0.44-1.76 | 0.71 | 0.99 | 0.49-1.98 | 0.98 |
| 3 vulnerable plaque features | 1.11 | 0.58-2.12 | 0.76 | 1.19 | 0.62-2.27 | 0.60 |
| ≥4 vulnerable plaque features | 1.54 | 0.82-2.87 | 0.18 | 1.65 | 0.89-3.06 | 0.11 |
All data stratified by study cohort. Test for Interaction for ≥4 vulnerable plaque features by CEA timing: p=0.05
Figure 1.
The relationship between overall plaque vulnerability and future stroke risk is time-dependent: Odd-ratios for the presence of multiple vulnerable plaque characteristics in relation to stroke risk (upper versus lower quartile)
Discussion
In the largest-ever study of carotid plaque histology, we have shown that various histological carotid plaque features correlate with predicted stroke risk, including plaque thrombus, fibrous content, macrophage infiltration, high micro-vessel density, and overall plaque instability. This is in line with the classical view on associations between composition of the vulnerable plaque and the risk of ipsilateral cerebrovascular events.12,33-36 Several features, including plaque inflammation, large lipid core, and low fibrous content, appear to have a highly temporal association with future stroke risk, being correlated in plaques removed within 30 days of last event, and not so in those removed greater than 30 days of event. Other key features targeted by current imaging modalities, such as cap thickness (MRA), intra-plaque haemorrhage (Ultrasound, MRA), plaque rupture (MRA, Ultrasound) and calcification (CT Angiography (CTA), were not significantly associated with calculated stroke risk.
High resolution magnetic resonance imaging is currently the most reliable and reproducible modality for the evaluation of carotid plaque morphology.21,37-38 It provides greater accuracy and atherosclerotic definition when compared to Colour Doppler ultrasonography and CT angiography, without the drawbacks of user-dependency in ultrasound and both radiation and contrast loads in CTA. MRA also enables the acquirement and combination of multiple different contrast weightings to distinguish tissue composition within the arterial wall. This modality has been validated with histology and shown to accurately quantify several key carotid plaque characteristics, including lipid-rich necrotic core,39-42 intraplaque hemorrhage,41-43 calcification,21,39 and cap rupture.43-44
Intraplaque hemorrhage is a frequently studied plaque feature, since it has been described to be associated with plaque progression. Turc et al showed that intraplaque hemorrhage detected with MRI in symptomatic patients was associated with the degree of stenosis, the type of cerebrovascular event, and the time from ischaemic event. These parameters are also important risk factors for future ipsilateral stroke risk. However in our study, intraplaque hemorrhage was not significantly associated with future stroke risk. The results are consistent throughout the two studies, which suggests that once important risk factors for ipsilateral stroke risk are taken into account, intraplaque hemorrhage is probably less useful in predicting ipsilateral stroke risk. An explanation for this might be found in the greater understanding of the different types and stages of intraplaque hemorrhage. For example, it has been shown that organized plaque hemorrhages with newly formed microvessels are associated with vulnerable plaque characteristics. Newly formed microvessels contain newly formed endothelium, which is frequently insufficient, leading to micro-vascular leakage, and thereby potentially resulting in plaque hemorrhage and progression. 45-47 This association may not be valid for other types of intraplaque hemorrhage. We could not analyze subgroups of intraplaque hemorrhage, because these data were not available for both studies.
At present the clinical utility of carotid plaque imaging is uncertain and no studies to-date have found a non-invasively identified plaque feature that is able to risk stratify patients. Our results suggest that out of the plaque features that are currently able to be imaged reliably, only the presence of plaque thrombus and large lipid-core (when assessed within 30 days of event) correlate significantly with predicted stroke risk. When considering the recent progress made in attempts to image and quantify features such as inflammation, neovascularisation, and cap thickness, we have also shown that out of these features, histological plaque inflammation (and not isolated cap inflammation) and high micro-vessel density correlate significantly with increased predicted stroke risk. It was not possible to perform an adjusted multivariate analysis for individual plaque characteristics in relation to stroke risk, as the features are too mutually correlated for a standard multivariate model to run effectively.
The strength of association between carotid plaque morphology and future predicted stroke risk appears to be greatest when the plaque is characterised shortly after the last symptomatic event with no significant plaque feature associations being found in plaques removed greater than 30 days from event. This has several important implications. First, this highlights a temporal relationship between plaque morphology and future stroke risk. Second, as our stroke risk model is based on the presence of traditional vascular risk factors in conjunction with details of event timing, type, and degree of carotid artery stenosis, it can also be inferred that plaque composition is related to these factors in a highly temporal manner. The reason for this is likely to be plaque stabilization over time after an event, as previously described.10,12,32 The plaques stabilise gradually after a cerebrovascular event and therefore it is likely that the results of patients that underwent surgery within 30 days most accurately reflect the situation preceding any event. Third, asymptomatic carotid plaques have mostly stable morphology35 and when considering time to intervention, can be viewed in a similar light to symptomatic plaques that are removed at a delayed point in time from presenting event. Our results could therefore also suggest that there will be limited clinical utility in imaging asymptomatic plaque morphological features in an attempt to risk-stratify these patients. Due to the lack of a valid prediction tool for asymptomatic patients with carotid stenosis we could not perform subgroup analyses, to answer the question whether the presented results can be extrapolated to this patient group.
When focusing on the overall strength of association between plaque morphology and future stroke risk, we can conclude that the number “vulnerable” plaque features present increases as predicted stroke risk rises, and this relationship is very strong in plaques assessed within 30 days of last event, and completely diminished those assessed greater than 30 days from event. Of interest for future prognostic imaging studies, the presence of 4 or more vulnerable plaque features is highly correlated with increased predicted stroke risk in plaques assessed within 30 days of event.
The current study was the largest-ever of carotid plaque histology and although we believe our results to be valid, certain limitations need to be addressed. First, the Oxford Plaque study examined the entire specimen along the longitudinal axis with 3mm intervals, whereas the Athero-Express study examined the culprit lesion, adjacent segments being processed for protein extraction.
However, both studies have shown previously that differences in sampling and sectioning technique do not have a major impact on categorisation of plaque histology.9,28 Second, many plaques from the Oxford study were collected in the 1980s and 1990s, since when time from symptoms to surgery has been reduced and use of antiplatelet agents and statins has increased. The time from event to intervention was included in the stroke risk prediction model and will therefore have been adjusted for in our analyses. Third, the carotid stenosis risk prediction model was developed based on patients on best medical treatment within ECST, which was conducted in the pre-statin era. This might, therefore, lead to an overestimation of stroke-risk, as some strokes may have been prevented if patients were on statin therapy. However, in our sub-group analysis, we have confirmed minimal statin influence on the majority of individual plaque feature – stroke risk correlations. Prior aggressive antiplatelet treatment may potentially have had an impact on these correlations. Unfortunately, however, we do not have accurate data on single versus dual antiplatelet use prior to event for a large proportion of cases and so we were unable to add perform additional analyses.
Fourth, the analyses of patients operated within 30 days after the last event is a subgroup analysis and therefore hypothesis generating. Nonetheless, we feel that the subgroup analysis has sufficient statistical power as it encompassed 553 patients. Since early carotid endarterectomy within two weeks of indicative event is part of current best medical practice, our subgroup results are relevant for current stroke management and provide important messages for carotid plaque imaging and risk-stratification studies. Imaging of plaques, including MRI, is ideally performed shortly after the event. Our results also suggest a stronger association between plaque characteristics and stroke risk, if the plaque is analysed shortly after an event. In our population the median time between the last event and surgery was 87 and 45 days, for the 2 studies respectively. This time is decreasing strongly over time due to current guidelines stating that patients with significant symptomatic carotid stenosis should be operated within 14 days of the last event. Due to this trend, future analyses on plaque composition and ipsilateral stroke risk could therefore find stronger correlations. One must note, however, that the risk prediction model is validated for the calculation of future ipsilateral stroke risk for cases who are 7-180 days post-event. In order to calculate stroke risk in patients who are within 6 days of event, further calibration and validation of the model would be required.
Fifth, the performance of the validated risk prediction model against actual stoke risk in the external NASCET cohort produced a c-statistic of 0.67, 95% CI 0.63-0.72 (p<0.0001). Therefore, as with all models, the risk prediction model is not perfect and it is therefore feasible that the plaque features found not to be associated with predicted stroke risk in our study could be associated with actual stroke risk in vivo. Future prognostic studies should focus on the follow-up of asymptomatic patients and those suffering symptomatic events who are believed to be at low future stroke risk using predictive tools such as the model used in this study. These studies should be guided by our findings and will be ideally placed to confirm if plaque features that are identifiable on non-invasive imaging correlate with future stroke risk in vivo.
Summary
This study shows that specific “vulnerable” carotid plaque characteristics correlate with predicted stroke risk. Plaques removed within 30-days of last symptomatic event are most strongly correlated with predicted stroke risk whereas those removed greater than 30-days reveal no such relation. Although, it remains uncertain whether any individual plaque feature can predict stroke independently of age, sex and more easily measured traditional vascular risk factors, this study provides a basis for plaque imaging studies focused on stroke risk stratification.
Supplementary Material
Acknowledgements
We are grateful to the staff of the Departments of Vascular Surgery and Histopathology at the John Radcliffe Hospital, Oxford and University Medical Center Utrecht and St Antonius Hospital, Nieuwegein, The Netherlands for the provision of plaques for this study.
Sources of Funding
Dominic Howard and the Oxford Plaque study are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre Programme (Oxford). PMR has a Wellcome Trust Senior Investigator Award and an NIHR Senior Investigator Award. The views expressed are those of the authors and not necessarily those of the NIHR or UK Department of Health.
Footnotes
Disclosure:
None
References
- 1.Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Carotid endarterectomy--an evidence-based review: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2005;65:794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Gutnikov SA, Eliasziw M, Fox AJ, Taylor W, Mayberg MR, et al. Carotid Endarterectomy Trialists’ Collaboration Pooled analysis of individual patient data from randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–16. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ, Carotid Endarterectomy Trialists Collaboration Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 4.Bond R, Rerkasem K, Shearman CP, Rothwell PM. Time trends in the published risks of stroke and death due to endarterectomy for symptomatic carotid stenosis. Cerebrovascular Diseases. 2004;18:37–46. doi: 10.1159/000078606. [DOI] [PubMed] [Google Scholar]
- 5.Barnett HJM, Meldrum HE, Eliasziw M. The appropriate use of carotid endarterectomy. CMAJ. 2002;166:1169–79. [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell PM, Warlow CP, ECST Collaborators Prediction of benefit from carotid endarterectomy in individual patients: A risk-modelling study. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. From subgroups to individuals: general principles and the example of carotid endartectomy. Lancet. 2005;365:256–65. doi: 10.1016/S0140-6736(05)17746-0. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Gibson R, Warlow CP, European Carotid Surgery Trialists’ Collaborative Group Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. Stroke. 2000;31:615–621. doi: 10.1161/01.str.31.3.615. [DOI] [PubMed] [Google Scholar]
- 9.Hellings WE, Pasterkamp G, Verhoeven BA, De Kleijn DP, De Vries JP, Seldenrijk KA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. 2007;45:289–7. doi: 10.1016/j.jvs.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 10.Redgrave JN, Lovett JK, Rothwell PM. Histological features of symptomatic carotid plaques in relation to age and smoking: the oxford plaque study. Stroke. 2010;41:2288–94. doi: 10.1161/STROKEAHA.110.587006. [DOI] [PubMed] [Google Scholar]
- 11.van Lammeren GW, Reichmann BL, Moll FL, Bots ML, de Kleijn DP, de Vries JP, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke. 2011;42:2550–2555. doi: 10.1161/STROKEAHA.110.607382. [DOI] [PubMed] [Google Scholar]
- 12.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113:2320–8. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 13.Peeters W, Hellings WE, de Kleijn DP, de Vries JP, Moll FL, Vink A, et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol. 2009;29:128–33. doi: 10.1161/ATVBAHA.108.173658. [DOI] [PubMed] [Google Scholar]
- 14.Virmani R, Burke A, Farb A. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. Eur Heart J. 1998;19:678–680. [PubMed] [Google Scholar]
- 15.Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 17.Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–81. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 18.Lovett JK, Gallagher PJ, Hands LJ, Walton J, Rothwell PM. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation. 2004;110:2190–7. doi: 10.1161/01.CIR.0000144307.82502.32. [DOI] [PubMed] [Google Scholar]
- 19.Golledge J, Siew DA. Identifying the carotid high risk plaque: is it still a riddle wrapped up in an enigma? Eur J Vasc Endovasc Surg. 2008;35:2–8. doi: 10.1016/j.ejvs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, et al. Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study (aces): A prospective observational study. Lancet Neurol. 2010;9:663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underhill HR, Hatsukami TS, Fayad ZA, Fuster V, Yuan C. MRI of carotid atherosclerosis: clinical implications and future directions. Nat Rev Cardiol. 2010;7:165–73. doi: 10.1038/nrcardio.2009.246. [DOI] [PubMed] [Google Scholar]
- 22.Turc G, Oppenheim C, Naggara O, Eker OF, Calvet D, Lacour JC, et al. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: The hirisc study. Arterioscler Thromb Vasc Biol. 2012;32:492–499. doi: 10.1161/ATVBAHA.111.239335. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging. 2012;5:798–804. doi: 10.1016/j.jcmg.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Annals of neurology. 2013;73:774–84. doi: 10.1002/ana.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with mri--initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 26.Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, Davis JW, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–185. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 27.Saam T, Cai J, Ma L, Cai YQ, Ferguson MS, Polissar NL, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo mr imaging. Radiology. 2006;240:464–472. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovett JK, Gallagher PJ, Rothwell PM. Reproducibility of Histological Assessment of Carotid Plaque: Implications for Studies of Carotid Imaging. Cerebrovascular Diseases. 2004;18:117–23. doi: 10.1159/000079259. [DOI] [PubMed] [Google Scholar]
- 29.Verhoeven BA, Velema E, Schoneveld AH, de Vries JP, de Bruin P, Seldenrijk CA, et al. Athero-express: Differential atherosclerotic plaque expression of mrna and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J Epidemiol. 2004;19:1127–1133. doi: 10.1007/s10564-004-2304-6. [DOI] [PubMed] [Google Scholar]
- 30.Kunte H, Amberger N, Busch MA, Ruckert RI, Meiners S, Harms L. Markers of instability in high-risk carotid plaques are reduced by statins. J Vasc Surg. 2008;47:513–22. doi: 10.1016/j.jvs.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Makris GC, Lavida A, Nicolaides AN, Geroulakos G. The effect of statins on carotid plaque morphology: a LDL-associated action or one more pleiotropic effect of statins? Atherosclerosis. 2010;213:8–20. doi: 10.1016/j.atherosclerosis.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 32.Verhoeven BA, Moll FL, Koekkoek JA, van der Wal AC, de Kleijn DP, de Vries JP, et al. Statin treatment is not associated with consistent alterations in inflammatory status of carotid atherosclerotic plaques: a retrospective study in 378 patients undergoing carotid endarterectomy. Stroke. 2006;37:2054–60. doi: 10.1161/01.STR.0000231685.82795.e5. [DOI] [PubMed] [Google Scholar]
- 33.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 34.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part i. Circulation. 2003;108:1664–1772. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeven B, Hellings WE, Moll FL, de Vries JP, de Kleijn DP, de Bruin P, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg. 2005;42:1075–1081. doi: 10.1016/j.jvs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Howard DP, van Lammeren GW, Redgrave JN, Moll FL, de Vries JP, de Kleijn DP, et al. Histological features of carotid plaque in patients with ocular ischemia versus cerebral events. Stroke. 2013;44:734–739. doi: 10.1161/STROKEAHA.112.678672. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe Y, Nagayama M. MR plaque imaging of the carotid artery. Neuroradiology. 2010;52:253–74. doi: 10.1007/s00234-010-0663-z. [DOI] [PubMed] [Google Scholar]
- 38.den Hartog AG, Bovens SM, Koning W, Hendrikse J, Luijten PR, Moll FL, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg. 2013;45:7–21. doi: 10.1016/j.ejvs.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo mri. Arterioscler Thromb Vasc Biol. 2005;25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 40.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 41.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi RA, U-King-Im J, Graves MJ, Horsley J, Goddard M, Kirkpatrick PJ, et al. Multi-sequence in vivo mri can quantify fibrous cap and lipid core components in human carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2004;28:207–213. doi: 10.1016/j.ejvs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: A high-resolution mri study. Stroke. 2004;35:1079–1084. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 44.Saba L, Potters F, van der Lugt A, Mallarini G. Imaging of the fibrous cap in atherosclerotic carotid plaque. Cardiovasc Intervent Radiol. 2010;33:681–9. doi: 10.1007/s00270-010-9828-8. [DOI] [PubMed] [Google Scholar]
- 45.Derksen WJ, Peeters W, van Lammeren GW, Tersteeg C, de Vries JP, de Kleijn DP, et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: A large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis. 2011;218:369–377. doi: 10.1016/j.atherosclerosis.2011.07.104. [DOI] [PubMed] [Google Scholar]
- 46.van Lammeren GW, Pasterkamp G, de Vries JP, Bosch L, de Haan JJ, de Kleijn DP, et al. Platelets enter atherosclerotic plaque via intraplaque microvascular leakage and intraplaque hemorrhage: A histopathological study in carotid plaques. Atherosclerosis. 2012;222:355–359. doi: 10.1016/j.atherosclerosis.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;28:1517–27. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.