Abstract
Background
Serotonin type 3 receptor (5-HT3R) antagonists are potentially useful therapeutic agents for diarrhea-predominant irritable bowel syndrome (IBS-D).
Aim
To identify biomarkers predicting effectiveness of the 5-HT3R antagonist (ramosetron) in IBS-D.
Methods
IBS-D Japanese subjects received 2.5 or 5μg of ramosetron once daily for 4 weeks. Colonic mucosal S100A and tryptophan hydroxylase (TPH) mRNA expression levels were measured before treatment. Genomic DNA was extracted from blood and polymorphisms of TPH1 and TPH2 were analyzed.
Key Results
Forty-two patients (27 men and 15 women, mean age 42 years) with IBS-D were included for analysis. Improvement of IBS symptoms was seen in 26 (61.9%). Baseline S100A10 (p=0.02) and TPH1 (p=0.02) expression were significantly higher in the ramosetron responders than in the non-responders. The frequencies of the TPH1 rs4537731 G allele in linkage disequilibrium with the TPH1 rs7130929 T allele (11.5% vs. 50%, p=0.003; OR 12 95%C.I. 2.1-69) along with TPH1 rs211105 C allele (3.8% vs. 43.8%, p=0.0003; OR 19 95%C.I. 2.1-181) were significantly lower in the responders than in the non-responders. The mean scores of diarrhea at baseline were significantly higher (5.2 vs 3.7, p=0.005) in patients with TPH1 rs211105 T/T than those with the G allele.
Conclusion & Inferences
TPH1 gene polymorphisms and S100A10 expression, which correlate with 5-HT signaling were associated with ramosetron effectiveness in IBS-D, and may possibly lead to prospective identification of the resistance to treatment.
Introduction
Serotonin [5-hydroxytryptamine (5-HT)] is an important neurotransmitter and paracrine signaling molecule in both the central nervous system and the gut regulating gastrointestinal (GI) motility, sensation and secretion. 1 5-HT is synthesized by the rate-limiting enzyme tryptophan hydroxylase (TPH) catalyzing the oxygenation of tryptophan,2, 3 which exists as two isoforms: TPH1 is expressed in the peripheral organs, especially enterochromaffin (EC) cells in the gut, while TPH2 is primarily expressed in the central nervous system (CNS) and peripheral serotonergic neurons. 4, 5 The 5-HT transporter (SERT, SLC6A4) is thought to play a critical role in the rapid re-uptake of 5-HT into presynaptic nerve terminals or epithelial cells of the GI mucosa thereby regulating the intensity and duration of serotonergic signaling.6-8 However, EC cell counts, 5-HT content, and mRNA expression of TPH1 and SLC6A4 in the colonic mucosa of irritable bowel syndrome (IBS) patients have been reported inconsistently. 9, 10
Both TPH gene variants have been evaluated intensively in psychiatric or behavioral disorders whose underlying pathophysiology is related to 5-HT. 11-14 The TPH1 promoter variant (11:g.18047335T>C, rs4537731), intron 3 variant (11:g.18033757T>G, rs211105), and intron 7 (11:g.18026269G>T, rs1800532) have been extensively studied with respect to psychiatric disorders. 15, 16 With respect to IBS, Jun S et al. 17 previously reported possible associations between two TPH1 gene single nucleotide polymorphisms (SNPs), rs4537731 and rs211105, and daily reporting of GI symptoms including diarrhea, bloating, and loose stools. The group also tested the correlation between a TPH2 gene promoter SNP (12:g.71938143G>T, rs4570625) and stool characteristics in European American women with IBS. Based on transcriptional regulatory studies in mice,18 a functional promoter variant was identified (rs7130929) in the TPH1 promoter in linkage disequilibrium with the rs4537731 SNP reported by Jun et al. 8, 17 The rs7130929 SNP variant forms a bona fide transcription factor binding site that along with the rs4537731 SNP generate a functional haplotype.8
5-HT transporter gene-linked polymorphic region (5-HTTLPR) consists of a 44 base pair (bp) insertion/deletion into the 5′ flanking promoter region of the gene creating long (l) and short (s) allelic variants, respectively. The variants of 5-HTTLPR impact the function of SLC6A4, and the s allele may result in the decrease of promoter activity reducing reuptake of 5-HT and thereby increasing 5-HT in the colonic mucosa. 19, 20 5-HTTLPR l/s or s/s genotype has been reported to occur with greater frequency in diarrhea predominant (IBS-D) than in controls, 21 whereas no association has also been reported. 22, 23, 24
Recent findings support a role for mucosal 5-HT in mediating visceral pain and hypersensitivity through 5-HT3R, and release of 5-HT from the colonic mucosa that correlates with the severity of abdominal pain/discomfort in patients with IBS. 25 Serotonin type 3 receptor (5-HT3R) antagonists have potential as a useful therapeutic agent for IBS-D. 23, 26, 27 Ramosetron hydrochloride (ramosetron), a tetrahydrobenzimidazole derivative, is one of 5-HT3R antagonists, which has potential as a useful treatment for patients with IBS-D. 28, 29 In a Japanese phase II trial including 418 patients with IBS-D, the monthly responder rate was significantly higher (43% vs 27%) in the ramosetron group compared to the placebo group. However, the significant efficacy of ramosetron compared to placebo at the final point was confirmed only in men but not in women, and the placebo effects were stronger and the incidence of drug-related adverse events was higher in women compared to men. 29 Therefore, ramosetron was approved only for men in Japan, although alosetron is indicated only for women with severe, chronic IBS-D in USA.
There are few studies investigating specific clinical parameters for predicting the effect of 5-HT3R antagonists in patients with IBS-D 23, 30 and no study for the ramosetoron. Camilleri et al. 23 previously demonstrated that 5-HTTLPR is associated with colonic transit response to alosetron in IBS-D patients. However, subsequent reports concerning the association of 5-HTTLPR with response to 5-HT3R antagonists have not yet been reported. Moreover, there are no published data using TPH polymorphisms to predict the effect of 5-HT3R antagonists.
Inflammatory and epithelial cells express S100A8 (also named calgranulin A; myeloid-related protein 8, MRP8) and S100A9 (calgranulin B; MRP14). These two myeloid cell proteins can also be induced in epithelial cells under inflammatory conditions. 31, 32 S100A8/A9 and calprotectin (the hetero-complex formed by non-covalent association of S100A8 and S100A9) are potential markers of gut inflammation and are also thought to be involved in the IBD pathogenesis. 33-36 S100A10 (also known as p11) co-localizes with the 5-HT1B receptor (5-HT1BR), and S100A10 expression is reduced in brain tissue of depressed patients but is increased in rodent brains by antidepressants.37 Stimulation of S100A10 results in relaxation of the gastric fundus and a delay stomach emptying. 38 The overexpression of S100A10 mRNA levels in the colonic mucosa of IBS patients has been previously reported. 24, 39
We conducted a pilot study to identify genetic variants that might predict the treatment response to ramosetron in patients with IBS-D and correlate with the colonic mucosal expression of 5-HTTLPR and TPH mRNAs.
Methods
This was a prospective interventional study of Japanese patients with IBS-D. The Kawasaki Medical School Ethical Committee approved the study. Written informed consent was obtained from each subject. The clinical trial has been register at UMIN Clinical Trials Registry (UMIN-CTR) as “Pathologic and therapeutic evaluation of IBS and ulcerative colitis (Trial ID: UMIN000004128).”
Subjects
Rome III criteria were used to subtype IBS patients as IBS-D. IBS-D patients were subsequently enrolled, while subjects with other IBS subtypes were excluded. 40 Subjects with a self-reported organic GI disorder (peptic ulcer disease, inflammatory bowel disease, malignancy, gallbladder disorder, pancreatitis, or liver disease), previous surgery of the GI tract were also excluded. All patients underwent colonoscopy to rule out other organic colon diseases. All new outpatients with IBS-D who met inclusion criteria and agreed attendance to the study were enrolled during the study registration period between February 2010 and January 2013. The enrolled patients received 5 μg of ramosetron once daily for 4 weeks, and the subjects were allowed to reduce the dose depending on monitored stool frequency and form to avoid severe constipation and ischemic colitis. They were prohibited from changing or adding other medications including antidiarrheal drugs. Patients taking ramosetron at the first visit and patients with constipation-predominant (IBS-C), a mixture of both diarrhea and constipation (IBS-M), and un-subtyped IBS were excluded.
Biopsy samples
Experienced endoscopists performed the colonoscopies. Two specimens from each sample site, rectum and sigmoid, were taken using endoscopic forceps (FB240U Olympus, Tokyo, Japan). The biopsy samples were immediately frozen with liquid nitrogen and stored at −80°C until use.
Laser-captured Microdissection (LCM)
The frozen samples obtained at endoscopy were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek U.S.A., Inc., Torrance, CA, US) and cut into serial 8 μm sections. Before microdissection, up to 8 sections from each block were mounted on slides and were stained using HistGene LCM Frozen Section Staining Kit (Arcturus Bioscience, Mountain View, CA, US). Colonic epithelium was isolated from the cryostat sections using a Leica LMD 7000 (Leica Microsystems, Wetzlar, Germany).
RNA extraction and quantitative polymerase chain reaction
RNA extraction and Quantitative mRNA analysis of S100A8, S100A9, A100A10, and TPH1 mRNA expression by reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) was performed as previously reported. 18 β-actin was used as an endogenous reference.
Genotyping
Genomic DNA was extracted from 200 μL of EDTA blood using FavoPrep Blood Genomic DNA Mini kits (FAVORGEN, Taiwan). To determine the SLC6A4, TPH1 and TPH2 polymorphisms, PCR reactions, PCR-restriction fragment length polymorphism (RFLP), or direct sequencing were performed. Based on previously reported association studies,17, 24 we selected 2 SNPs in SLC6A4, 6 SNPs in the TPH1 gene, and one SNP in the TPH2 gene for genotyping. The primers and restriction enzymes used to determine the polymorphisms are shown in supplemental Table 1. The samples for direct sequencing were run on an Applied Biosystems 3130xl Genetic Analyzer (Applied Biosystems) according to the manufacturer's recommendations.
Patients reported assessment
During the treatment phase, patients recorded their daily IBS symptoms (severity of abdominal discomfort and/or pain, stool form, stool frequency, bowel urgency and the feeling of incomplete bowel movement) on paper diary cards. The severity of abdominal discomfort and/or pain was assessed on a 5-point scale (0: None, 1: Mild, 2: Moderate, 3: Severe, 4: Intolerable) and stool form was scored on a 7-point ordinal scale according to the Bristol Stool Form Scale. A responder was defined as a patient who reported a decrease by one point of scores of abdominal discomfort and/or pain together with improvement of the score of stool form in daily IBS symptoms for at least 2 weeks of the 4-week treatment. 29
Questionnaire
Clinical symptoms were also assessed by the GI symptom rating scale (GSRS) and self-rating depression scale (SDS) before and at the end of the 4-week ramosetron treatment period. SDS is a 20-item self-report questionnaire, and the items are scored on a Likert scale ranging from 1 to 4. The score of patients with depression ranged between 50 and 69. A score of 70 and above indicates severe depression. 41, 42 The severity of GI symptoms was assessed by the GI Symptom Rating Scale (GSRS). The scale depends on how uncomfortable the symptom has been during the previous week. It is a validated self-administered questionnaire including 15 questions on a scale of 1 to 7 and a higher score indicates more discomfort. 42, 43 Combination scores can assess symptoms of reflux, abdominal pain, indigestion, diarrhea and constipation.
Analyses
Values are expressed as the mean ± SD or the median with a 25%-75% range, whichever was appropriate depending on whether the data were normally distributed. Mantel-Haenszel Chi square analysis and the unpaired t test were performed to measure differences in demographic and clinical characteristics. Statistical analyses for significant differences between the two groups were performed using the unpaired t test for GSRS and SDS scores and using the nonparametric Mann-Whitney U test for mRNA levels. Differences in genotype frequencies between the two groups and Hardy-Weinberg equilibrium of allele frequencies at individual loci were assessed using the Chi-square test or the Fisher's exact probability test by comparing the observed and expected genotype frequencies. The odds ratio (OR) and 95% confidence interval (CI) were obtained by Mantel-Haenszel Chi square analysis and stepwise regression analysis. A two-sided p value of less than 0.05 was considered statistically significant. All statistical computations were performed using SPSS (version 11.0 for Windows, SPSS Inc, Chicago, IL).
Results
A total of 72 patients were candidates for the study. Thirteen patients with IBS mixed type, 5 with constipation-predominant, 2 with possible UC and 2 diagnosed with collagenous colitis did not meet the inclusion criteria and were excluded. Eight patients finally refused the study and informed consents were not obtained. The 42 remaining patients included in the final analysis (27 men and 15 women, mean age 42 years) were designated as IBS-D by the ROME III criteria.
The demographic and clinical data are shown in Table 1. The frequencies of concomitant medicines were not significantly different between the responder group and the non-responder group. The overall response rate was 61.9% (26 / 42); 59.2% (16/27) for men and 66.7% (10/15) for women. In the responder group, 13 patients reduced the dose and one patient temporarily discontinued the drug due to complete relief from diarrhea and abdominal pain. Nine responders reduced the dose due to the adverse events, such as abdominal discomfort (abdominal distention and upper abdominal pain) and constipation, and the adverse events disappeared. IBS symptoms improved after a reduction in the dose. In the non-responder group, abdominal discomfort (7 patients), constipation (1 patient) and headache (1 patient) were reported., one patient reduced the dose, and 10 patients discontinued treatment due to abdominal discomfort (7 patients), worsened IBS symptoms (2 patients), and headache (one patient). However IBS symptoms in those patients did not improve. There was no serious adverse event such as severe constipation or ischemic colitis in either the responder or non-responder group.
Table 1.
Clinical characteristics of ramosetron responders and non-responders
| Total n=42 | Non-responders n=16 | Responders n=26 | p | |
|---|---|---|---|---|
| Age mean (SD) | 42 (16) | 43 (15) | 42 (17) | 0.80 |
| Gender men (%) | 27 (67.5) | 11 (68.8) | 16 (61.5) | *0.64 |
| Concomitant medicationa | 20 | 8 | 12 | 1.0 |
| Calcium Polycarbophil | 12 | 5 | 7 | 1.0 |
| Mepenzolate bromide | 4 | 2 | 2 | 0.63 |
| Bifidobacterium | 3 | 0 | 3 | 0.28 |
| Antidepressant | 3 | 2 | 1 | 0.55 |
| Ramosetron dose | ||||
| 5μg | 17 | 5 | 12 | |
| 5μg to 2.5μg | 14 | 1 | 13 | |
| Discontinuation | 11 | 10 | 1 | |
| Adverse event | ||||
| Abdominal discomfort | 9 | 7 | 2 | |
| Constipation | 8 | 1 | 7 | |
| Headache | 1 | 1 | 0 |
Concomitant medication for IBS symptoms
p Values, by unpaired t test or by chi square analyses
Questionnaire
Baseline scores for diarrhea tended to be higher (5.3 vs. 4.5, p=0.06) in the ramosetron responder group than in the non-responder group, and the other GSRS scores and SDS scores were not significantly different between the two groups (Table 2).
Table 2.
Comparison of GSRS and SDS scores for ramosetron responders and non-responders
| Non-responder n=16 | Responder n=26 | p values | |
|---|---|---|---|
| GSRS scores | |||
| Reflux | 1.9 (1.0) | 2.3 (1.4) | 0.31 |
| Abdominal pain | 2.3 (1.0) | 2.3 (1.0) | 0.94 |
| Indigestion | 2.4 (1.4) | 2.5 (1.2) | 0.78 |
| Diarrhea | 4.5 (1.4) | 5.3 (1.2) | 0.06 |
| Constipation | 2.4 (1.0) | 2.6 (1.0) | 0.73 |
| Total score | 3.4 (1.0) | 3.8 (0.9) | 0.28 |
| SDS scores | 43 (7) | 43(9) | 0.84 |
p Values: by unpaired t test
Expression of S100A and TPH1 mRNA at baseline
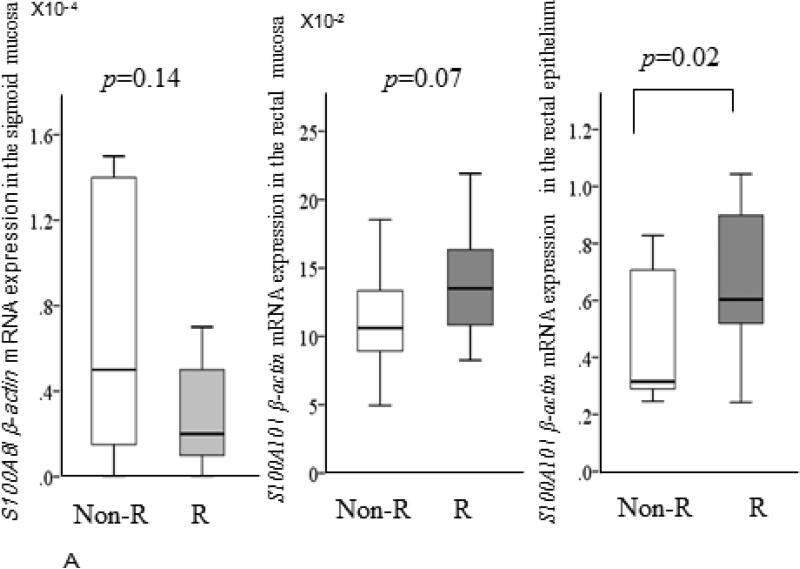
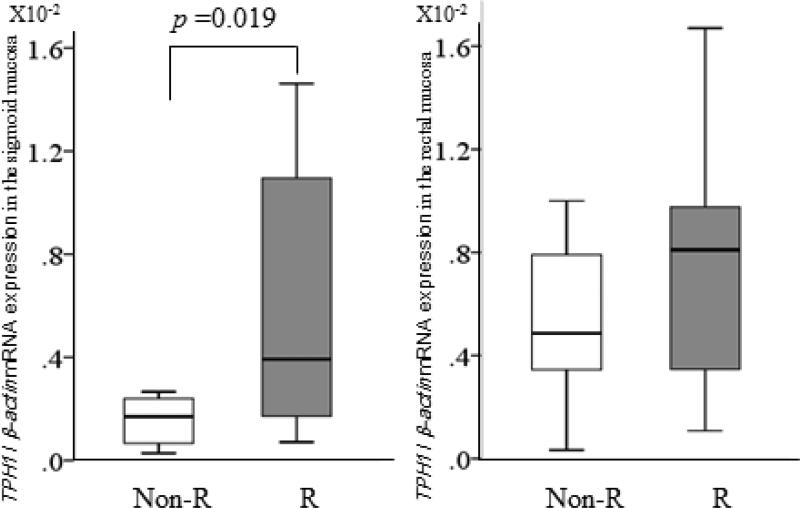
Baseline median S100A8 expression levels in the sigmoid (0.2 X10−4 vs 0.5 X10−4, p=0.14) and rectal (0.3 X10−4 vs 0.6 X10−4, p=0.23) mucosa of the responders tended to be lower than in the non-responders. However both baseline S100A8 and S100A9 expression levels in the biopsy samples including the LCM samples taken from both the rectum and sigmoid were not significantly different between the two groups (Figure 1A). Median S100A10 expression levels in the rectal mucosa (13.5X10−2 vs 10.6 X10−2, p=0.07) and the rectal epithelium (6.0 X10−1 vs 3.2 X10−1, p=0.02) of the responders were higher compared to the non-responders (Figure 1B), but not in the sigmoid. However, median TPH1 expression levels in the sigmoid colon of the responders were significantly higher (3.9 X10−3 vs 1.7 X10−3, p=0.02) compared to the non-responders (Figure 2), but not in the rectum nor in the epithelium.
Figure1.
Comparisons of baseline relative expressions of S100A 8, S100 A9, and S100A 10 mRNA between the ramosetron non-responders group (light bars) and the responders group (dark bars). Horizontal bar= median; Box=25th-75th interquartile range; Vertical lines= range of values. The cycle passing threshold (Ct) was recorded for each mRNA, and β-actin was used as the endogenous control for data normalization. p Values were calculated using the nonparametric Mann-Whitney U test between the two groups.
Figure 2.
Comparisons of baseline relative expressions of TPH 1 mRNA between the ramosetron non-responders group (light bars) and the responders group (dark bars). Horizontal bar= median; Box=25th-75th interquartile range; Vertical lines= range of values. The cycle passing threshold (Ct) was recorded for each mRNA, and β-actin was used as the endogenous control for data normalization. p Values were calculated using the nonparametric Mann-Whitney U test between the two groups.
SLC6A4 and TPH genotypes
All 42 subjects were successfully genotyped and the genotypes and allele frequencies are shown in Table 3. SLC6A4 genotypes were not significantly different between the two groups.
Table 3.
Comparison of genotype frequencies between ramosetron responders and non-responders
| Allele frequencies pa for HWE | Genotype | Ramosetron Non-responders n=16 | Ramosetron responders n=26 | p values | |
|---|---|---|---|---|---|
| SLC6A4 5-HTTLPR del(s)/ins(l) | l=0.18, s=0.82 p=0.38 |
ll/ls/ss ss (%) |
0/8/8 50 |
0/7/19 73.1 |
0.13 |
| SLC6A4 intron 2 VNTR | 10rep=0.12 12rep=0.88 p =0.69 |
10/12 & 12/12rep | 2/14 | 8/18 | 0.27 |
| TPH1 rs4537731 −1066 T > C | T=0.87, C=0.13 p =0.93 |
TT/TC/CC C allele % |
8/7/1 50 |
24/2/0 11.5 |
0.003 |
| TPH1 rs7130929 −347 C > A | C=0.87, A=0.13 p =0.93 |
CC/CA/AA A allele % |
8/7/1 50 |
24/2/0 11.5 |
0.003 |
| TPH1 rs684302 7465 C > T | C=0.42, T=0.68 p =0.34 |
CC/CT/TT C allele % |
2/7/7 56.2 |
3/10/13 50 |
0.69 |
| TPH1 rs211105 12517 T > G | T=0.89, G=0.11 p =0.58 |
TT/GT/GG G allele % |
9/6/1 43.8 |
25/1/0 3.8 |
0.003 |
| TPH1 rs1800532 218 G>T | G=0.33, T=0.67 p =0.66 |
GG/GT/TT T allele % |
3/6/7 56.2 |
13/10/3 50 |
0.69 |
| TPH1 rs1799913 779 G > T | G=0.32, T=0.68, p =0.90 | GG/GT/TT G allele % |
2/7/7 56.2 |
13/10/3 50 |
0.69 |
| TPH2 rs4570625 −709 G > T | G=0.49 T=0.51 p =0.18 |
GG/GT/TT G allele % |
3/11/2 87.5 |
4/16/6 76.9 |
0.69 |
p Values by the chi-square test
Hardy-Weinberg equilibrium (HWE) of allele frequencies at individual loci was assessed by comparing the observed and expected genotype frequencies.
As reported by Grasberger et al. 8, we also found that the two proximal promoter TPH1 SNPs at -1066 (11:g.18047335T>C, rs4537731) and -347(11:g.18046616C>A, rs7130929) were in complete linkage disequilibrium. The frequencies of the TPH1 rs4537731 minor C allele (11.5% vs. 50%, p=0.003; OR 12 95%C.I. 2.1-69) and TPH1 rs211105 minor G allele (3.8% vs. 43.8%, p=0.003; OR 19 95%C.I. 2.1-181) were significantly lower in the responders than in the non-responders. Those SNPs were significantly associated with ramosetron resistance, and the others including the TPH2 SNP were not associated with resistance (Table 3). The TPH1 rs211105 minor G allele was only significantly associated with ramosetron resistance (OR 19 95%C.I. 2.1-181) in the stepwise regression analysis.
The mean scores of diarrhea at baseline were significantly higher (5.2 vs 3.7, p=0.005) in patients with TPH1 rs211105 T/T than in patients with G allele. However the scores after treatment were not significantly different between the two groups. The other SNPs examined did not influence IBS symptoms including diarrhea.
Discussion
In the present study, we show that ramosetron effectiveness correlated with TPH1 SNPs and increased TPH1mRNA levels in the colon. TPH1 rs211105 (11:g.18033757T>G) located within intron 3 as well as rs4537731 (11:g.18047335T>C) and rs7130929 (11:g.18046616C>A) located within the promoter region at -1066 and -347 respectively upstream from the TPH1 transcriptional start site exhibited the most significant association with ramosetron efficacy. TPH1 11:g.18047335T>C (rs4537731) and 11:g.18046616C>A (rs7130929) SNPs were only 724 base pairs apart and were in complete linkage disequilibrium with each other, as recently reported.8 The major alleles (at -1066 T and -347 C on the sense strand relative to the transcriptional start site) of the two promoter SNPs (rs4537731 and rs7130929) both correlated with IBS bowel subtypes and a trend towards higher TPH1 mRNA levels compared to the minor C and A alleles, respectively. 8, 44 Therefore, the clinical response to ramosetron seems to correlate best with increased TPH1 expression in the colonic mucosa and presumably increased 5-HT tissue levels. Our results are indeed consistent with those reported by Grasberger et al. 8 indicating that the rs7130929 C/C genotype was more common in IBS-D subjects.
There are no studies reporting the function of the TPH1 rs211105 intronic SNP and little is known about the impact of DNA variants in the TPH1 gene. The mean diarrhea scores were significantly higher in our patients with the TPH1 rs211105 T/T genotype than those carrying the minor G allele indicating that the SNPs might exhibit function and reflect the level of 5-HT biosynthesis. In contrast, Jun et al. investigated TPH1 SNPs in Caucasian women with mainly the IBS constipation bowel subtype (IBS-C) and reported that the severity of diarrhea symptoms associated with the TPH1 rs211105 G/G or G/T instead of the T/T genotype. However, they failed to confirm a significant association of the individual SNPs with any IBS subtypes.
Camilleri et al. 23 previously demonstrated that 5-HTTLPR genotypes in IBS-D patients were associated with colonic transit in response to alosetron, a 5-HT3R antagonist. Specifically, the observed a greater response in those subjects with the minor 5-HTTLPR l/l genotype compared to the major s/s or s/l genotypes. However, in our study, there were no subjects with the l/l genotype. Therefore we compared the frequency of the s/s and l/s genotype between the two groups and observed a greater response with the s/s compared to l/s genotype, although the difference was not significant. The prevalence of the l/l genotype has been consistently reported to be less than 6% in Korea and Japan, which is in contrast to the Far Eastern, Turkish and US studies showing that a genotype frequency greater than 20%. 21, 22, 24, 45 The TPH1 and 5-HTTLPR polymorphisms differ according to race. Moreover, the variation in background prevalence seems to not only influence the statistical power but also might confound detection precluding genotype-related associations.
A recent phase II clinical trial demonstrated the efficacy of an oral TPH1 inhibitor acting locally on the GI mucosa in relieving symptoms of non-constipating IBS. The clinical response to the TPH1 inhibitor correlated with a decrease in urine excretion of a 5-HT metabolite reflecting reduced 5-HT biosynthesis. 46-48 Therefore, inhibiting the TPH1 enzyme seems to improve IBS-D symptoms by reducing 5-HT biosynthesis. The TPH1 SNPs correlating with enhanced TPH1 expression perhaps increase 5-HT in the colonic mucosa which ostensibly could worsen IBS-D symptoms. 4, 5, 8, 44 Baseline diarrhea scores tended to be higher (more severe symptoms) in our responders than in the non-responders. Ramosetron seemed to be more effective for the patients with severe symptoms, especially diarrhea and probably abdominal pain. On the other hand, there is the possibility that specific TPH1 genotypes might be important in identifying patients whose IBS-D is truly related to abnormal serotonergic function rather than to other causative mechanisms such as food tolerance or bile acid malabsorption. 49-51 Additional studies that correlate TPH1 genotypes, a specific dose of ramosetron and symptom response are desirable to implement functional genomics assessments for IBS.
To our knowledge, this study is the first study to demonstrate a significant association of decreased S100A10 rectal expression in patients with IBS-D with ramosetron ineffectiveness. S100A10 expression levels in the rectal epithelium isolated by LCM were significantly higher in the responders than in the non-responders, but not in the rectal biopsy samples. In a recent study, S100A10 expression in the rectal epithelium of patients with IBS-D was significantly higher than in controls, but not mucosal expression. 24 This result may be due to the fact that epithelial cells express S100A10 and biopsy samples taken from the patients with IBS-D contains small amount of inflammatory cells and other stromal cells. In fact relative S100A10 / β-actin mRNA expression in the rectal epithelium were higher in the LCM samples compared to the biopsy samples. S100A10 was recently reported to induce 5-HT4 receptor (5-HT4R) surface expression thereby facilitating 5-HT4R signaling, as well 5-HT1BR. 39, 52 In our recent study, colonic mucosal S100A10 expression in the rectal mucosa was significantly higher in the IBS-D patients than in controls or UC patients. 24 It is still unclear whether 5-HT4R or 5-HT1BR signaling plays some role in determining the effectiveness of ramosetron. Further investigation of histological inflammation and expression of inflammatory cytokines in colonic tissue is required, and investigation of S100A expression levels in response to ramosetron is also required.
In a previous Japanese clinical trial, 163 of 270 (60.4%) IBS-D patients taking 5μg ramosetron reported adverse events. GI disorders such as hard stool, constipation, abdominal distension, and upper abdominal pain were the most frequently reported adverse events, which occurred in 25.9% of participants.29 Moreover, in other clinical trials of 5-HT3R antagonists, significant constipation occurred in approximately 25% of patients, leading to withdrawal of up to 10% of patients from the treatment. 53 Because constipation related adverse events induced by 5-HT3R antagonists seem to reduce compliance, we allowed our subjects to reduce their dose to avoid adverse events such as constipation and ischemic colitis. Our response rate to ramosetron was about 62% and is much greater compared to the previous Japanese study, 29 although their response might have been weaker after accounting for placebo effects. Moreover, our higher responder rate might be due to the improvement in compliance by allowing our subjects to reduce their ramosetron dose. Indeed, baseline scores for diarrhea tended to be higher in the responders than in the non-responders and the frequency of reducing the dose due to constipation was higher in the responders than in the non-responders. These results suggest that the ramosetron effects were stronger especially against diarrhea symptoms in the responders compared to the non-responders.
The major limitations of the present study are possible selection bias especially relating to the relatively small number of cases. To avoid accounting for placebo effects, a placebo-controlled study is always ideal. However, the aim of the present study was to identify underlying genetic variants predictive of a clinical response to ramosetron therapy. Although our results cannot exclude the possibility that some responses were due to a placebo effect, we wish to emphasize that this is the first study to collectively examine various SNPs in several IBS-related genes that can form the basis for a placebo-controlled study. Ramosetron is only approved for men in Japan, and alosetron is indicated only for women with severe chronic D-IBS in USA because of serious GI events, which are sometimes fatal, including ischemic colitis and bowel motor dysfunction. We strictly selected the patients with IBS-D excluding mixed type to avoid adverse events, and therefore the enrollment of sufficient numbers of patients at a single center was considered to be difficult. Therefore to demonstrate significant prediction of symptom relief with ramosetron using multiple genetic variables will require a large-scale multicenter double-blind, placebo-controlled study. Moreover, future investigations could employ a genome-wide screening approach that might reveal additional genetic variants that correlate with the effect of a 5-HT3R antagonist in IBS-D patients.
In summary, the baseline diarrhea score, S100A10 expression levels and an increase in the frequency of the TPH1 rs4537731 T/T, rs7130929 C/C and rs211105 T/T genotypes were significantly higher in the ramosetron responder group than those in the non-responder group. Increased S100A10 and TPH1 mRNA expression as well TPH1 promoter and intronic SNPs appear to predict higher 5-HT signaling, and correlates not only with diarrhea symptoms, but also with greater ramosetron effectiveness in IBS-D patients. The minor alleles for TPH1 rs211105 may possibly lead to prospective identification of ramosetron non-responders.
Supplementary Material
Key Messages.
The aim was to identify biomarkers predicting effectiveness of the 5-HT3R antagonist (ramosetron) in IBS-D.
Colonic mucosal S100A and TPH mRNA expression levels and TPH1 SNPs were analyzed in 42 treated patients.
Increased S100A10 and TPH1 expression as well TPH1 high producer SNPs appears to be associated with not only diarrhea symptoms, but also greater ramosetron effectiveness in IBS-D patients.
TPH1 gene polymorphisms and S100A10 expression correlating with 5-HT signaling may possibly lead to prospective identification of ramosetron non-response
Acknowledgement
The authors thank Ms. Maki Nomura and Ms. Tomoko Yobimoto for assistance of laboratory work. Ken Haruma was supported by donations from Takeda Pharmaceutical, AstraZeneca Co Ltd, Astellas Pharmaceutical, Otsuka Pharmaceutical, Daiichi Sankyo Co Ltd, and Eisai Co Ltd. Juanita L Merchant received NIH grant support (R01-DK55732). The funding sources played no role in the design, practice or analysis of this study.
Footnotes
Competing Interests: the authors have no competing interests.
AS performed the research, analyzed the data and wrote the paper; HK, MI, HI, NM, TK enrolled patients and collected samples; JM result analysis and manuscript preparation; KH designed the research.
References
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Tenner K, Walther D, Bader M. Influence of human tryptophan hydroxylase 2 N- and C-terminus on enzymatic activity and oligomerization. J Neurochem. 2007;102:1887–94. doi: 10.1111/j.1471-4159.2007.04664.x. [DOI] [PubMed] [Google Scholar]
- 3.Harvey M, Shink E, Tremblay M, et al. Support for the involvement of TPH2 gene in affective disorders. Mol Psychiatry. 2004;9:980–1. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- 4.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–80. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 5.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 6.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–64. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 8.Grasberger H, Chang L, Shih W, et al. Identification of a functional TPH1 polymorphism associated with irritable bowel syndrome bowel habit subtypes. Am J Gastroenterol. 2013;108:1766–74. doi: 10.1038/ajg.2013.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Kerckhoffs AP, ter Linde JJ, Akkermans LM, et al. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053–60. doi: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 11.Gizatullin R, Zaboli G, Jonsson EG, et al. Haplotype analysis reveals tryptophan hydroxylase (TPH) 1 gene variants associated with major depression. Biol Psychiatry. 2006;59:295–300. doi: 10.1016/j.biopsych.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AP, Binder EB, Bowman D, et al. A common TPH2 haplotype regulates the neural processing of a cognitive control demand. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:829–40. doi: 10.1002/ajmg.b.32090. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Li H, Qin W, et al. Association of TPH1 with suicidal behaviour and psychiatric disorders in the Chinese population. J Med Genet. 2006;43:e4. doi: 10.1136/jmg.2004.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zill P, Baghai TC, Zwanzger P, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–6. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- 15.Andreou D, Saetre P, Werge T, et al. Tryptophan hydroxylase gene 1 (TPH1) variants associated with cerebrospinal fluid 5-hydroxyindole acetic acid and homovanillic acid concentrations in healthy volunteers. Psychiatry Res. 2010;180:63–7. doi: 10.1016/j.psychres.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Wilson ST, Stanley B, Brent DA, et al. Interaction between tryptophan hydroxylase I polymorphisms and childhood abuse is associated with increased risk for borderline personality disorder in adulthood. Psychiatr Genet. 2012;22:15–24. doi: 10.1097/YPG.0b013e32834c0c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun S, Kohen R, Cain KC, et al. Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:233–9, e116. doi: 10.1111/j.1365-2982.2010.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essien BE, Grasberger H, Romain RD, et al. ZBP-89 regulates expression of tryptophan hydroxylase I and mucosal defense against Salmonella typhimurium in mice. Gastroenterology. 2013;144:1466–77. 1477, e1–9. doi: 10.1053/j.gastro.2013.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 20.Lesch KP, Balling U, Gross J, et al. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 21.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–8. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pata C, Erdal ME, Derici E, et al. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97:1780–4. doi: 10.1111/j.1572-0241.2002.05841.x. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–32. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 24.Shiotani A, Kusunoki H, Kimura Y, et al. S100A expression and interleukin-10 polymorphisms are associated with ulcerative colitis and diarrhea predominant irritable bowel syndrome. Dig Dis Sci. 2013;58:2314–23. doi: 10.1007/s10620-013-2677-y. [DOI] [PubMed] [Google Scholar]
- 25.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–8. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 26.Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13(Suppl 2):77–82. doi: 10.1046/j.1365-2036.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey PP, Bountra C, Clayton N, et al. Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13(Suppl 2):31–8. [PubMed] [Google Scholar]
- 28.Matsueda K, Harasawa S, Hongo M, et al. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–35. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 29.Matsueda K, Harasawa S, Hongo M, et al. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202–11. doi: 10.1080/00365520802240255. [DOI] [PubMed] [Google Scholar]
- 30.Jarcho JM, Chang L, Berman M, et al. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Aliment Pharmacol Ther. 2008;28:344–52. doi: 10.1111/j.1365-2036.2008.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–71. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 32.Roth J, Vogl T, Sorg C, et al. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 33.Manolakis AC, Kapsoritakis AN, Tiaka EK, et al. Calprotectin, calgranulin C, and other members of the s100 protein family in inflammatory bowel disease. Dig Dis Sci. 2011;56:1601–11. doi: 10.1007/s10620-010-1494-9. [DOI] [PubMed] [Google Scholar]
- 34.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859–68. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 35.Foell D, Wittkowski H, Ren Z, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–92. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- 36.Leach ST, Yang Z, Messina I, et al. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321–31. doi: 10.1080/00365520701416709. [DOI] [PubMed] [Google Scholar]
- 37.Svenningsson P, Chergui K, Rachleff I, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 38.Coulie B, Tack J, Maes B, et al. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am J Physiol. 1997;272:G902–8. doi: 10.1152/ajpgi.1997.272.4.G902. [DOI] [PubMed] [Google Scholar]
- 39.Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Gabrys JB, Peters K. Reliability, discriminant and predictive validity of the Zung Self-rating Depression Scale. Psychol Rep. 1985;57:1091–6. doi: 10.2466/pr0.1985.57.3f.1091. [DOI] [PubMed] [Google Scholar]
- 42.Oka T, Tamagawa Y, Hayashida S, et al. Rikkunshi-to attenuates adverse gastrointestinal symptoms induced by fluvoxamine. Biopsychosoc Med. 2007;1:21. doi: 10.1186/1751-0759-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 44.Sun HS, Fann CS, Lane HY, et al. A functional polymorphism in the promoter region of the tryptophan hydroxylase gene is associated with alcohol dependence in one aboriginal group in Taiwan. Alcohol Clin Exp Res. 2005;29:1–7. doi: 10.1097/01.alc.0000150635.51934.6d. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida K, Ito K, Sato K, et al. Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:383–6. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 46.Brown PM, Drossman DA, Wood AJ, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–16. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camilleri M. LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil. 2011;23:193–200. doi: 10.1111/j.1365-2982.2010.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tack J, Janssen P, Wouters M, et al. Targeting serotonin synthesis to treat irritable bowel syndrome. Gastroenterology. 2011;141:420–2. doi: 10.1053/j.gastro.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Farup PG, Monsbakken KW, Vandvik PO. Lactose malabsorption in a population with irritable bowel syndrome: prevalence and symptoms. A case-control study. Scand J Gastroenterol. 2004;39:645–9. doi: 10.1080/00365520410005405. [DOI] [PubMed] [Google Scholar]
- 50.Ghoshal UC, Kumar S, Misra A, et al. Lactose malabsorption diagnosed by 50-g dose is inferior to assess clinical intolerance and to predict response to milk withdrawal than 25-g dose in an endemic area. J Gastroenterol Hepatol. 2013;28:1462–8. doi: 10.1111/jgh.12273. [DOI] [PubMed] [Google Scholar]
- 51.Gracie DJ, Kane JS, Mumtaz S, et al. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil. 2012;24:983–e538. doi: 10.1111/j.1365-2982.2012.01953.x. [DOI] [PubMed] [Google Scholar]
- 52.Warner-Schmidt JL, Flajolet M, Maller A, et al. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–46. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mertz H. Psychotherapeutics and serotonin agonists and antagonists. J Clin Gastroenterol. 2005;39:S247–50. doi: 10.1097/01.mcg.0000156113.14974.f5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.