Abstract
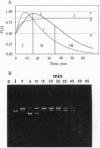
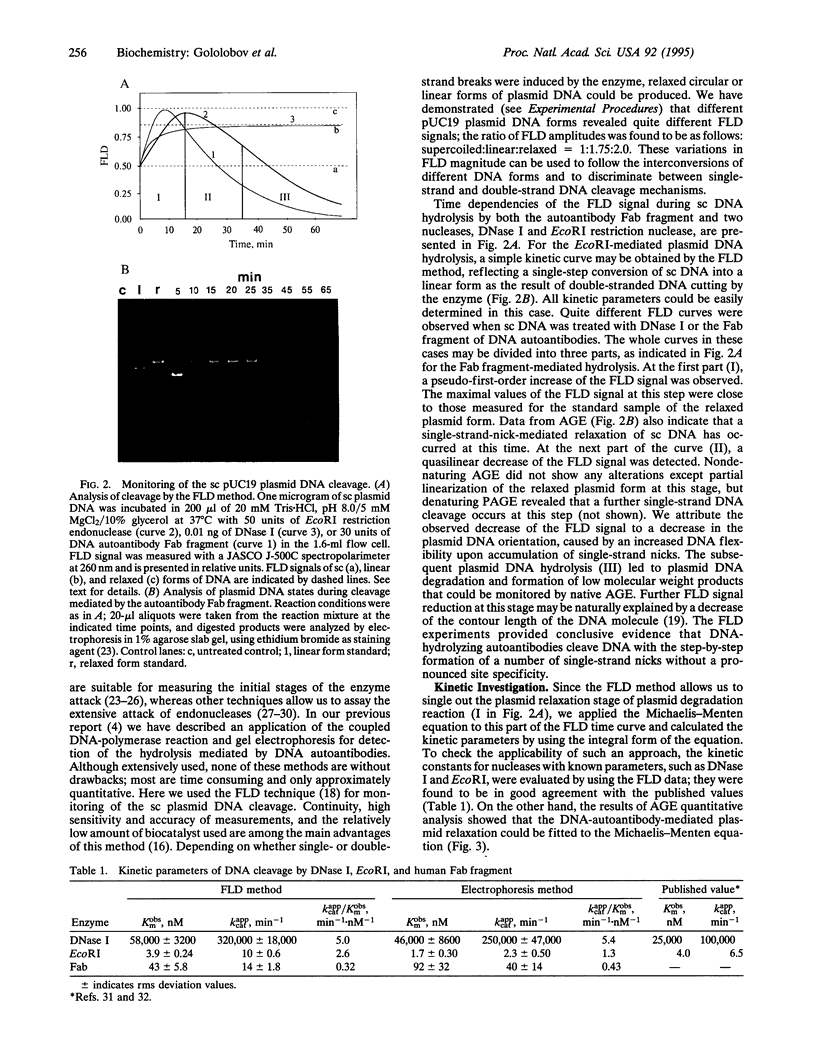
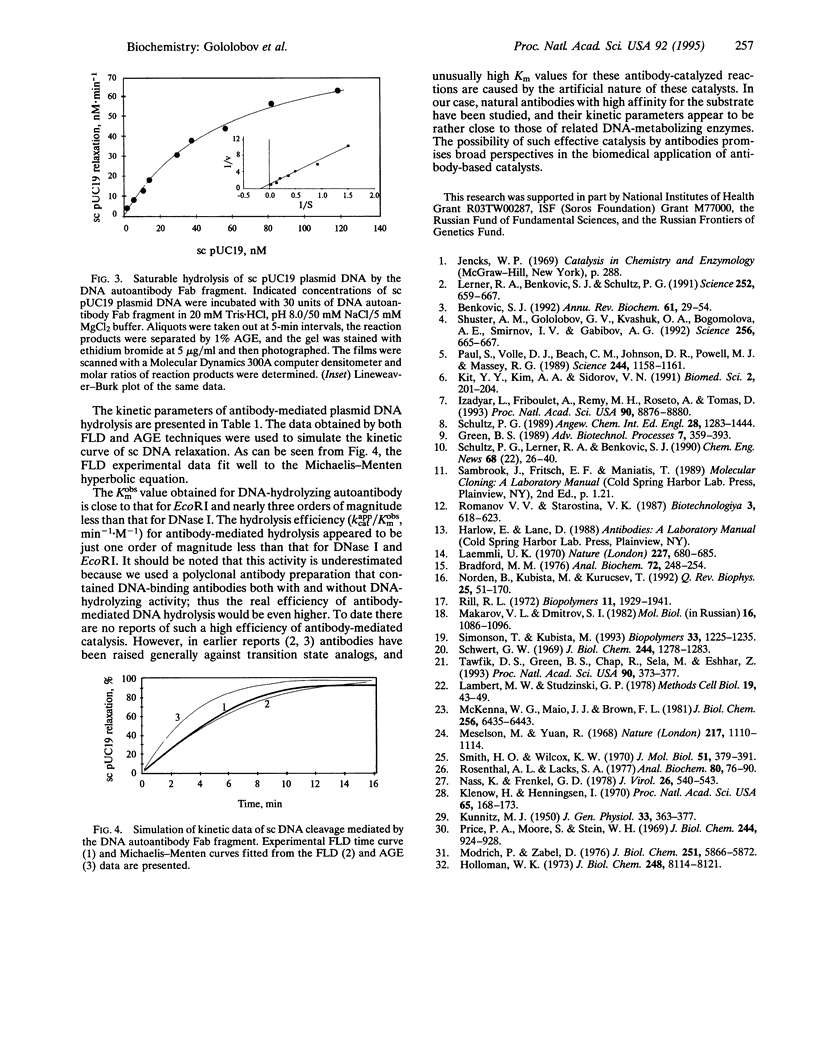
A highly effective method consisting of two affinity chromatography steps and ion-exchange and gel-filtration chromatography steps was developed for purification of autoantibodies from human sera with DNA-hydrolyzing activity. Antibody Fab fragment, which had been purified 130-fold, was shown to catalyze plasmid DNA cleavage. The flow linear dichroism technique was used for quantitative and qualitative studying of supercoiled plasmid DNA cleavage by these autoantibodies in comparison with DNase I and EcoRI restriction endonuclease. The DNA autoantibody Fab fragment was shown to hydrolyze plasmid DNA by Mg(2+)-dependent single-strand multiple nicking of the substrate. Kinetic properties of the DNA autoantibody Fab fragment were evaluated from the flow linear dichroism and agarose gel electrophoresis data and revealed a high affinity (Kobsm = 43 nM) and considerable catalytic efficiency (kappcat/Kobsm = 0.32 min-1.nM-1) of the reaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J. Catalytic antibodies. Annu Rev Biochem. 1992;61:29–54. doi: 10.1146/annurev.bi.61.070192.000333. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Green B. S. Monoclonal antibodies as catalysts and templates for organic chemical reactions. Adv Biotechnol Processes. 1989;11:359–393. [PubMed] [Google Scholar]
- Holloman W. K. Studies on a nuclease from Ustilago maydis. II. Substrate specificity and mode of action of the enzyme. J Biol Chem. 1973 Dec 10;248(23):8114–8119. [PubMed] [Google Scholar]
- Izadyar L., Friboulet A., Remy M. H., Roseto A., Thomas D. Monoclonal anti-idiotypic antibodies as functional internal images of enzyme active sites: production of a catalytic antibody with a cholinesterase activity. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8876–8880. doi: 10.1073/pnas.90.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; digestion of thymus nucleic acid; the kinetics of the reaction. J Gen Physiol. 1950 Mar;33(4):363–377. doi: 10.1085/jgp.33.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit Y. Y., Kim A. A., Sidorov V. N. Affinity-purified secretory immunoglobulin A possesses the ability to phosphorylate human milk casein. Biomed Sci. 1991;2(2):201–204. [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert M. W., Studzinski G. P. Methods for assessment of DNase activity. Methods Cell Biol. 1978;19:43–49. doi: 10.1016/s0091-679x(08)60008-4. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J., Schultz P. G. At the crossroads of chemistry and immunology: catalytic antibodies. Science. 1991 May 3;252(5006):659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Dimitrov S. I. Izuchenie strukturnykh izmenenii v khromatine v prisutstvii mono- i divalentnykh karionov metodom lineinogo dikhroizma v potoke. Mol Biol (Mosk) 1982 Sep-Oct;16(5):1086–1096. [PubMed] [Google Scholar]
- McKenna W. G., Maio J. J., Brown F. L. Purification and properties of a mammalian endonuclease showing site-specific cleavage of DNA. J Biol Chem. 1981 Jun 25;256(12):6435–6443. [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Nass K., Frenkel G. D. Adenovirus-induced inhibition of cellular DNase. J Virol. 1978 May;26(2):540–543. doi: 10.1128/jvi.26.2.540-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden B., Kubista M., Kurucsev T. Linear dichroism spectroscopy of nucleic acids. Q Rev Biophys. 1992 Feb;25(1):51–170. doi: 10.1017/s0033583500004728. [DOI] [PubMed] [Google Scholar]
- Paul S., Volle D. J., Beach C. M., Johnson D. R., Powell M. J., Massey R. J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989 Jun 9;244(4909):1158–1162. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- Price P. A., Moore S., Stein W. H. Alkylation of a histidine residue at the active site of bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):924–928. [PubMed] [Google Scholar]
- Rill R. L. The linear dichroism of oriented helical and superhelical polymers. Biopolymers. 1972;11(9):1929–1941. doi: 10.1002/bip.1972.360110913. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Schwert G. W. Use of integrated rate equations in estimating the kinetic constants of enzyme-catalyzed reactions. J Biol Chem. 1969 Mar 10;244(5):1278–1284. [PubMed] [Google Scholar]
- Shuster A. M., Gololobov G. V., Kvashuk O. A., Bogomolova A. E., Smirnov I. V., Gabibov A. G. DNA hydrolyzing autoantibodies. Science. 1992 May 1;256(5057):665–667. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Tawfik D. S., Green B. S., Chap R., Sela M., Eshhar Z. catELISA: a facile general route to catalytic antibodies. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):373–377. doi: 10.1073/pnas.90.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]