Abstract
The placenta is a highly vascularized organ that mediates fetal-maternal exchange during pregnancy and is thereby vital for the survival and growth of the developing embryo. In addition to this well-established role, the placenta was recently unveiled as a major fetal hematopoietic organ. The placenta is unique among other fetal hematopoietic organs as it is capable of both generating HSCs/progenitors de novo and establishing a major HSC pool in the conceptus while protecting HSCs from premature differentiation. The mouse placenta contains two distinct vascular regions that support hematopoiesis: the large vessels in the chorionic plate where HSCs/progenitors emerge and the labyrinth vasculature where nascent HSCs/progenitors colonize for expansion and possible functional maturation. Defining how this cytokine- and growth factor rich organ supports HSC generation, maturation and expansion may ultimately help to establish culture protocols for HSC expansion or de novo generation from pluripotent cells.
Keywords: Placenta, hematopoietic stem cell, niche, allantois, mesoderm, hemogenic endothelium
Introduction
Continuous blood cell production throughout the lifetime of an individual is ensured by hematopoietic stem cells (HSCs), which are rare bone marrow cells that possess extensive self-renewal capacity and ability to differentiate to all blood cell lineages. Due to the unique properties of HSCs, they can regenerate the entire hematopoietic system of a recipient upon transplantation and thereby provide a cure for inherited and acquired blood diseases. As such, HSCs are of substantial therapeutic interest. However, the limited availability of human leukocyte antigen (HLA) matched bone marrow donors and the relatively low yield of HSCs in more accessible sources, such as cord blood, limits the number of patients that can be treated. Therefore, a great need exists for improving culture methods that would facilitate HSC expansion, or de novo HSC generation from pluripotent cells such as human embryonic stem cells (ES) or induced pluripotent stem (iPS) cells. To succeed in these endeavors, major emphasis has been put on understanding HSC development and the microenvironmental niches that support these processes. As a result, a revised model for HSC development is emerging that includes a greater understanding of both the anatomical sites and cellular niches where HSCs develop as well as the cell of origin for HSCs.
HSCs are formed only within a limited time window during embryogenesis, after which the HSC pool is maintained by self-renewing cell divisions. Unlike post-natal hematopoiesis that is confined to the bone marrow, fetal hematopoiesis occurs in multiple different anatomical sites in a temporally defined manner. The utilization of multiple hematopoietic sites during fetal development facilitates both the rapid generation of the first differentiated blood cells that the embryo needs for survival and growth, as well as the establishment of a large pool of undifferentiated HSCs that are required for post-natal hematopoiesis. The first hematopoietic activity is observed in the extra-embryonic yolk sac, which generates large cohorts of uni- or oligolineage progenitors that are non-self-renewing and restricted to erythroid and myeloid fates, whereas the truly multipotential, self-renewing HSCs are generated later. These long-term reconstituting HSCs emerge in multiple anatomical sites, and once generated, convene in the fetal liver for expansion and differentiation prior to their colonization of the bone marrow. During this journey, the developing HSCs are exposed to distinct extracellular cues that promote their generation, expansion, and functional maturation to acquire the adult HSC phenotype and functional properties. Until recently, the aorta-gonad mesonephros (AGM) region and adjacent vitelline and umbilical arteries were considered to be the main source of HSCs during embryogenesis (Muller et al. 1994; Cumano et al. 1996; Medvinsky and Dzierzak 1996; Godin et al. 1999; de Bruijn et al. 2000). However, due to the low number of transplantable HSCs found in the AGM, it was questioned whether this scarce population could be the sole contributor of the substantial amount of HSCs found in the fetal liver. As the fetal liver is incapable of de novo generation of HSCs and hematopoietic progenitors, it was hypothesized that other embryonic sites such as the yolk sac, or yet unknown sites, may contribute to the seeding of the HSCs to the fetal liver (Kumaravelu et al. 2002). Studies in recent years have revealed that the placenta also harbors a major HSC pool, which may in part account for the “missing HSCs” from the equation. Notably, the placenta appears to be unique among the other fetal hematopoietic organs, as it not only has the capacity for de novo hematopoiesis but also accrues a large reservoir of HSCs and protects them from signals that promote immediate differentiation. The placenta is a highly vascularized organ that is rich in cytokines and growth factors that likely contribute to the hematopoietic microenvironment (Cross 2005). Hence, the discovery of the placenta as a major fetal HSC niche has opened new avenues of research focused on defining the unique microenvironmental cues that support HSC development, and offered access to larger numbers of developing HSCs from both mice and humans (Gekas et al. 2005; Ottersbach and Dzierzak 2005; Barcena et al. 2009a; Barcena et al. 2009b; Robin et al. 2009). Here, we review how the placenta was unveiled as a fetal hematopoietic organ and discuss its unique function as a specialized niche for HSC development.
Discovery of hematopoietic stem- and progenitor cell activity in the mouse placenta
The placenta has not traditionally been regarded as a hematopoietic organ, although the first report suggesting a possible role for the placenta in blood development was published already in 1961 (Till and McCulloch 1961). That study discovered that transplantation of mouse placental tissue into irradiated recipient mice leads to the formation of hematopoietic colonies in the spleen (CFU-S, colony forming unit spleen; Table1). Although it is now known that the hematopoietic cells that form CFU-S are not necessarily true long-term repopulating HSCs (LTR-HSCs), this early study evidenced that the placenta harbors a robust population of clonogenic hematopoietic cells with high proliferative potential. Another study published in 1977 provided compelling evidence that the placenta harbors HSCs, as placental tissue exhibited the capacity to rescue the anemic phenotype of W/Wv recipient mice upon transplantation (Dancis et al. 1977) (Table 1). Interestingly, separation of placenta from the embryonic circulation did not abolish hematopoietic reconstitution ability implying that accumulation of HSCs in the placenta was not dependent on the continuous supply of blood to the placenta. Notably, these authors had already previously reported the presence of immunologically competent fetal hematopoietic cells in the placenta similar to the spleen, as graft-versus-host disease ensued after transplantation of placenta from C57 mice into BALB/C mice (Dancis et al. 1962). Furthermore, another study showed that the placenta is an early reservoir for B cell precursors, as plaque-forming assays documented robust B-cell potential in the midgestation placenta prior to the fetal liver (Melchers 1979). In spite of these intriguing findings that hinted at an important role for the placenta in fetal hematopoiesis, further work on the placenta as a hematopoietic organ did not commence until decades later.
Table 1. Historical perspective to the discovery of HSCs in the placenta.
| Discovery | Methods | Authors | Year |
|---|---|---|---|
| Transplantable CFU-S10 were found in E11-12 mouse placenta |
Transplantation into lethally irradiated mice | Till and McCulloch | 1961 |
| Graft-versus-host disease upon transplantation of placental cells into allogeneic recipients suggest presence of immunologically competent hematopoietic cells. |
Transplantation into lethally irradiated allogeneic mice |
Dancis et al. | 1962 |
| Sustained rescue of anemia of W/Wv mice after transplantation of E15, but not E18-19, placenta cells, suggested transient presence of HSCs |
Transplantation into sub-lethally irradiated W/Wv mice |
Dancis et al. | 1977 |
| Plaque-forming B-cell precursors were found in the midgestation placenta, prior to the fetal liver |
In-vitro stroma co-culture assays | Melchers | 1979 |
| Multipotent hematopoietic cells, suggestive of HSCs, were found in the avian allantois |
Chick-to-quail grafting experiments | Caprioli et al. | 1998, 2001 |
| Multipotent hematopoietic progenitors of fetal origin were found in the mouse placenta (E9.0 onwards), prior to the fetal liver |
In vitro clonogenic assays using GFP+ fetal cells |
Alvarez-Silva et al. | 2003 |
| A large pool of transplantable HSCs were found in midgestation placenta |
Serial transplantations into lethally irradiated mice, in vitro clonogenic assays |
Gekas et al. | 2005 |
| HSCs were found in placental labyrinth | Localization of HS/PCs in Sca1GFP model, transplantation into lethally irradiated mice |
Ottersbach and Dzierzak |
2005 |
| Allantoic and chorionic mesoderm are capable of generating hematopoietic progenitors de novo |
Localization of hemogenic precursors in Runxl- LacZ embryos, in vitro stroma co-culture assays |
Zeigler et al. | 2006 |
| CD41+ hematopoietic cells are generated autonomously from precirculation allantois |
Allantois explant culture | Corbel et al. | 2007 |
| The placenta was shown to be capable of de novo generation of multipotent myelo-lymphoid hematopoietic progenitors independent of blood flow |
Localization of HS/PCs in Runx1-LacZ embryos, in vitro stroma co-culture assays using Ncx1−/− heartbeat mutant embryos |
Rhodes et al. | 2008 |
| The human placenta was shown to harbor myelo-lymphoid hematopoietic progenitors |
Colony-forming assay and stroma co-culture | Barcena et al. | 2009a |
| The human placenta was shown to harbor myelo-lymphoid hematopoietic progenitors throughout gestation |
Colony-forming assay and stroma co-culture | Barcena et al. | 2009b |
| The human placenta was shown to harbor HSCs that were engraftable to immunodeficient mice |
Transplantation of human placental tissues to NOD-SCID and Rag2−/−γC−/− mice |
Robin et al. | 2009 |
Interest in the placenta as a hematopoietic organ was revived after work by Dieterlen-Liévre, Jaffredo and colleagues showed that the avian allantois harbors multipotential hematopoietic precursors prior to the onset of circulation (Caprioli et al. 1998; Caprioli et al. 2001) (Table 1). The avian allantois is a mesodermal appendage derived from the primitive streak, and has both a similar developmental origin and function in nutrient and gas exchange as the placenta in eutherial mammals. This discovery that the avian allantois possesses de novo hematopoietic activity, again brought up the question whether the mammalian placenta is also capable of generating HSCs.
Studies by Dieterlen-Liévre and colleagues indeed revealed that the mouse placenta harbors a major pool of multipotential hematopoietic progenitors that are of fetal origin (Alvarez-Silva et al. 2003) (Table 1). To verify that the placental hematopoietic progenitors were not maternal cells that were circulating through the placenta, GFP-reporter transgenic male mice were bred with non-transgenic females, allowing distinction between fetal (GFP+) and maternal (GFP−) cells. Methylcellulose colony forming assays showed that the placenta harbors clonogenic progenitors by the 20sp (somite pair) stage (E9.0), shortly after the yolk sac (15sp) and the caudal half of the embryo (18sp), which contains the para-aortic splanchopleura (pSP) that gives rise to the AGM region (Table 1). In contrast, colony-forming units (CFUs) were not found in the fetal liver until later in development. Furthermore, the distribution of CFU types in the placenta and the fetal liver were different: the frequency of the multipotential, as compared to committed, unilineage, progenitors in the placenta was much higher than in the fetal liver, implying that the hematopoietic programs in the placenta and the fetal liver and the microenvironments that support these programs are different from one another (Alvarez-Silva et al. 2003).
However, progenitor activity does not necessarily correlate with the presence of true HSCs. As indicated before; lineage-restricted, transient progenitors develop during embryogenesis before the appearance of the first definitive HSCs. Therefore, it was critical to assess HSC activity in vivo by stringent transplantation assays. Two independent studies verified that the midgestation placenta harbors true adult-reconstituting HSCs that can generate all blood cell lineages and self-renew through serial transplantations (Gekas et al. 2005; Ottersbach and Dzierzak 2005) (Table 1). To verify fetal origin of hematopoietic cells, Ottersbach and Dzierzak used transgenic male mice expressing human beta-globin from a ubiquitous promoter or GFP from the Sca1 (Ly-6A) promoter to generate transgenic fetuses. In the other study, Gekas and colleagues bred embryos that were heterozygous for the two different alleles of the pan-hematopoietic marker CD45 (CD45.1 and CD45.2), distinguishing them from maternal cells that only expressed the CD45.2 allele. These studies showed that the placenta harbors adult reconstituting HSCs at the same developmental stage as the AGM (E10.5-11.0, 33-40sp). At that stage, no HSCs were found in circulating blood or the fetal liver rudiment, supporting the hypothesis that placental HSCs could at least in part be generated de novo in the placenta (Figure 1A). Hematopoietic reconstitution by placental HSCs was multilineage and sustained through serial transplantations, as shown by flow cytometry analysis of blood and bone marrow from primary and secondary recipients. Furthermore, functional analysis of prospectively isolated sub-fractions of placental cells revealed that at E12.5, placental HSCs and multipotential progenitors possess the classical CD34medc-kithi fetal HSC surface phenotype, similar to the fetal liver HSCs (Figure 2A). Although these studies were not able to unequivocally reveal the origin of placental HSCs, they were the first to describe the placenta as a hematopoietic organ that harbors classical HSCs with all the essential properties of long-term reconstituting HSCs.
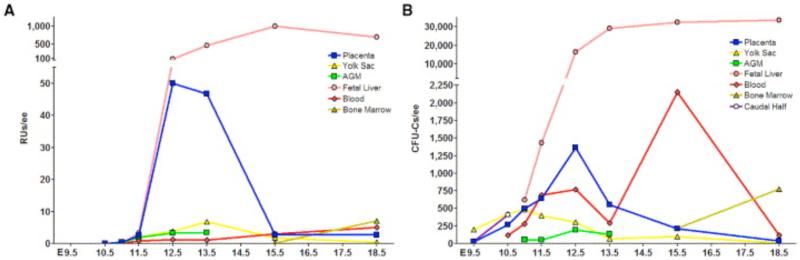
Figure 1. Kinetics of Long Term Reconstituting HSCs and Clonogenic Progenitors in the Embryo and Extraembryonic Hematopoietic Tissues.
(A)The graph depicts the number of HSCs with long term reconstitution capability per tissue during development. The midgestation placenta harbors a large pool of HSCs, which diminishes towards the end of gestation while the fetal liver HSC pool is expanding. (B) The diagram shows numbers of clonogenic progenitors (CFU-C) per embryo equivalent of tissue. Despite the major increase of HSCs in placenta between E11.5-12.5 (Fig. 1A), the number of CFU-Cs increases only modestly. In contrast, a substantial expansion of CFU-Cs can be noted in fetal liver throughout gestation. Note that presence of circulating progenitors in the bloodstream does not correlate with hematopoietic kinetics in placenta. RU, reconstituting unit; ee, embryo equivalent, CFU-C, colony forming unit-colony. Caudal half (E 10.5) includes the dorsal aorta and fetal liver rudiment. (Gekas et al. 2005)
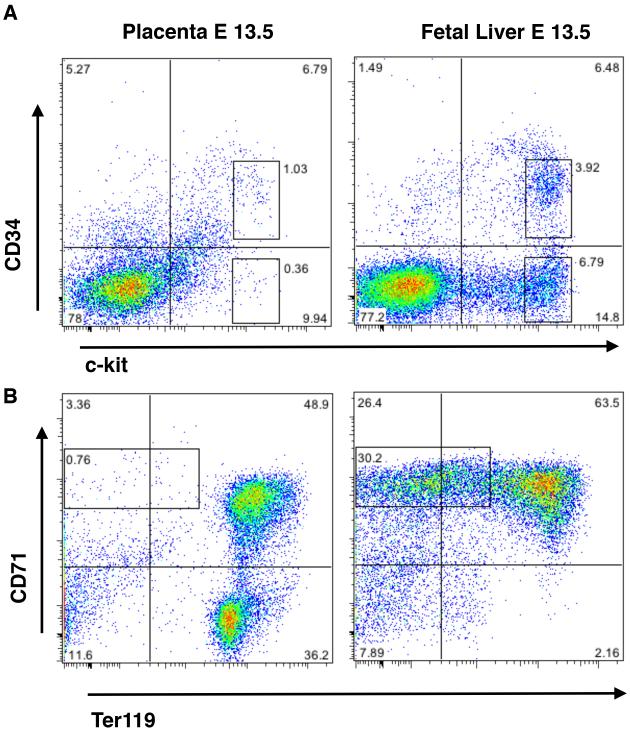
Figure 2. Distribution of Placental and Fetal Liver HSCs/multipotential Progenitors and Committed progenitors.
(A) Surface phenotype analysis of HSCs and progenitors in E13.5 placenta and fetal liver by flow cytometry for expression of CD34 and c-kit. The CD34+ckithi fraction (upper boxed region) harbors HSCs and multipotential progenitors, whereas the c-kit+ fraction (lower boxed region) harbors progenitors that are committed to erythroid and megakaryocytic lineages. The fetal liver contains a much higher propertion of the lineage-committed progenitors than the placenta. (B) Distribution of erythroid precursors in the placenta and the fetal liver documents an abundance of CD71+Ter117− proerythroblasts (boxed region), indicative of active definitive erythropoiesis, in the liver but not the placenta. The CD71+Ter119+ cells represent either primitive or definitive erythroblasts that contain a nucleus, whereas the Ter119+ cells represent enucleated fetal or maternal (placenta only) erythrocytes.
Kinetics of HSCs and progenitors between fetal hematopoietic organs
The potential impact of the discovery of the placenta as a novel source of HSCs was further strengthened by the finding that the number of HSCs in the placenta surpassed that in the AGM and yolk sac, the two known sites with de novo hematopoietic activity. In contrast to the AGM where the number of HSCs remains low, the HSC pool in the placenta increases drastically and, at its peak at E12.5-13.5, the placenta harbors 15-fold more HSCs than the AGM or the yolk sac (Gekas et al. 2005) (Figure 1A). The dramatic increase in HSCs occurring in the placenta between E11.5 and E12.5 (~20×) is comparable to that of the fetal liver (~28×), which is not seen in any other fetal hematopoietic organ. Interestingly, although the number of reconstituting units in the fetal liver was higher than in the placenta at E12.5, the average reconstitution level from placental HSCs vs. fetal liver HSCs was higher, implying that the placental HSCs at this stage are more potent (Gekas et al. 2005). After E13.5, the placental HSC pool decreases greatly and a concomitant increase of HSC number can be seen in the fetal liver; at this stage the average reconstitution ability of fetal liver HSCs is also higher. These data suggested that the placental HSCs might seed the fetal liver, which is directly downstream of the placenta in fetal circulation.
The analysis of clonogenic progenitor frequencies throughout gestation by Gekas and colleagues provided further insight into the distinct roles of the different hematopoietic tissues. Whereas CFU-C numbers increased in the fetal liver between E11.5 to E12.5 (~13×), CFU-C numbers in the placenta only increased two-fold between E11.5-12.5 (Figure 1B) despite the much greater increase in functional HSCs during this time. This provided functional evidence that the placental microenvironment is geared towards supporting the expansion and/or maturation of HSCs without promoting their concomitant differentiation into myeloerythroid progenitors (Figure 1B). In contrast, the fetal liver produces major populations of CD34−c-kithi erythro-megakaryocytic progenitor cells and proerythroblasts, indicative of ongoing definitive erythropoiesis (Figure 2A and B). In contrast, the only major erythroid populations in the midgestation placenta consist of primitive erythroblasts that are circulating through the placenta, as well as enucleated maternal red blood cells (Figure 2B). These data implicate inherent differences between fetal liver and placental HSC/progenitor populations and hematopoietic microenvironments, where the fetal liver, but not the placenta, promotes active erythropoiesis during midgestation. These results are in accordance with the study from Alvarez-Silva et al., which showed that the placenta specifically harbors multipotential, high-proliferative potential progenitors that can be serially replated whereas the fetal liver is rich in unilineage progenitors (Alvarez-Silva et al. 2003). These studies pinpointed the uniqueness of the placental microenvironment as a niche that facilitates the establishment of a large pool of adult-type HSCs that are not directed towards lineage differentiation during their residence in the placenta.
De novo hematopoiesis in the placenta
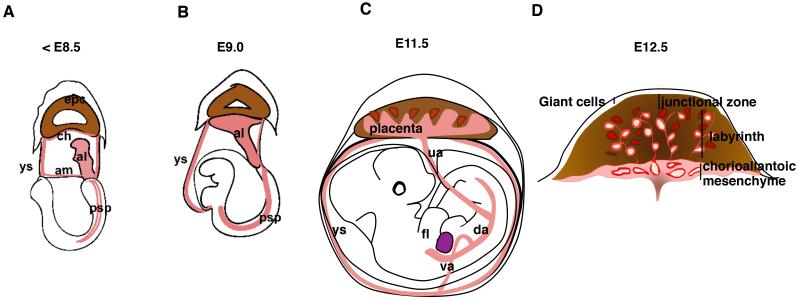
In order to define the origin of placental HSCs and the niches that support their development, it is essential to understand how the placenta develops. The placenta is an extra-embryonic organ that forms from trophoectoderm and mesodermal tissues. The two mesodermal components that form the placenta are the chorionic mesoderm, which lines the exocoelomic cavity, and the allantois, which grows from posterior primitive streak of the epiblast into the exocoelomic cavity (Figure 3A). The allantoic bud grows toward the ectoplacental cone and fuses with the chorionic mesoderm by E8.5 of mouse gestation (5sp stage), coinciding with the initiation of heartbeat and the onset of circulation in the embryo. The proximal part of the allantoic mesoderm becomes the umbilical cord that connects the placenta to the embryo, whereas the distal mesoderm that has fused with the chorionic mesoderm will both form the chorioallantoic mesenchyme in the chorionic plate and start to invade the trophoblast layer to establish the fetal vascular compartment of the placental labyrinth (Figure 3B, C, D). After midgestation, two regions of fetal vasculature can be distinguished in the placenta: the chorioallantoic vessels and the labyrinth vessels.
Figure 3. Development of the Mouse Placenta.
(A) At E7.5-8.25 the allantois (red) has formed from mesodermal precursors from the primitive streak, and is growing towards the ectoplacental cone (brown). (B) Fusion of the allantois with the chorionic mesoderm occurs at E8.5, concomitant with the onset of heartbeat. Subsequently chorioallantoic mesoderm interdigitates with the trophoblasts and the placental vasculature starts to form. (C) By E11.5 large vessels that connect to the umbilical cord have formed in the chorioallantoic mesenchyme, and the feto-placental circulation is fully established. The placenta labyrinth is still developing and is therefore an active site of vasculogenesis/angiogenesis. (D) A E12.5 cross-section of the placenta displays the different regions of the placenta, namely the chorioallantoic mesenchyme including the large vessels of the placenta (in red) and the placenta labyrinth, which is a unique region including trophoblast-lined maternal blood spaces (red spaces surrounded by brown trophoblasts) and fetal vessels lined by fetal endothelium (red vessels with lumens). al, allantois; ch, chorion; am, amnion; epc, ectoplacental cone; ys, yolk sac; psp, para-aortic splanchnopleura; da, dorsal aorta; ua, umbilical artery; va, vitelline artery; fl, fetal liver.
The chorioallantoic vasculature is comprised of large vessels that are surrounded by mesenchyme and directly connect to the placenta to the dorsal aorta and fetal liver through the umbilical cord vessels (Figure 3C, D). This region of the placenta is primarily of mesodermal origin, although during midgestation it harbors unique tubular structures derived from ectoplacental endoderm (Figure 4). Later during development, these endodermal cells form a cavity between chorioallantoic mesenchyme and the labyrinth known as the Crypt of Duval (Duval 1891; Ogura et al. 1998). The labyrinth is composed of an intricate fetal vascular network that is intertwined with the trophoblast-lined maternal blood spaces (Figure 3D). This region directly mediates fetal-maternal exchange of gases, waste products and nutrients. (Figure 3D). Next to the labyrinth layer is the spongiotrophoblast layer, or junctional zone, which provides both structural support for the placenta and a gateway for the maternal spiral arteries that bring the maternal blood to the placenta. Giant cells, which are large, multinucleated trophoblasts, form a thin layer between the spongiotrophoblasts and the maternal decidua. Hence, most of the placental tissue is of fetal origin, except for the maternal blood cells that circulate in the trophoblast spaces.
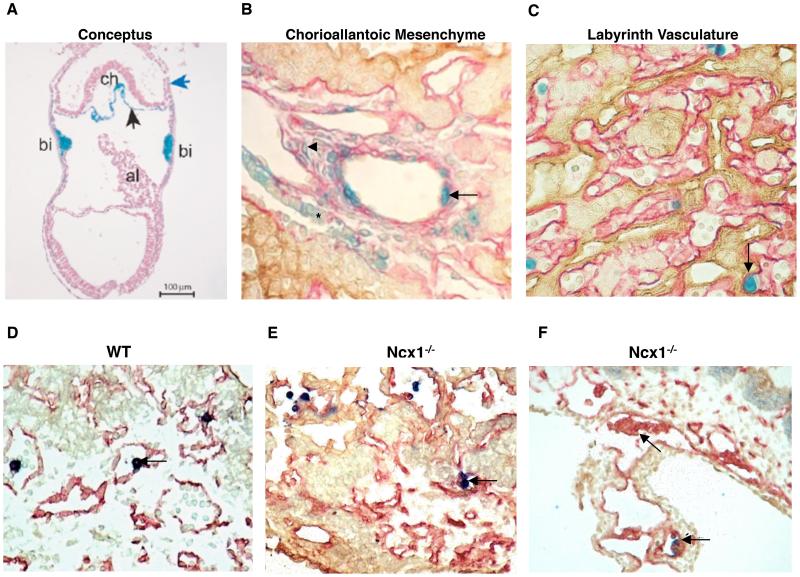
Figure 4. Localization of Developing HSCs in in the Placenta.
(A) A cross-section of a precirculation Runx1-LacZ conceptus documenting Runx1 expression in the chorionic mesoderm (black arrow) and the blood-islands of the yolk sac. The blue arrow denotes the ectoplacental cone. Image is a courtesy of Dr. Nancy Speck. (B) The large vessels of the chorioallantoic mesenchyme harbor LacZ+ cells within the wall of the vessel (arrow) at E11.5, the time when HSCs emerge. The mesenchyme contains two distinct populations of LacZ+ cells: oblong-shaped cells that straddle within the stromal cells (arrowhead), and cuboidal LacZ+/cytokeratin+ cells derived from ectoplacental endoderm that have an organized structure (asterisk). (C) LacZ+ definitive hematopoietic stem/progenitor cells localize to the fetal labyrinth vasculature. (A, B, C) Runx1-LacZ is in blue. (B,C) Laminin (pink) marks mesodermal tissues while cytokeratin (brown) marks trophoblasts and ectoplacental endoderm in the placenta. Bi, blood island; al, allantois. D) A wild-type placenta harboring CD41+ hematopoietic cells in the placental vasculature (arrow). E) CD41+ hematopoietic cells emerge in the, vasculature in Ncx1−/− placenta in the absence of circulation (arrows). (F) Ncx1−/− placentas harbor clusters of putative hemogenic endothelial intermediates that protrude into the vascular lumen (arrows). (D, E, F) CD41 (blue) marks hematopoietic cells, whereas CD31 (red) identifies endothelium. Cytokeratin (brown) marks the trophoblasts.
As all blood is derived from mesoderm, the chorionic and allantoic mesoderm are the candidate tissues of HSC origin in the placenta. To define whether the allantoic and/or chorionic mesoderm have a capacity for de novo hematopoiesis, these mesodermal tissues were isolated prior to chorioallantoic fusion and circulation and assayed for hematopoietic progenitor potential. Earlier studies had shown that the pre-circulation allantois does not harbor hematopoietic progenitors that form colonies in standard methylcellulose progenitor assays (Downs et al. 1998; Palis et al. 1999). Therefore, to assess whether the placenta contains the precursors that can generate hematopoietic stem/progenitor cells, hematopoietic potential had to be assessed by stimulating the cells from the tissues by in vitro co-culture on OP-9 stroma followed by methylcellulose culture, or by using an explant culture where hemogenic precursors remain in contact with their neighboring cells (Zeigler et al. 2006; Corbel et al. 2007)(Table 1). These studies revealed that both the chorionic and allantoic mesoderm are capable of generating multipotential myeloerythroid colonies, suggesting that the mesodermal tissues destined to become the placenta have innate potential to generate multipotential hematopoietic precursors. Furthermore, an earlier study in mice had shown that an ectopically transplanted murine allantois can contribute to the dorsal aorta and surrounding tissues (Downs and Harmann 1997). Although hematopoietic potential of the transplanted allantoic mesoderm was not addressed in this study, these findings imply a similar developmental potential of the allantoic mesoderm as the lateral plate mesoderm in the embryo proper.
Verification of the origin of HSCs in the more developed embryo and placenta has been hindered by the fact that definitive HSCs do not emerge until a few days after the onset of heartbeat, after which blood cells are circulating freely in the conceptus, Once circulation is established, the origin of HSCs and progenitors found within a tissue is difficult to prove. This issue was overcome using Ncx1 knockout embryos that lack heartbeat and circulation (Rhodes et al. 2008). These embryos are defective for the sodium calcium exchange pump 1 (Ncx1), which abolishes the initiation of heartbeat and thus prevents circulation (Koushik et al. 2001; Lux et al. 2007). As Ncx1−/− embryos die by E10.5, it is not possible to functionally verify HSC potential by in vivo transplantation assays due to the immaturity of nascent HSCs, which precludes engraftment and reconstitution of adult bone marrow. Nevertheless, these studies showed that placental tissue, alike AGM and yolk sac, from E9.5 Ncx1−/− embryos, has robust multilineage myelo-lymphoid hematopoietic potential when cultured on supportive stroma (Rhodes et al. 2008). Specifically, the ability to generate myeloerythroid as well as B- and T-lymphoid cells indicates that placental hematopoietic cells have the differentiation potential characteristic of HSCs that is distinct from earlier, transient progenitor populations in the yolk sac, which solely generate myeloerythroid progeny. Taken together these studies implied that the placenta can initiate multilineage hematopoiesis de novo.
Placental hematopoietic niches
To understand what regions of the placenta support hematopoiesis, localization of developing hematopoietic stem- and progenitor cells in the placenta was mapped using markers that are expressed in nascent HS/PCs. To that end, Runx1-LacZ reporter embryos have been used to pinpoint the putative HSC precursors and the specific cellular niches in the conceptus where HSCs may initially be specified. The transcription factor Runx1 is essential for the onset of definitive hematopoiesis; lack of Runx1 abolishes HSC formation leading to embryonic lethality by E12.5 (Wang et al. 1996). Runx1 is expressed in all HSCs throughout ontogeny and therefore its expression indicates putative sites of definitive hematopoiesis. In the AGM, Runx1 expression is observed both in the endothelium and subendothelial mesenchyme on the ventral side of the dorsal aorta where HSCs emerge (North et al. 1999). The Runx1 locus is also active in the homozygous Runx1-LacZ null embryos, which are unable to generate HSCs, but display LacZ expression in precursors that are attempting to generate HSCs. Interestingly, Runx1 expression can first be observed in the conceptus at E7.5 at blood islands in the yolk sac, the chorionic mesoderm and parts of the allantoic mesoderm (Figure 4A) (Zeigler et al. 2006). By E9.5-10.5, the large chorioallantoic vessels in the placenta harbor Runx1-LacZ+ cells that are integrated within the wall of the vessel lumen, highly reminiscent of the LacZ+ cells found within the ventral wall of the dorsal aorta (Rhodes et al. 2008) (Figure 4B). As LacZ+ cells are found in the large vessels of the chorioallantoic mesenchyme in both the heterozygote Runx1LacZ/+ and homozygote Runx1LacZ/LacZ, it was suggested that these vessels are a site of HSC origin. In comparison, as round LacZ+ definitive hematopoietic cells localized to the fetal labyrinth vessels in placentas of Runx1LacZ/+ embryos, but not Runx1LacZ/LacZ knockout embryos, it was suggested that the placental labyrinth vasculature might be a niche that becomes colonized by HSCs generated in the placenta and other sources (Figure 4C) (Table 1). Furthermore, many of the round LacZ+ cells within the labyrinth vessels were mitotically active and formed small clusters, prompting the hypothesis that the placental labyrinth vasculature is a site for HSC expansion (Rhodes et al. 2008). In summary, these data suggested that the large vessels in the chorioallantoic mesenchyme may generate HSCs, similar to the large dorsal aorta and adjacent large vessels in the AGM region, while the small fetal labyrinth vessels of the placenta provide a niche in which HSCs may expand and mature. Indeed, many Runx1-LacZ+ cells in the chorioallantoic and labyrinthine blood vessels co-expressed the embryonic HSC/progenitor marker CD41 (Rhodes et al. 2008). However, in addition to being expressed in the nascent HSCs/progenitors, Runx1 is also expressed in non-hematopoietic cells. In the dorsal aorta, Runx1-LacZ cells are not only found in endothelial cells, but in the mesenchyme underneath. In the placenta, oblong-shaped LacZ+ cells fill the chorioallantoic mesenchyme and may in part represent precursors for primitive macrophages that are found in the stroma (Rhodes et al. 2008). In addition, the Crypts of Duval, which are derived from ectoplacental endoderm, also were LacZ-positive (Rhodes et al. 2008) (Figure 4B). Due to the observed expression of Runx1 in other cells than definitive HSCs/progenitors, hematopoietic specific markers had to be used to verify the hematopoietic nature of the cells. Indeed, expression of the hematopoietic specific marker CD41 was observed in cells attached to the luminal side of the large vessels in the the placenta in both control and circulation-deficient Ncx1−/− embryos, implying that the placental vasculature is capable for de novo hematopoiesis (Table 1, Figure 4D, E).
Hemogenic endothelium as a source of HSCs
Definition of the precursor from which HSCs are formed has been controversial. One of the two major theories of HSC origin proposes that HSCs are generated from hemogenic endothelium, whereas an alternative theory posits that HSCs are specified in the subendothelial mesenchyme and migrate through the endothelial wall into circulation. Common ancestry for HSCs and endothelium has been proposed for a long time based on the fact that a large number of transcription factors and surface markers are shared between both lineages. All blood and endothelial cells originally develop from Flk1+ mesodermal precursor; however, Flk1+ becomes sharply downregulated in HSCs after their emergence. In contrast, the pan-endothelial marker CD31 (PECAM) remains expressed in HSCs throughout their ontogeny (Baumann et al. 2004; Taoudi and Medvinsky 2007), while CD34 is expressed in endothelium as well as fetal HSCs, but is downregulated in the quiescent adult LTR-HSCs during early post-natal life (Ogawa et al. 2001).VE-Cadherin, another vascular marker that is a component of the endothelial cell adherens junctions, is expressed in HSCs from their emergence through midgestation (Kim et al. 2005; Taoudi et al. 2005). Nevertheless, the shared marker expression alone does not prove their common origin. Recent studies have verified the hypothesis that HSCs are generated from the vasculature. First, lineage tracing experiments using VE-Cadherin as a marker revealed hematopoietic potential in the endothelial cells in the AGM, the placenta and the yolk sac, but not the fetal liver (Zovein et al. 2008). Furthermore, conditional deletion of Runx1 in VE-Cadherin expressing cells led to a drastic reduction of hematopoietic activity throughout the conceptus, including the AGM and the placenta (Chen et al. 2009). As VE-Cadherin is expressed in the endothelium and not in subendothelial mesenchymal cells, these results confirmed that HSCs emerge from the endothelium. The capacity of the endothelium to generate hematopoietic cells was visualized by time-lapse experiments (Eilken et al. 2009). In that study, individual ES-cell derived endothelial sheet colonies were monitored over many days and shown to lose endothelial morphology, marker expression and functional properties, while up-regulating hematopoietic markers CD41 and CD45 and acquiring hematopoietic progenitor morphology. Furthermore, our recent studies show that sorted endothelial cells from the placenta, similar to the AGM and the yolk sac, give rise to hematopoietic progenitors when co-cultured on supportive stroma (Rhodes, Van Handel et al. unpublished data). Studies on the circulation-deficient Ncx1−/− embryos further suggested that hematopoietic stem/progenitor cells develop from endothelium, as the blood vessels of these placentas harbored unique clusters of cells containing putative hemogenic endothelial intermediates that were protruding into the lumen (Figure 4F). All together, these studies present compelling evidence that definitive hematopoietic stem- and progenitor cells arise from an endothelial precursor, It still remains to be defined how hemogenic endothelium is established, and what makes it distinct from other non-hemogenic arterial endothelium. The study by Zovein et al. suggests that unique precursor cells in the mesenchyme underlying the dorsal aorta and other hemogenic arteries could be the precursors that give rise to hemogenic endothelium (Zovein et al. 2008). Defining these precursors that give rise to hemogenic endothelium and definitive HSCs will be required to understand the regulatory mechanisms that drive the fate decisions required for generating functional HSCs de novo.
Conservation of the placenta or analogous vascular organs as sites of hematopoiesis across species
The establishment of the hematopoietic system during embryogenesis is a highly conserved process between vertebrate species such as mouse, human and avian. In all organisms named above, HSCs emerge from the dorsal aorta in the embryo proper and other major arterial vasculature, and the same transcription factors and signaling pathways appear to direct this process (Martinez-Agosto et al. 2007). Although the placenta is unique to eutherian mammals, structures with similar mesodermal origin are also found in other species (Figure 5).
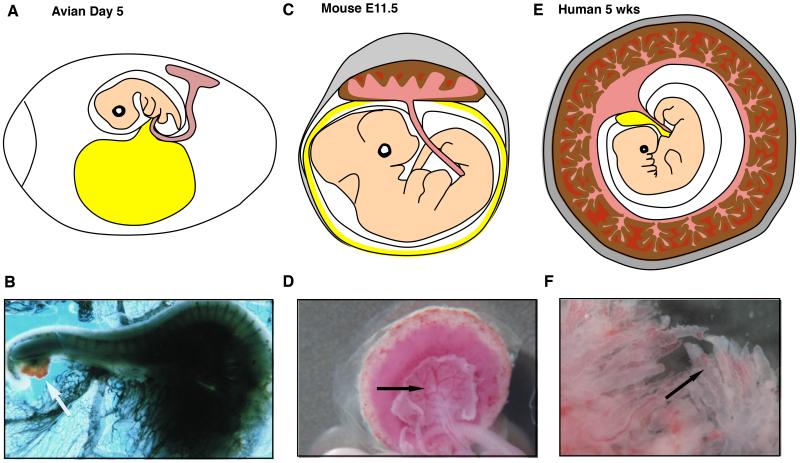
Figure 5. Allantoic Mesoderm and the Placenta as a Source of Hematopoietic Stem- and Progenitor Cells Across Vertebrate Species.
(A, B) In avian embryos, hematopoietic potential was observed in the allantois (B, white arrow, (Caprioli et al. 1998). (C, D) In mice, the allantois forms the mesodermal components of the placenta, including the chorioallantoic mesenchyme at its large vessels (D, black arrow) and the vascular labyrinth. These regions have the ability to generate and expand hematopoietic stem cells, respectively. (E, F) The human placenta is formed as the allantoic mesoderm invades the trophoblast-derived primary villi and forms the fetal vasculature and mesenchyme. The chorionic plate vessels and the vasculature in the villi (F, black arrow) are putative hematopoietic niches. Light red, allantois and its derivatives; brown, trophoblast; dark red, maternal blood: yellow, yolk sac.
As noted earlier, the avian allantois is a mesodermal appendage with comparable developmental origin as the mammalian allantois, and it harbors multipotential hematopoietic cells that possess bone marrow reconstitution ability (Caprioli et al. 1998; Caprioli et al. 2001). Hematopoietic potential of the avian allantois was demonstrated by a xenograft assay where the allantois of a quail embryo was isolated before the initiation of circulation to exclude cells derived from other tissues, and grafted into a chick embryo. This approach revealed that the cells derived from the quail allantois could contribute to the bone marrow of a post-natal chick, indicative of HSC activity. In addition, the presence of both hematopoietic and endothelial cells from quail origin in the chick host suggested that these cells have originated from the hemangioblast, a common progenitor of blood and endothelium, in the allantois (Figure 5A, B).
It has also been suggested that in zebrafish the vascular plexus termed the caudal hematopoietic tissue (CHT) or posterior blood island (PBI), which supports de novo generation, expansion and maturation of hematopoietic cells, may have similar functions in hematopoiesis as the mammalian placenta or the fetal liver (Burns and Zon 2006; Murayama et al. 2006). Indeed, the CHT/PBI is directly downstream of the dorsal aorta in the embryonic circulatory route, similar to the placenta in mammals. It is unknown, however, whether this vascular plexus also has exchange functions as the placenta.
Recent studies have shown that the human placenta functions also as a hematopoietic organ throughout gestation (Barcena et al. 2009a; Barcena et al. 2009b; Robin et al. 2009). Similar to the mouse placenta, the human placenta contains two main vascular structures. The chorionic plate in human is analogous to the chorioallantoic mesenchyme in mice that harbor the large blood vessels that connect to the fetus, and the villi in human placenta are analogous to the mouse placental labyrinth, which form the site of fetal-maternal exchange (Figure 5 C, D, E, F). The human placenta forms as mesoderm from the chorionic plate invades the primary villi that are comprised of trophoblast, thus giving rise to the mesodermal core that will provide the mesenchyme and vasculature of the villi. Fetal-maternal exchange takes place via the interaction between trophoblasts that sheathe the villi and maternal blood in that bathe the blood lacunae. The villous ultra-structure differentiates the human placenta from the labyrinthine mouse placenta at the macroscopic level (Fig. 5B, D); however, the placentas are remarkably similar at the cellular and molecular level (Georgiades et al. 2002) and thereby it is plausible that similar niche cells coordinate hematopoiesis in the placenta in both species. In support of this, Barcena et al. showed that the human placenta harbors progenitors with myeloerythroid potential throughout gestation and with lymphoid potential from the second trimester to birth (Barcena et al. 2009a: Barcena et al. 2009b). Importantly, the authors demonstrate an enrichment of phenotypic hematopoietic progenitors early in gestation (5-8 weeks) as compared to later gestational ages, suggesting a similar peak in hematopoietic potential in both mouse and human placentas at analogous developmental stages. Extending these findings, Robin et al. transplanted cells isolated from human placenta throughout gestation into immunocompromised mice. Analysis of the recipients via PCR and flow cytometry revealed the presence of engraftable human cells capable of producing low-level hematopoietic reconstitution as early as the first trimester and until term (Robin et al. 2009). Additionally, data from our lab indicates that the human placenta may also have the capacity to generate various hematopoietic progenitor types de novo, before circulating blood cells have arrived to the placenta (Van Handel et. al, unpublished data). Together, these findings nominate the human placenta, like the mouse placenta, as an important hematopoietic organ that may potentially generate and expand HSCs/progenitors and thus provide an accessible tissue source for studying the development of human HSCs, and the niche cells that support this process.
Conclusions and future perspectives
Proper development and function of the placenta is essential for supporting healthy pregnancy. However, its vital functions are not only limited to its requirement in mediating fetal-maternal exchange, but also include a novel role as a fetal HSC niche. The uniqueness of the placenta as a hematopoietic organ relies on the findings that it is capable of de novo hematopoiesis, similar to the AGM and the yolk sac, but that it also provides a true hematopoietic niche that allows the establishment of the first major HSC pool in the conceptus. Although not formally proven, the establishment of the large pool of HSCs in the placenta is likely due to the combination of at least four processes: de novo HSC generation in the chorioallantoic vasculature of the placenta; colonization of HSCs from the umbilical artery; the AGM and possibly yolk sac, expansion of HSCs in the placental vascular labyrinth and functional maturation of functionally immature “pre-HSCs” into HSCs that can engraft in standard transplantation assays. Remarkably, when HSCs reside in the placenta, they are not directed towards differentiation. These data imply that the placental microenvironment has unique properties that can support both HSC emergence and maintenance/expansion. It has been known for a long time that the placenta is rich in growth factors and cytokines that are secreted by various placental cells such as the endothelium, stromal cells, ectoplacental endoderm/Crypts of Duval, trophoblast and others. Indeed, the placenta is the largest vascular bed at this stage of development, and endothelial cells have been implicated as an important niche cell for HSCs. Furthermore, Robin et al. recently documented the presence of phenotypic pericytes surrounding vessels in the human placenta that support the ex vivo expansion of hematopoietic stem- and progenitor cells (Robin et al. 2009). While these, and other putative niche cell types are shared with other hematopoietic organs, the trophoblasts are unique to the placenta. Their importance in supporting hematopoiesis may not be restricted to being a source of vasculogenic/angiogenic factors that are essential for forming proper placental vasculature, but may also be due to other functions that relate to placenta’s role in facilitating feto-maternal exchange. Therefore it is plausible that defects in the trophoblasts and placental vasculature may not only affect hematopoiesis in the placenta, but also in the embryo proper. On the other hand, the question has been raised how the placenta could initiate de novo hematopoiesis without the proximity of gut endoderm found in the AGM, which polarizes the dorsal aorta and induces hematopoiesis specifically on the ventral side of the aorta. Interestingly, the cells that form the Crypts of Duval are composed of ectoplacental endoderm, and secrete signaling molecules such as Ihh (Indian Hedgehog) that are important for hematopoietic specification from mesoderm. As they are in close proximity to the large placental vessels where HSCs emerge during midgestation, it is possible that the Crypts of Duval provide local signals required for initiating hematopoiesis in the placenta (Ogura et al. 1998; Rhodes et al. 2008).
Functional and molecular analysis of the putative niche cells in the placenta and defining the cues that dictate distinct stages of HSC development from HSC specification, emergence, and maturation through expansion will be an important task for the future. Ultimately, defining the microenvironmental cues dictating HSC development may have a major impact on clinical therapeutic applications that depend on recreating the fetal hematopoietic niches for in vitro expansion of HSCs from cord blood, or de novo generation of HSCs from pluripotent cells.
References
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130(22):5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- Barcena A, Kapidzic M, Muench MO, Gormley M, Scott MA, Weier JF, Ferlatte C, Fisher SJ. The human placenta is a hematopoietic organ during the embryonic and fetal periods of development. Developmental biology. 2009a;327(1):24–33. doi: 10.1016/j.ydbio.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barcena A, Muench MO, Kapidzic M, Fisher SJ. A new role for the human placenta as a hematopoietic site throughout gestation. Reprod Sci. 2009b;16(2):178–187. doi: 10.1177/1933719108327621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104(4):1010–1016. doi: 10.1182/blood-2004-03-0989. [DOI] [PubMed] [Google Scholar]
- Burns CE, Zon LI. Homing sweet homing: odyssey of hematopoietic stem cells. Immunity. 2006;25(6):859–862. doi: 10.1016/j.immuni.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lievre F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci U S A. 1998;95(4):1641–1646. doi: 10.1073/pnas.95.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli A, Minko K, Drevon C, Eichmann A, Dieterlen-Lievre F, Jaffredo T. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Developmental biology. 2001;238(1):64–78. doi: 10.1006/dbio.2001.0362. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009 doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel C, Salaun J, Belo-Diabangouaya P, Dieterlen-Lievre F. Hematopoietic potential of the pre-fusion allantois. Developmental biology. 2007;301(2):478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice--a review. Placenta. 2005;26(Suppl A):S3–9. doi: 10.1016/j.placenta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86(6):907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- Dancis J, Jansen V, Brown GF, Gorstein F, Balis ME. Treatment of hypoplastic anemia in mice with placental transplants. Blood. 1977;50(4):663–670. [PubMed] [Google Scholar]
- Dancis J, Samuels BD, Douglas GW. Immunological competence of placenta. Science. 1962;136:382–383. doi: 10.1126/science.136.3514.382. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19(11):2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs KM, Gifford S, Blahnik M, Gardner RL. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development. 1998;125(22):4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- Downs KM, Harmann C. Developmental potency of the murine allantois. Development. 1997;124(14):2769–2780. doi: 10.1242/dev.124.14.2769. [DOI] [PubMed] [Google Scholar]
- Duval M. Le placenta des rongeurs. J Anat Physiol. 1891 [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23(1):3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J Exp Med. 1999;190(1):43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106(3):903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. Faseb J. 2001;15(7):1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development (Cambridge, England) 2002;129(21):4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging prior to E10 in the mouse embryo are products of the yolk sac. Blood. 2007 doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21(23):3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Melchers F. Murine embryonic B lymphocyte development in the placenta. Nature. 1979;277(5693):219–221. doi: 10.1038/277219a0. [DOI] [PubMed] [Google Scholar]
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25(6):963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Tajima F, Ito T, Sato T, Laver JH, Deguchi T. CD34 expression by murine hematopoietic stem cells. Developmental changes and kinetic alterations. Ann N Y Acad Sci. 2001;938:139–145. doi: 10.1111/j.1749-6632.2001.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Takakura N, Yoshida H, Nishikawa SI. Essential role of platelet-derived growth factor receptor alpha in the development of the intraplacental yolk sac/sinus of Duval in mouse placenta. Biology of reproduction. 1998;58(1):65–72. doi: 10.1095/biolreprod58.1.65. [DOI] [PubMed] [Google Scholar]
- Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8(3):377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin C, Bollerot K, Mendes S, Haak E, Crisan M, Cerisoli F, Lauw I, Kaimakis P, Jorna R, Vermeulen M, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5(4):385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104(22):9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132(18):4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133(21):4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]