Abstract
Proteomics analysis is important for characterizing tissues to gain biological and pathological insights, which could lead to the identification of disease-associated proteins for disease diagnostics or targeted therapy. However, tissues are commonly embedded in optimal cutting temperature compound (OCT) or they are formalin-fixed and paraffin-embedded (FFPE) in order to maintain tissue morphology for histology evaluation. Although several tissue proteomics analyses have been performed on FFPE tissues using advanced mass spectrometry technologies, high throughput proteomic analysis of OCT-embedded tissues has been difficult due to the interference of OCT in the mass spectrometry analysis. In addition, molecules other than proteins present in tissues further complicate tissue proteomic analysis. Herein, we report the development of a method using Chemical Immobilization of Proteins for Peptide Extraction (CIPPE). In this method, proteins are chemically immobilized onto a solid support; interferences from tissues and OCT embedding are removed by extensive washing of proteins conjugated on the solid support. Peptides are then released from the solid phase by proteolysis, enabling mass spectrometry analysis. This method was first validated by eliminating OCT interference from a standard protein, human serum albumin, where all the unique peaks contributed by OCT contamination were eradicated. Finally, this method was applied for the proteomic analysis of frozen and OCT-embedded tissues using iTRAQ labeling and 2D liquid chromatography tandem mass spectrometry. The data showed reproducible extraction and quantitation of 10,284 proteins from 3,996 protein groups and a minimal impact of OCT embedding on the analysis of the global proteome of the stored tissue samples.
Keywords: OCT, proteomics, protein chemical immobilization, polymer contamination, tissues
Introduction
Tissue proteins play important roles in biological processes. The quantitative analysis of tissue proteins facilitates the understanding of molecular mechanisms that differentiate between normal and disease states. Disease-specific tissue-derived proteins can also be used as biomarkers for disease diagnosis or as novel drug targets for therapeutic drug development1. In the diseased state, tissues secrete or shed disease-specific proteins into the body fluids such as serum, which can be used as biomarkers. However, the secreted proteins from a diseased tissue are present in higher concentrations at the tissue site and become diluted in the serum, which contains proteins from other tissues1, 2. If disease-specific proteins can be identified in tissues, tissue-targeted approaches can be used to detect tissue-derived proteins in serum 1. An example of this type of approach was shown for the detection of prostate cancer proteins in serum using TOF/TOF mass spectrometry3.
Traditionally, tissue proteins are analyzed using immunoassays, which rely on the development of high quality antibodies. Advances in mass spectrometry (MS) and high performance liquid chromatography (HPLC) systems have led to the increasingly widespread applicability of proteomics4. Increases in sensitivity, resolution, and speed of the mass spectrometers have enabled the rapid identification of large numbers of proteins with high confidence, thereby enabling the analysis of complex samples such as tissue. The analysis of the tissue proteome, located at the primary site of pathology, aids the understanding of the molecular mechanism of diseases and facilitates the identification of potential biomarkers and therapeutic targets.
Tissue proteomics requires tissues to be stored by snap freezing. However, flash frozen tissues without embedding medium are difficult to section, thereby making histopathology or immunohistochemistry evaluation difficult. Instead, tissues are embedded in optimal cutting temperature medium (OCT) or they are formalin-fixed and paraffin-embedded (FFPE) to retain their morphology5. Recently, proteomic analyses have been performed on FFPE-embedded tissues6-8. However, FFPE tissues undergo extensive cross linking between protein/DNA/RNA during formalin fixation, which creates inter- and intra-cross-linked proteins5, 9, 10. Some of the peptide modifications that occur in the proteomic analysis of FFPE tissues are Metylol derivatives, Schiff bases and methylene bridges9. The duration of FFPE processing and storage can also lead to different levels of protein degradation and protein modifications. In contrast, OCT-embedded tissues are instantly frozen and stored for histological studies; therefore, the protein content likely remains unchanged and is representative of the tissue proteome.
OCT is widely used for tissue embedding prior to storage. However, the proteomic analysis of OCT-embedded tissues is difficult, largely due to the presence of water soluble synthetic polymers. OCT can compete with peptides for ionization during mass spectrometry analysis11. OCT can also generate ion suppression in Matrix Assisted Laser Desorption Ionization (MALDI) mass spectrometry and ionization competition in Electrospray ionization (ESI) mass spectrometry12. In addition, OCT creates a deleterious effect on the peptide chromatographic separation required for tissue proteomics. OCT has a high affinity to the reversed phase stationary medium commonly used in shotgun proteomics. OCT competes with peptides for binding to the C18 reversed phase column. OCT also decreases the sensitivity of detection due to its propensity to co-elute with peptides during chromatographic separation. Thus, it is necessary to remove OCT prior to the LC-MS/MS analysis of tissues.
Due to the malicious effect of OCT on the mass spectrometer, only a small number of proteomics studies have been performed on OCT-embedded tissues13-17. OCT-embedded tissues are studied using either two-dimensional gel electrophoresis (2D DIGE) technology or shotgun proteomics using LC-MS/MS. 2D DIGE can separate proteins from OCT; however, most of the LC-MS/MS studies of OCT-embedded tissue did not completely remove OCT, resulting in fewer protein identifications15-17. Recently, we demonstrated that OCT-embedded tissues could be used for glycoproteomic analysis using solid-phase extraction of glycopeptides (SPEG)18. The glycopeptides were chemically immobilized to the solid support using oxidized glycan tags, whereas the non-glycopeptides and OCT were removed from the immobilized peptides before the enzymatic release of N-glycopeptides. While the SPEG method was used to effectively remove the OCT interference prior to glycoprotein analysis, proteins without glycans were not conjugated to the solid-phase and were excluded from the proteomic analysis.
In this study, we describe a method using Chemical Immobilization of Proteins for Peptide Extraction (CIPPE) from tissues for tissue proteomic analysis. In this method, proteins are chemically immobilized onto a solid support, which allows for sample cleaning to remove interferences from tissues before peptides are released from the solid support using proteolysis. We applied the method to the quantitative analysis of frozen and OCT-embedded tissues to determine the impact of OCT on the mass spectrometry-based detection of the tissue proteome.
Methods
Materials
Human serum albumin (HSA), 1× PBS buffer, dithiothreitol (DTT), sodium cyanoborohydride, and iodoacetamide were purchased from Sigma Aldrich (St. Louis, MO). Protein estimation BCA kit, polyacrylamide desalting spin columns and AminoLink coupling resin were purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Sequencing grade trypsin was purchased from Promega (Madison, WI). iTRAQ 4-plex reagents were purchased from AB Sciex (Framingham, MA). PNGase F was obtained from New England Biolabs (Ipswich, MA).
Protein extraction
Mouse kidney tissue was collected from NIH01a mice and snap frozen in Dr. Kemp's laboratory at the Fred Hutchinson Cancer Research Cancer19, 20. Mouse kidney tissue was cut in half. One half was embedded in OCT followed by storage at −80°C. The other half was stored as fresh-frozen tissue. The OCT-embedded or frozen mouse kidney tissue was lysed in 1 mL of RIPA buffer (100 mM sodium citrate, 50 mM sodium carbonate, and 1% NP-40 ) by vortexing for 2-3 min and sonicating for 4 min in an ice bath to homogenize the tissues. After the tissues were homogenized, the buffer of the extracted proteins was exchanged with 1 mL of pH 10 binding solution (40 mM sodium citrate and 20 mM sodium carbonate) using polyacrylamide desalting spin columns. The protein concentration was determined by BCA assay. Protein (1 mg) from each tissue was used for AminoLink resin capture using the chemical immobilization of proteins to beads protocol as previously described21, 22. Briefly, AminoLink resin (800 μL) was loaded onto snap-cap spin-column and centrifuged at 2000 g for 1 minute. Resin was washed with 800 μL of pH 10 buffer (100 mM sodium citrate and 50 mM sodium carbonate) followed by centrifugation. The wash step was repeated twice. The sample in pH 10 buffer (1 mg/200 μL protein:beads) was added to the washed AminoLink resin.
The sample-resin mixture was incubated at room temperature overnight on a mixer. The mixture was centrifuged at 2000 g to remove any unbound protein. The resin was rinsed three times with 450 μL 1× PBS buffer. Fifty mM sodium cyanoborohydride in PBS (400 μL) was added to the resin. After a 4 h incubation, the supernatant was removed via centrifugation (2000 g), and 400 μL of 1 M Tris-HCl (pH 7.6) in the presence of 50 mM sodium cyanoborohydride was added to block any un-reacted aldehyde sites on the resin. The blocking process was terminated after 1 h. Then, the beads were washed twice with 1× PBS, twice with 1.5 M NaCl, and three times with deionized water.
Peptide extraction by proteolysis
Proteins bound to the beads were treated with 10 mM DTT in 50 mM ammonium bicarbonate for 30 min at 60 ºC followed by a wash with 50 mM ammonium bicarbonate. Afterwards, the beads were treated for 1 h with 15 mM iodoacetamide in 50 mM ammonium bicarbonate in the dark. Finally, proteins were digested using trypsin (1:50 enzyme:substrate) in the presence of 50 mM ammonium bicarbonate. The proteins were digested at 37 °C overnight. The released peptides were collected from the supernatant of the beads and the following water wash step of the beads. iTRAQ labeling was performed according to the manufacturer's protocol23.
In-solution digestion
HSA protein with and without OCT was incubated with 10 mM DTT at 60 °C for 1 h and alkylated for 30 min with 10 mM iodoacetamide in the dark at room temperature. The pH of the solution was adjusted to 7.5 with 50 mM NH4HCO3 and protein was enzymatically digested with trypsin (1:50 enzyme:substrate) with overnight incubation at 37 °C.
Mass spectrometric analysis of peptides by direct infusion to TSQ Quantum
A TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Scientific, Rockford, IL) with an electrospray ionization (ESI) source was used for the analysis of HSA peptides using direct infusion. The flow rate was set at 5 μL/min. Peptides were scanned from m/z 300 to 1000 using a spray voltage of 3000 V a capillary temperature of 180 °C.
High-pH RPLC fractionation
Two hundred μg iTRAQ-labeled peptides were subjected to high-pH RPLC fractionation with a 1200 Infinity LC system (Agilent Technology, Santa Clara, CA) and a 4.6 × 100 mm BEH130-C-18 column (Waters, Milford, MA). Samples were adjusted to a basic pH using 1% ammonium hydroxide and injected in 2 mL solvent A (7 mM tri-ethyl ammonium bicarbonate (TEAB) in water). Solvent B was 7 mM TEAB in 90% acetonitrile.
The separation gradient was set as follows: 0 % B for 18 min, 0 to 31% B in 42 min, 31 to 50% B in 10 min, 75 to 100% B in 15 min, and 100% B for an additional 10 min. Ninety-six fractions were collected throughout the LC separation and were concatenated into 24 fractions according to the following scheme: fractions 1, 25, 49, and 73; fractions 2, 26, 50, and 74, etc. The samples were dried in a Speed-Vac and stored at −80°C until LC-MS/MS analysis.
LC-MS/MS Analysis
A Dionex Ultimate 3000 RSLC nano system (Thermo Scientific, Rockford, IL) was used with a 75 μm × 25 cm Acclaim PepMap100 separating column (Thermo Scientific, Rockford, IL). Peptides were separated using a flow rate of 300 nL/min with mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in 95% acetonitrile). The gradient profile was set as follows: 4-35% B in 70 min, and 35-95% B in 5 min. MS analysis was performed using a Q Exactive mass spectrometer (Thermo Scientific, Rockford, IL). The spray voltage was set at 2.0 kV. Orbitrap spectra were collected at a resolution of 70K followed by data-dependent HCD MS/MS (at a resolution of 17500 and collision energy 33) of the twenty most abundant ions. A dynamic exclusion time of 25 sec was used.
Database Search
Data generated using Orbitrap was searched using SEQUEST with Proteome Discoverer 1.3 (Thermo Scientific, Rockford, IL) against the IPI mouse v. 3.87 database containing 59,534 protein entries. Peptides were searched with fully tryptic specificity allowing only two missed cleavages. The search parameters used were 10 ppm precursor tolerance and 0.06 Da fragment ion tolerance, a static modification of 4-plex iTRAQ at the N-terminus and carbamidomethylation of cysteine. The following variable modifications were used: oxidation of methionine, deamidation of asparagine and 4-plex iTRAQ modification of lysine. Filters used for data analysis included peptide rank 1, 2 peptides per protein, and 2% FDR threshold.
Data Analysis
Removal of OCT from Human Serum Albumin (HSA)
Peaks were selected from ESI spectra acquired on a TSQ Quantum with a threshold of 20% intensity of the base peak intensity. Peaks were obtained from HSA protein digested in the presence of OCT, without OCT, and with OCT followed by the removal of OCT. Afterwards, the spectra were aligned and compared. The comparison was performed between HSA, HSA with OCT, and HSA with OCT followed by OCT removal using CIPPE.
The Pearson's correlation coefficient of the peptide spectra obtained between replicate analyses of OCT-embedded tissues (iTRAQ 116, 117) and frozen tissue (iTRAQ 114, 115) using CIPPE was calculated to assess the reproducibility of the OCT removal method. Protein expression from the OCT-embedded tissue (116, 117) and frozen tissue (114, 115) was quantified and normalized using Proteome Discoverer 1.3. The log2 ratios between replicates 116 and 117 were used as the “null” distribution, and the values for 5% cut-off (2.5th and 97.5th percentiles) of the histogram were selected as the thresholds for increased and decreased expression. Similarly, the Pearson's correlation coefficient of the peptide spectra between the OCT-embedded tissues/ frozen tissues (116/114 and 117/115) was calculated to assess the impact of embedding the tissue in OCT.
Results and Discussion
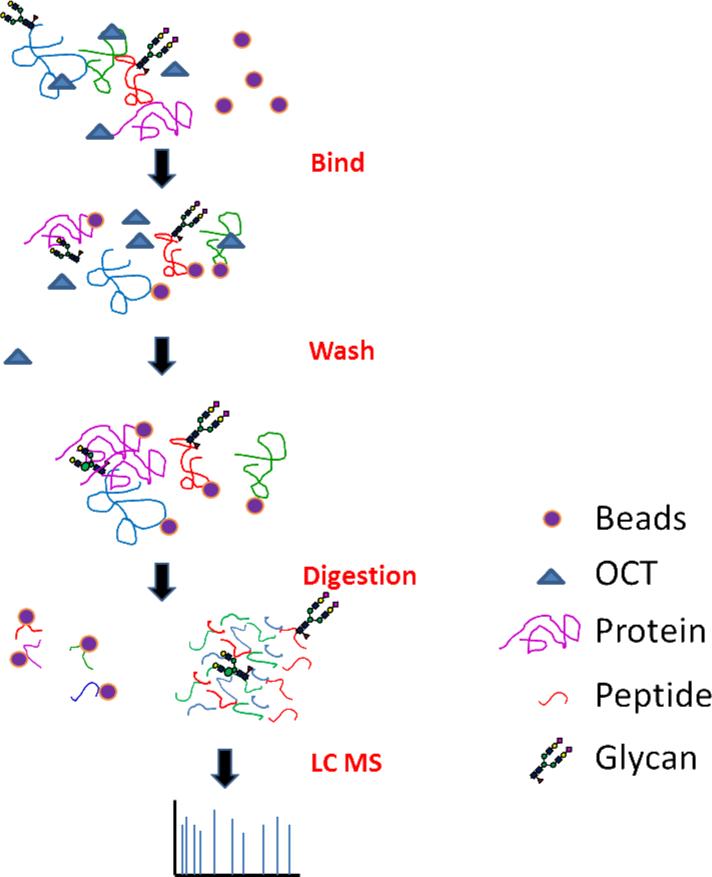
To analyze the global proteome of tissues, we therefore employed a chemical immobilization of proteins for peptide extraction (CIPPE) method based on the capture of proteins by conjugation of amino groups of proteins to beads containing aldehyde groups (Figure 1). After washing, the proteins conjugated to beads were reduced, carbamidomethylated, and proteolyzed to release the peptides for proteomics analysis (Figure 1). Using this method, proteins were extracted from tissues and chemically immobilized onto the solid phase by reductive amination and peptides are released from solid phase by proteolytic digestion. However, OCT polymers from OCT-embedding and other MS interfering molecules from tissues were not immobilized on the beads or not released from beads. Therefore, they were removed by washing the beads..
Figure 1.
Scheme for Chemical Immobilization of Proteins for Peptide Extraction (CIPPE). Proteins are conjugated onto the solid support. Unbound compounds are washed away. Peptides are released from the solid support using proteolysis and are analyzed using LC-MS/MS.
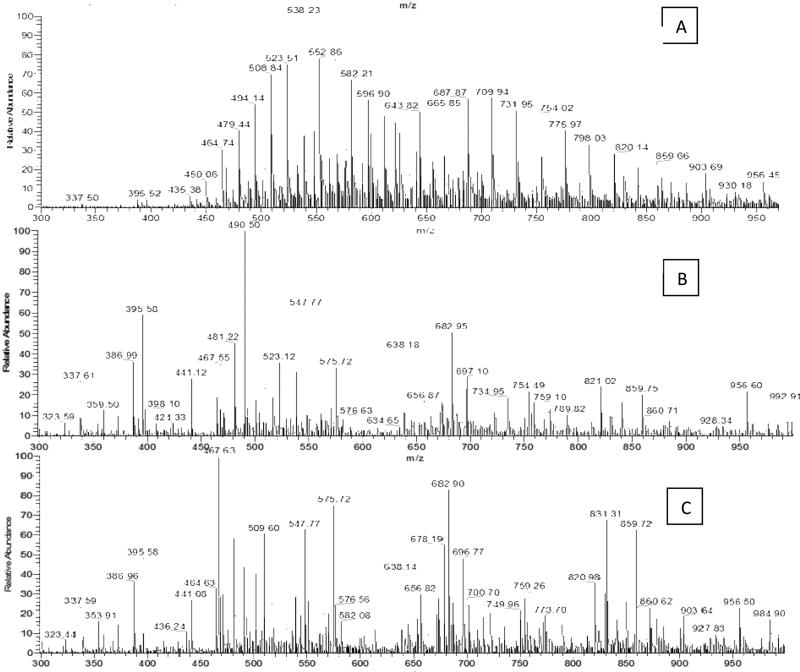
To develop a procedure to remove the OCT, HSA was used as a model protein. The tryptic peptides from HSA with OCT contamination were directly analyzed by a TSQ Quantum mass spectrometer using direct infusion ESI. Figure 2A shows the ESI spectrum of OCT-contaminated HSA digested with trypsin demonstrating an MS pattern with a regular bell-shaped curve with mass values that are 44 Da, 22 Da and 14.6 Da apart. These readily observed peaks correspond to different charge states of the polyethylene glycol present in OCT. The OCT polymer peaks mask those of the HSA peptides. In MS, OCT dominates the mass spectrum, indicating the preferential ionization of OCT compared to the HSA peptides in a set mixture of OCT to the HSA peptide. At 20% of the base peak intensity, only 11 peptide HSA peaks were detected in the OCT-contaminated HSA (10% OCT volume/HSA weight) (Figure 2A). In contrast, OCT-contaminated HSA had 46 unique polymer peaks that suppressed the ionization of HSA peptides and overshadowed these peptides in the mass spectrum. To remove OCT interferences from the sample, OCT contaminated HSA was first chemically immobilized onto beads using reductive amination, the beads were then washed with various conditions, and the immobilized HSA was digested using trypsin. The released peptides were analyzed (Figure 2B). We found that after washing the beads with PBS, 1.5 M NaCl and water, no OCT peaks were detected and the HSA tryptic peptide peaks were visible in the mass spectra. None of the 46 polymer peaks uniquely observed in the OCT sample was observed after CIPPE. In this method, proteins were bound to the solid phase and released by trypsin. The OCT polymers were removed from released HSA tryptic peptides, resulting in the complete removal of OCT from chemically immobilized proteins. The results showed that CIPPE removed OCT contaminants from the protein sample, thereby enabling the mass spectrometric analysis of proteins with OCT contamination.
Figure 2.
Mass spectrometric detection of the tryptic peptides from HSA with and without OCT. A) A representative ESI spectrum of the tryptic peptides from OCT-contaminated HSA digested in solution. B) A representative ESI spectrum of OCT-contaminated HSA after OCT removal using CIPPE. C) A representative ESI spectrum of clean HSA digested in solution.
CIPPE is a chemical immobilization method for protein immobilization via protein amino groups and peptide release by protease digestion. To compare the peptide spectral pattern from protein directly digested by trypsin in solution and the peptide spectral pattern released from immobilized protein, HSA without OCT contamination was digested in solution. The tryptic peptides from HSA digested in solution were directly analyzed by a TSQ Quantum mass spectrometer using direct infusion ESI and the peptides were compared to the tryptic peptides from the HSA digested on beads. The spectrum of HSA tryptic peptides digested in solution showed that the mass fingerprint of the albumin tryptic peptides differed between CIPPE (Figure 2B) and the in solution digestion of HSA (Figure 2C). The difference may have resulted from the OCT contamination of HSA or the sample process using CIPPE. To determine whether peptides, especially the Lys-containing peptides, were lost due to the conjugation of protein to solid phase using CIPPE, the peptide samples from the CIPPE method and the in solution digestion were analyzed by LC-MS/MS using a Q Exactive mass spectrometer. The same amount of HSA peptides prepared from the two methods was analyzed in triplicate LC-MS/MS runs (Supplementary Table 1). Overall, 94 peptides were identified in all 6 LC-MS/MS runs, 10 peptides were only identified from the peptides prepared using CIPPE method and 9 of them were Lys-containing peptides, and 10 peptides were only identified from the in solution digestion and all of them were lys-containing peptides (Supplementary Table 1). The peptide spectral counts of HSA from the two methods were also used for relative quantitation (Supplemental Table 1). We identified the 74 common peptides from the in solution digestion and the on-bead digestion using CIPPE method, but with different spectral counts. The spectral counts of 10 peptides (9 lys-containing peptides) were reduced at least of 2 fold in the in solution digested sample compared to the on-bead digested sample. The spectral counts of 18 peptides (14 lys-containing peptides) were increased at least 2 fold in the in solution digested peptides compared to the on-bead digested sample.
The results show that there are no significant losses of lys-containing peptides from the on-bead digested samples using CIPPE method. However, peptides prepared from on bead digestion using CIPPE method and in solution digestion showed different patterns (Figure 2). This could be due to the fact that protein amino groups are randomly conjugated to the solid support and conjugated peptides could be lost in abundance but the lys-containing peptides are still detectable and identified due to random conjugation on beads. In CIPPE method, the proteolytic digestion of proteins will not release chemically immobilized amino terminal or Lys-containing peptides. However, proteins may not be immobilized onto the beads at the same amino group-containing peptides. Our data shows that identification of most of the peptides on the immobilized protein is possible, provided that there are various amino-groups present in the protein. In a complex sample analyzed via a shotgun proteomics approach, commonly, only two peptides are required for protein identification. In addition, protease digestion efficiency could differ between on-bead digestion and in solution digestion based on the availability of different protease digestion sites, which could result in the altered quantitation of different peptides between on-bead digestion and in solution digestion. However, this random conjugation of amino group-containing peptides and changes in protease digestion efficiency will not affect the relative quantitation among the protein samples because different protein samples are expected to have similar conjugation and protease digestion efficiency.
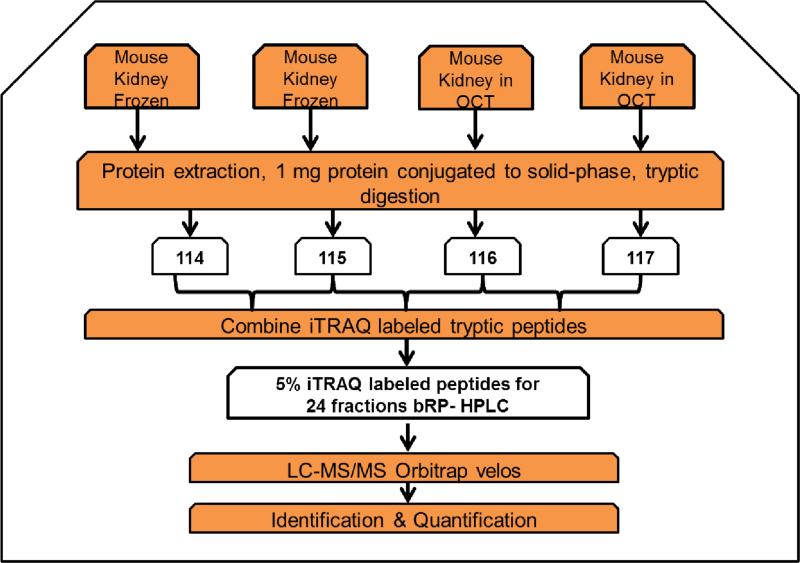
To determine the impact of embedding tissue with OCT prior to tissue proteomics analysis, we applied the developed method to OCT-embedded tissues and frozen tissues. A complex biological tissue, mouse kidney, was analyzed. Mouse kidney tissue was divided into two halves. One half was embedded in OCT and the other half was directly frozen. An equal amount of protein from the four tissues (1 mg) was used for quantitative proteomic profiling using chemical immobilization and iTRAQ methodology (Figure 3). Protein from each sample was first bound to beads. The quantity of unbound protein was determined by BCA assay and was estimated to be less than 5% of the protein input for protein immobilization. Beads containing sample were rigorously washed. Proteins were further reduced and alkylated on the beads. Finally, proteins were released from the beads using proteolysis, and all the released peptides were iTRAQ labeled and mixed. Technical replicates of an OCT-embedded tissue (labeled with iTRAQ 116 and 117) and technical replicates of a frozen tissue (labeled with iTRAQ 114 and 115) were lysed. Two hundred micrograms of sample was used for global proteomic analysis. In the global proteomic analysis, basic reverse phase liquid chromatography was used to generate 24 offline fractions, and each fraction was subjected to LC-MS/MS analysis using a Q Exactive mass spectrometer (Figure 3).
Figure 3.
Schematic diagram for the relative quantification method used to study the impact of OCT on tissue samples using the CIPPE method. Mouse kidney was split in half. One half was embedded in OCT, and the second half was directly frozen at −80 °C. Proteins were extracted from the two OCT-embedded tissues and the two frozen tissues using CIPPE. Peptides were labeled with iTRAQ tags and the labeled peptides were combined. The iTRAQ-labeled tryptic peptides were analyzed using 2D LCMS/MS.
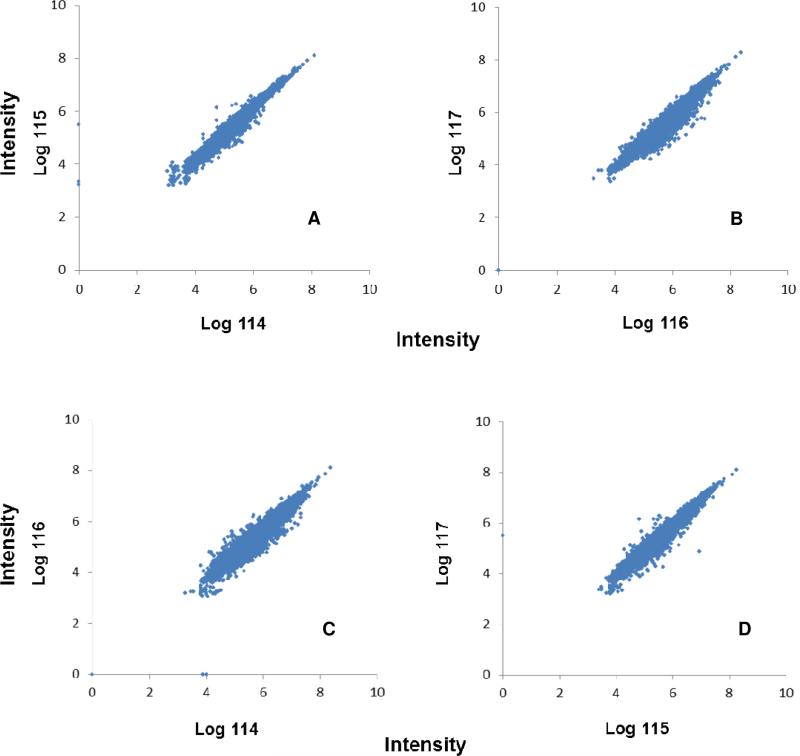
From the proteomic analysis of the iTRAQ labeled tryptic peptides, 10,284 proteins belonging to 3,996 protein groups were identified on the basis of at least two unique peptides exceeding a threshold score of 2% FDR (Supplementary Table 2). The quantification results are depicted in Figure 4. Each dot represents an identified peptide. The replicate of 114/115 and 116/117 showed little variance. The correlation between channel 116 and 117 was 0.990 for the replicate global proteomic analysis of OCT-embedded tissues. Similarly, the correlation between iTRAQ channel 114 and 115 was 0.996 for the replicate proteomic analysis of frozen tissues. From the analysis of replicate OCT-embedded tissues using CIPPE and MS/MS, we estimated that 95% of proteins had a ratio between 0.850 and 1.180, which indicated good analytical reproducibility of the frozen and OCT-embedded tissues using the CIPPE method based on the chemical immobilization of tissue proteins. Our results showed that accurate quantitation could be achieved on OCT-embedded tissues and frozen tissues using chemical immobilization, iTRAQ labeling, and tandem mass spectrometry.
Figure 4.
Quantitative analysis of proteins isolated from tissues using CIPPE. The scatter plot represents iTRAQ channel 115 and 114 (A) and 116 and 117 (B) 114:116 (C), 115:117(D). The 116 and 117 channels were used for the quantitative analysis of two OCT-embedded tissues using CIPPE. The intensities observed for peptides in the 114:115, 116:117 114:116 and 115:117 iTRAQ channels were plotted on the X-axis and Y–axis, respectively for each peptide spectrum match. The scatter plot represents the quantitative linearity between reporter ion groups. The sample and the reporter ion intensity scatter plot are clustered around a 45° line indicating the symmetric distribution of fold-change across the scatter plot.
To determine the impact of OCT-embedding on tissue proteomics, we further analyzed the quantitative data from the OCT-embedded tissues and compared it to the data from the frozen tissues. The scatter plot of intensities of the iTRAQ channels 116 vs. 114 and 117 vs. 115 (OCT-embedded tissue/frozen tissue) showed similar patterns as the technical replicates of the OCT-embedded tissues (Figure 4). The quantitative distribution is roughly symmetrical with only a minimal spread from the R2 = 1 line in the scatter plot, indicating high quantitative similarity between the frozen and OCT-embedded tissues. A vast majority of the proteins from OCT-embedded tissues vs. frozen tissues showed a 1:1 ratio, with 91.0% (117/115) and 82.6% (116/114) of the proteins exhibiting a ratio within the 0.850 - 1.180 cut-off determined from the replicate analysis of OCT-embedded tissues. The correlation of 0.923 between 114 and 116 and 0.956 between 115 and 117 is similar to the replicate analysis of the OCT-embedded tissues. The data showed that there were no significant differences between the OCT-embedded and frozen tissues.
Our results demonstrate that quantitative proteomic analysis of OCT-embedded tissue is feasible using CIPPE. Using this method, we successfully removed the interference from OCT and identified thousands of tissue proteins from OCT-embedded tissues. CIPPE has the potential to be used for PTM analysis such as glycosylation, phosphorylation, ubiquitination and acetylation.
In addition to the removal of OCT from OCT-embedded tissues, this method could be used to extract peptides from tissue proteins for tissue proteomics. Compared to the proteins from body fluids, the proteins from tissues are more difficult to extract in order to obtain a complete proteome due to the three-dimensional structures of tissues and solubility of certain tissue proteins. During the proteomic analysis of tissues, detergents such as sodium dodecyl sulfate (SDS), NP-40, or Triton X-100 are often used to solubilize the membrane proteins from tissues for protein extraction (NP-40 was included in the RIPA buffer to extract kidney tissue proteins in this study). However, detergents also impede the mass spectrometric detection of peptides, similar to the spectra from the OCT-contaminated HSA (Figure 2A). In addition, these detergents, similar to OCT, bind to reversed phase columns that are commonly used online with mass spectrometers, further impairing the capability of tissue proteomics using LC-MSMS/MS. The CIPPE method described here is also capable of removing detergents from the tissue samples that were introduced during the protein extraction for proteomics analysis.
One of the limitations of this method is the incomplete release of all tryptic peptides from the beads. After the proteins are chemically immobilized onto the beads and washed, peptides are then released from the beads using trypsin digestion. We have shown that for protein identification and quantification, it is not necessary to recover all tryptic peptides. However, in the cases where all tryptic peptides are required for proteomic analysis, a cleavable linker to the solid phase could be used to capture and release all peptides. Another limitation of the current method is using same amino group of the lysine residue for protein capture and the relative quantification. The captured lysine residues are not cleaved by trypsin and so blocked for labeling reagents such as iTRAQ. This will cause reduced quantity of lysine containing peptides. However, the relative quantitation of the lysine containing peptides from different samples still remains quantitative given the fact that lysine residues from each sample have similar coupling efficiency. For absolute quantitation of specific lysine containing peptides, other amino acids residues like cysteine can be used for coupling of the proteins..
Conclusion
This study shows that tissue proteins can be analyzed using shotgun proteomics incorporating CIPPE and tandem mass spectrometry. The CIPPE methodology described here was used to conduct global proteomic analyses of tissues OCT-embedded and frozen tissues. This method is highly efficient in the removal of contaminants and other molecules and extracting peptides for shotgun proteomics using mass spectrometry. Our data indicate that OCT does not appear to impact the tissue proteome. Therefore, CIPPE can be used for the proteomic and PTM analysis of OCT-embedded tissue, leading to the possibility of the tissue proteomics of OCT embedded tissues.
Supplementary Material
Acknowledgement
This work was supported in part by the National Institutes of Health under grants and contracts of National Cancer Institute, Clinical Proteomics Tumor Analysis Consortium (U24CA160036), the Early Detection Research Network (EDRN, U01CA152813 and U24CA115102), and R01CA112314; National Heart Lung Blood Institute, Programs Excellence in Glycosciences (P01HL107153), NHLBI Proteomic Center (N01-HV-00240). We acknowledge Dr. Christopher Kemp at the Fred Hutchinson Cancer Research Center for providing mouse tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang H, Chan DW. Cancer biomarker discovery in plasma using a tissue-targeted proteomic approach. Cancer Epidemiology Biomarkers & Prevention. 1915-1917;200716(10) doi: 10.1158/1055-9965.EPI-07-0420. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin G, Novitskaya V, Sadej R, Pochec E, Litynska A, Hartmann C, Williams J, Ashman L, Eble JA, Berditchevski F. Tetraspanin CD151 regulates glycosylation of α3β1 integrin. Journal of biological chemistry. 2008;283(51):35445–35454. doi: 10.1074/jbc.M806394200. [DOI] [PubMed] [Google Scholar]
- 3.Tian Y, Kelly-Spratt KS, Kemp CJ, Zhang H. Identification of glycoproteins from mouse skin tumors and plasma. Clinical Proteomics. 2008;4(3):117–136. doi: 10.1007/s12014-008-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantscheff M, Lemeer S, Savitski MM, Kuster B. Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Analytical and bioanalytical chemistry. 2012:1–27. doi: 10.1007/s00216-012-6203-4. [DOI] [PubMed] [Google Scholar]
- 5.Turbett GR, Sellner LN. The use of optimal cutting temperature compound can inhibit amplification by polymerase chain reaction. Diagnostic molecular pathology: the American journal of surgical pathology, part B. 1997;6(5):298. doi: 10.1097/00019606-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ralton LD, Murray GI. The use of formalin fixed wax embedded tissue for proteomic analysis. Journal of clinical pathology. 2011;64(4):297–302. doi: 10.1136/jcp.2010.086835. [DOI] [PubMed] [Google Scholar]
- 7.Vincenti DC, Murray GI. The proteomics of formalin-fixed wax-embedded tissue. Clinical Biochemistry. 2012 doi: 10.1016/j.clinbiochem.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Shi S-R, Liu C, Balgley BM, Lee C, Taylor CR. Protein extraction from formalin-fixed, paraffin-embedded tissue sections: quality evaluation by mass spectrometry. Journal of Histochemistry & Cytochemistry. 2006;54(6):739–743. doi: 10.1369/jhc.5B6851.2006. [DOI] [PubMed] [Google Scholar]
- 9.Magdeldin S, Yamamoto T. Toward deciphering proteomes of formalin-fixed paraffin-embedded (FFPE) tissues. Proteomics. 2012;12(7):1045–1058. doi: 10.1002/pmic.201100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahram M, Flaig MJ, Gillespie JW, Duray PH, Linehan WM, Ornstein DK, Niu S, Zhao Y, Petricoin EF, Emmert†Buck MR. EvaluaIon of ethanol†fixed, paraffin†embedded tissues for proteomic applications. Proteomics. 2003;3(4):413–421. doi: 10.1002/pmic.200390056. [DOI] [PubMed] [Google Scholar]
- 11.Setou M. Imaging mass spectrometry: protocols for mass microscopy. Vol. 2010. Springer; [Google Scholar]
- 12.Chaurand P, Schwartz SA, Caprioli RM. Peer Reviewed: Profiling and Imaging Proteins in Tissue Sections by MS. Analytical chemistry. 2004;76(5):86–93. [PubMed] [Google Scholar]
- 13.Asomugha C, Gupta R, Srivastava O. Identification of crystallin modifications in the human lens cortex and nucleus using laser capture microdissection and CyDye labeling. Molecular vision. 2010;16476 [PMC free article] [PubMed] [Google Scholar]
- 14.Somiari RI, Sullivan A, Russell S, Somiari S, Hu H, Jordan R, George A, Katenhusen R, Buchowiecka A, Arciero C. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3(10):1863–1873. doi: 10.1002/pmic.200300560. [DOI] [PubMed] [Google Scholar]
- 15.Nirmalan NJ, Hughes C, Peng J, McKenna T, Langridge J, Cairns DA, Harnden P, Selby PJ, Banks RE. Initial development and validation of a novel extraction method for quantitative mining of the formalin-fixed, paraffin-embedded tissue proteome for biomarker investigations. Journal of proteome research. 2010;10(2):896. doi: 10.1021/pr100812d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer-Toy DE, Krastins B, Sarracino DA, Nadol JB, Jr, Merchant SN. Efficient method for the proteomic analysis of fixed and embedded tissues. Journal of proteome research. 2005;4(6):2404–2411. doi: 10.1021/pr050208p. [DOI] [PubMed] [Google Scholar]
- 17.Scicchitano MS, Dalmas DA, Boyce RW, Thomas HC, Frazier KS. Protein extraction of formalin-fixed, paraffin-embedded tissue enables robust proteomic profiles by mass spectrometry. Journal of Histochemistry & Cytochemistry. 2009;57(9):849–860. doi: 10.1369/jhc.2009.953497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian Y, Bova GS, Zhang H. Quantitative glycoproteomic analysis of optimal cutting temperature-embedded frozen tissues identifying glycoproteins associated with aggressive prostate cancer. Analytical chemistry. 2011;83(18):7013–7019. doi: 10.1021/ac200815q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Y, Kelly-Spratt KS, Kemp CJ, Zhang H. Mapping tissue-specific expression of extracellular proteins using systematic glycoproteomic analysis of different mouse tissues. Journal of proteome research. 9(11):5837–5847. doi: 10.1021/pr1006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Y, Gurley K, Meany DL, Kemp CJ, Zhang H. N-linked glycoproteomic analysis of formalin-fixed and paraffin-embedded tissues. Journal of proteome research. 2009;8(4):1657–1662. doi: 10.1021/pr800952h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah P, Yang S, Sun S, Aiyetan P, Yarema KJ, Zhang H. Mass Spectrometric Analysis of Sialylated Glycans with Use of Solid-Phase Labeling of Sialic Acids. Analytical chemistry. 2013;85(7):3606–3613. doi: 10.1021/ac3033867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang SJ, Li Y, Shah PK, Zhang H. Glycomic analysis using glycoprotein immobilization for glycan extraction. Analytical chemistry. 2013 doi: 10.1021/ac400761e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular & Cellular Proteomics. 2004;3(12):1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.