Abstract
Background:
Quorum sensing (QS) in Pseudomonas aeruginosa plays a key role in virulence factor production, biofilm formation and antimicrobial resistance. Because of emerging antimicrobial resistance in P. aeruginosa, there is a need to find an alternate nonantibiotic agent for the control of infections caused by this organism.
Objective:
To evaluate anti-QS activity of Adenanthera pavonina L., a medicinal plant used in traditional medicine.
Materials and Methods:
Preliminary screening for anti-QS activity of ethanol extract of A. pavonina was carried out using Chromobacterium violaceum CV026 biosensor strain and inhibition of QS-regulated violacein production was quantified using C. violaceum ATCC12472. Bioassay guided fractionation of ethanol extract resulted in ethyl acetate fraction (AEF) with strong anti-QS activity and AEF was evaluated for inhibition of QS-regulated pyocyanin production, proteolytic, elastolytic activity, swarming motility and biofilm formation in P. aeruginosa PAO1.
Results:
AEF, at 0.5 mg/ml, inhibited pyocyanin production completely and at 1 mg/ml of AEF, complete inhibition of proteolytic and elastolytic activities were observed. However, viability of P. aeruginosa PAO1 was not affected at the tested concentrations of AEF as observed by cell count. Swarming motility was inhibited at the concentration of 0.1 mg/ml of AEF. Thin layer chromatography and biosensor overlay of AEF showed violacein inhibition zone at Rf value 0.63.
Conclusion:
From the results of this study, it can be concluded that A. pavonina extracts can be used as effective anti-QS agents.
Keywords: Adenanthera pavonina, Pseudomonas aeruginosa, Quorum sensing, Virulence
INTRODUCTION
Many pathogenic Gram-negative bacteria rely on quorum sensing (QS) circuits as central regulators of virulence expression. QS is the regulation of gene expression in accordance with population density through chemical signal molecules.[1] QS is well-established in Chromobacterium violaceum where violacein production is under the control of N-acyl homoserine lactone (AHL)-mediated QS system.[2] Similarly, in Pseudomonas aeruginosa, the production of virulence factors and formation of biofilms are mediated by QS-regulated gene expression.[3] Therefore, QS has been suggested as an ideal target for the development of novel anti-infective drugs, which would function to efficiently interfere with QS signal molecules, and thereby be capable of inducing chemical attenuation of pathogens. As QS is not directly involved in processes essential for growth of the bacteria, inhibition of QS does not impose harsh selective pressure for development of resistance as with antibiotics.[4]
Importance of QS system has been demonstrated in various infections like cystic fibrosis, burn wound, respiratory tract infections, microbial keratitis and urinary tract infection caused by P. aeruginosa.[5] To orchestrate synchronous production of virulence factors and biofilm formation, P. aeruginosa relies on two major LuxI/R quorum-sensing systems, the Las and Rhl systems, which are arranged in a hierarchical manner such that the las system activates the rhl system. Virulence factors of P. aeruginosa namely pyocyanin, proteases, elastases, exotoxin A and rhamnolipids are QS-dependent.[6,7]
Plants have evolved numerous chemical strategies for deterring pathogen attack, including the production of bactericidal and anti-infective compounds, leading to their use as medicines. Adenanthera pavonina L. (Family: Fabaceae) known as red bead tree, found in the Western Ghat region of India has been part of the Indian Ayurvedic medicine. Various parts of this plant have been used in the treatment of diarrhea, gout, inflammations, tumor and ulcers, and as a tonic.[8] Previous phytochemical studies on this plant revealed the presence of secondary metabolites, mainly flavonoids, steroids, saponins, tannins and triterpenoids.[9,10] As a part of the screening studies for identifying the potential sources for anti-QS activity, A. pavonina leaf extract was tested and it showed positive for anti-QS activity. Hence, detailed study using the solvent extract of A. pavonina was undertaken to demonstrate the anti-QS activity.
MATERIALS AND METHODS
Bacterial strains, media and culture conditions
C. violaceum ATCC12472, a mini-Tn5 mutant C. violaceum CV026 and P. aeruginosa PAO1 were used to study the anti-QS potential of A. pavonina. All the bacterial strains were grown in LB (Luria-Bertani) medium by incubating at 32°C except P. aeruginosa PAO1, which was incubated at 37°C for 24 h. C. violaceum CV026 is a mutant that produces violacein pigment only in the presence of exogenous C6 -AHL. For all the experiments, inoculum was prepared by growing the bacteria in 10 ml LB broth under shaking (130 rpm) for 24 h. The cell density was measured spectrophotometrically (UV-1800, Shimadzu, Japan).
Collection of plant materials and extract preparation
The fresh leaves of A. pavonina were collected from the foothills of Western Ghat region in Karnataka, India, washed in sterile water and air dried. The dried leaves were pulverized in an analytical mill (IKA, Germany) to fine powder. For the preparation of ethanolic extract, 100 g of powdered leaves of A. pavonina was repeatedly extracted with 90% ethanol in the soxhlet extractor at 70°C for 16 h. The extract was concentrated to dryness and stored at 4°C until further use.
Biosensor bioassay
For the detection of anti-QS activity of the extract, disc diffusion agar plate assay was carried out using the reporter strain C. violaceum CV026 as described earlier.[2] Different concentrations of ethanol extract of A. pavonina (10 μl) loaded onto 6 mm sterile discs (Himedia, India) were placed on the LB agar plates that were seeded with 100 μl of C. violaceum CV026 and supplemented with 100 μl of 5 μg/ml of C6 -AHL. Inhibition of QS was detected after 24 h incubation by the presence of a zone of pigmentless but viable cells around the disc. The experiment was also repeated with wild type strain C. violaceum ATCC12472.
Bioassay-guided fractionation of ethanol extract
The ethanolic extract was fractionated into hexane fraction (HF), water soluble (WF) and water insoluble (WI) fractions. The WF fraction was repeatedly re-extracted with ethyl acetate to obtain ethyl acetate fraction (AEF). All the fractions were concentrated, redissolved in dimethyl sulfoxide and subjected to biosensor bioassay using 10 μl of the concentrated fractions in sterile discs. The most active fraction, AEF, was used for further studies.
Quantification of inhibition of violacein production in C. violaceum 12472
Inhibition of violacein production in the presence of solvent extracts was quantified using previously described method with modification.[11] Briefly, 10 ml LB broth containing AEF (0.25-1 mg/ml) was inoculated with 100 μl of C. violaceum ATCC12472 and incubated at 32°C for 24 h under shaking (130 rpm). After incubation, culture (1 ml) was centrifuged and the cell pellet was mixed with equal volume of water saturated n-butanol and centrifuged again. The supernatant containing violacein was quantified spectrophotometrically at OD585. The cell viability in the culture medium was tested by standard plate count method.
Effect of AEF on QS-controlled virulence factors production in P. aeruginosa PAO1
Effect of active fraction, AEF, on widely studied QS-controlled virulence factors such as pyocyanin, proteolytic, elastolytic activity, swarming motility and biofilm formation was tested in P. aeruginosa PAO1. To determine the effect of plant extract on pyocyanin production, P. aeruginosa PAO1 was grown in glycerol alanine minimal medium in the presence or absence of AEF for 24 h at 37°C.[12] Pyocyanin from the cell-free supernatant (5 ml) was extracted with 3 ml of chloroform and subsequently, the chloroform layer was acidified with HCl. The pyocyanin-rich acid layer was quantified by recording OD520 spectrophotometrically. Swarming assay was performed in LB semisolid (0.5% agar) medium supplemented with AEF. LB plates were point inoculated with P. aeruginosa PAO1 and incubated at 37°C for 24 h. The extent of swarming was determined by measuring the swarming diameter.[13]
Inhibition of proteolytic and elastolytic activities was assessed according to previously described methods.[14,15] Briefly, P. aeruginosa PAO1 was grown in LB medium supplemented with different concentrations of AEF and incubated at 37°C for 16 h. Culture supernatant (100 μl) was added to 900 μl of elastin congo red (ECR) buffer (100 mM Tris, 1 mM CaCl2, pH 7.5) containing 20 mg ECR (Sigma) and incubated for 3 h at 37°C. Insoluble ECR was removed by centrifugation, and the absorbance of the supernatant was measured at 495 nm. For proteolytic activity, 100 μl culture supernatant was added to 900 μl of ECR buffer containing 3 mg of azocasein (Sigma) and were incubated at 37°C for 30 min. Trichloroacetic acid (10%, 100 μl) was added to each reaction tube. After 30 min, the tubes were centrifuged and absorbance of the supernatant was determined at 440 nm. Cell-free LB medium alone and with extract were used as negative controls for both the assays.
Biofilm studies were carried out as described by Vandeputte et al.[16] Briefly, 50 μl of overnight grown P. aeruginosa PAO1 culture (106 CFU/ml) was diluted to 3 ml with fresh tryptone broth containing CEA and incubated statically for 18 h. The biofilm formed was assessed by crystal violet staining method by recording OD590 spectrophotometrically.
Separation of anti-QS compounds by thin layer chromatography
The active fraction AEF was spotted on a silica gel TLC plate and chromatographed using chloroform: methanol (80:20) solvent system. After elution, the plate was dried and overlaid with sterile LB medium containing exogenous C6-AHL inoculated with C. violaceum CV026 biosensor strain. The TLC overlay was incubated at 32°C for 24 h and anti-QS activity was detected by the presence of turbid halo region in purple background.
RESULTS
Inhibition of QS by A. pavonina extracts
The ethanol extract of A. pavonina showed anti-QS activity in C. violaceum CV026 biosensor bioassay. After 24 h incubation, clear turbid halo zone of 12 mm diameter of violacein inhibition was observed at the concentration of 200 μg/disc. The antimicrobial activity was ruled out as the sample from the halo zone region showed the growth of bacterial cells on LB agar medium, which further confirms the inhibition of violacein production by A. pavonina. Bioassay-guided fractionation resulted in AEF with more pronounced anti-QS activity with 24 mm diameter of violacein inhibition zone at the concentration of 100 μg/disc [Figure 1a]. HF, WI did not show anti-QS activity in bioassay.
Figure 1.
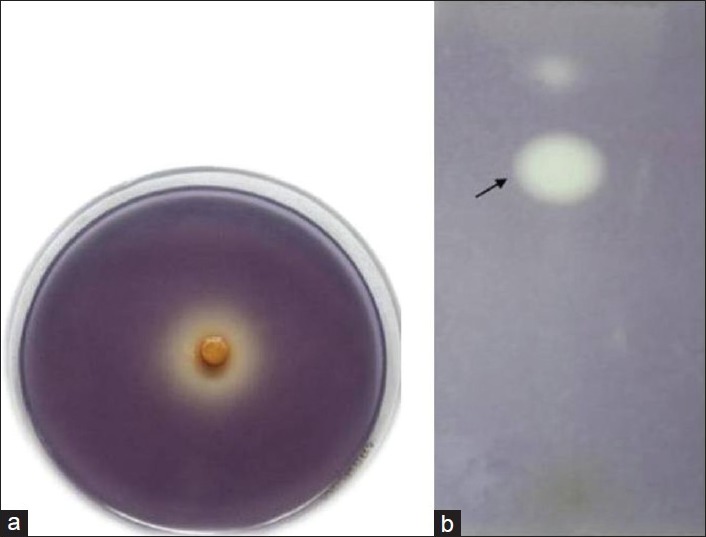
Anti-QS activity of active fraction (AEF) of A. pavonina. (a) Biosensor bioassay of AEF showing inhibition of C6-AHL-mediated violacein production in bioreporter C. violaceum CV026 (b) TLC overlay assay of AEF illustrating the inhibition of C6-AHL-mediated violacein production in C. violaceum CV026. Areas of pigment clearing (indicated by arrow) show region of AEF compounds that inhibit AHL-regulated violacein production in C. violaceum CV026
Inhibition of violacein production in C. violaceum ATCC12472
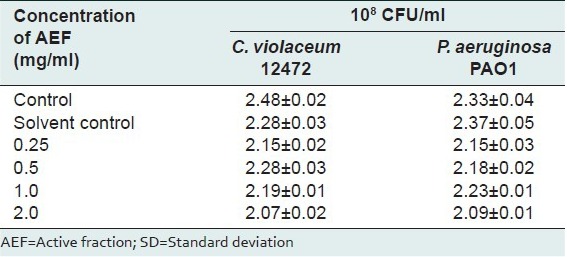
The active fraction, AEF, inhibited violacein production in a concentration-dependent manner as evidenced by the quantitative assay conducted using C. violaceum ATCC12472. The tested concentrations of AEF (0.25–1.0 mg/ml) showed a significant drop in violacein content [Figure 2] without interfering with the bacterial growth [Table 1].
Figure 2.
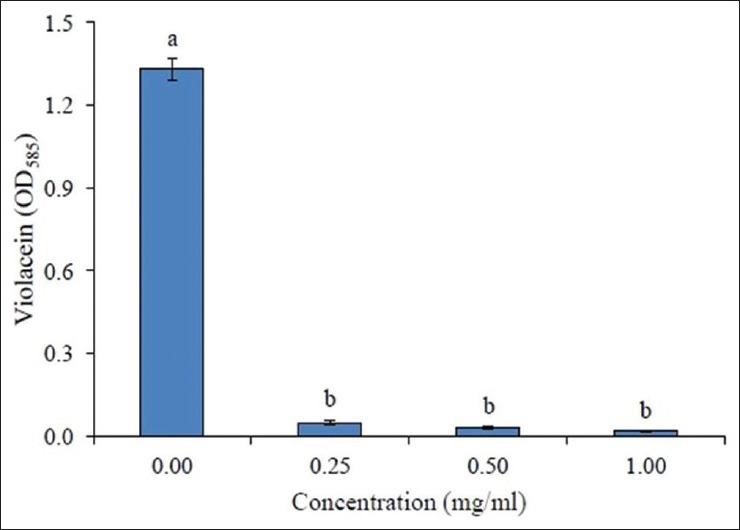
Inhibition of violacein production in C. violaceum ATCC12472 by different concentrations of active fraction (AEF) of A. pavonina. Values are expressed as mean ± SD, n = 4. Same letters in the columns (bar) are not significantly different (P < 0.001)
Table 1.
Effect of active fraction of A. pavonina on the viability of the bacterial cells in the culture medium as estimated by the standard plate count method after 24 h incubation. Values are mean±SD, n=4
Effect of AEF of A. pavonina on virulence factor production in P. aeruginosa PAO1
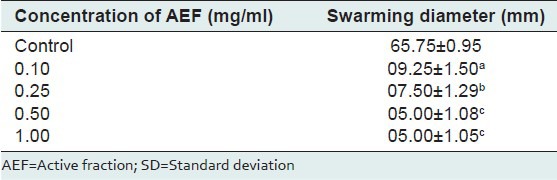
The AEF fraction significantly inhibited the production of pyocyanin in P. aeruginosa PAO1 [Figure 3a]. At 0.25mg/ml of AEF, 50% inhibition of pyocyanin production was obtained and complete inhibition of pyocyanin production was attained at 1.0 mg/ml concentration of AEF. The swarming motility was completely inhibited in P. aeruginosa PAO1 at a very low concentration of 100 μg/ml of AEF [Table 2]. Under control conditions, the swarming diameter was 65 mm and in the presence of AEF, the bacteria were able to grow and form a colony in the center with diameter not exceeding 12 mm, and tendril formation or other features indicative of swarming motility were not observed. When P. aeruginosa PAO1 was grown in the presence of AEF, significant decrease in the elastolytic and proteolytic activities was observed in a concentration-dependent manner [Figure 3b]. At 1.0 mg/ml concentration, AEF showed complete inhibition of elastolytic and proteolytic activities in P. aeruginosa PAO1. Since biofilm formation is partially controlled by QS mechanisms, the effect of AEF on biofilm formation in P. aeruginosa PAO1 was assessed after 18 h of growth. At 1.0 mg/ml concentration of AEF, the biofilm formation was decreased by 81% [Figure 3a].
Figure 3.
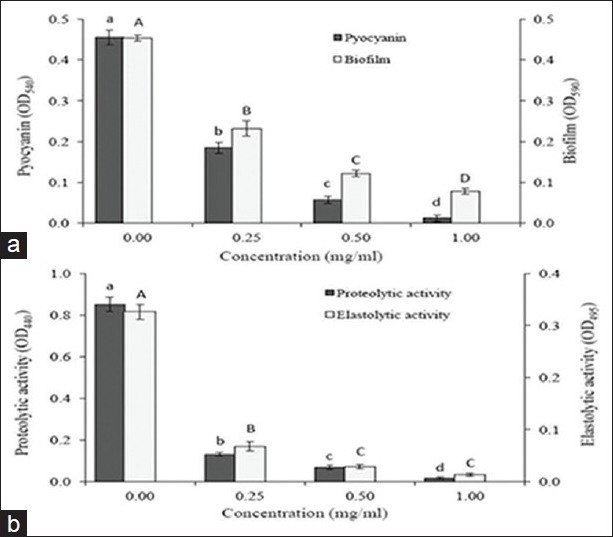
The effect of AEF on QS-regulated virulence factor production in P. aeruginosa PAO1. (a) Pyocyanin production and biofilm formation (b) Proteolytic activity and elastolytic activities. Values are expressed as mean ± SD, n = 4. Same letters in the columns (bar) are not significantly different (P < 0.001)
Table 2.
Inhibition of swarming motility in Pseudomonas aeruginosa PAO1 by different concentrations of active fraction of A. pavonina. Values are mean±SD. n=4. Same letters in the columns are not significantly different (P<0.001)
Phytochemical screening of anti-QS compounds separated by TLC
The AEF fraction was spotted onto a silica gel TLC plate and eluted with chloroform: Methanol, after which the TLC plate was cast into agar containing C. violaceum CV026 biosensor strain. After incubation, a band showing anti-QS zone was observed with Rf value 0.63 [Figure 1b].
DISCUSSION
QS plays an important role in the regulation of cell physiology in many Gram-negative bacteria. QS system consisted of inducer and regulator proteins of las and rhl components, which work interdependently in a hierarchal manner to regulate the expression of various genes, including virulence ones in P. aeruginosa.[17] In the present study, A. pavonina inhibited of both las- and rhl-mediated phenotypes in P. aeruginosa PAO1. Preliminary screening of anti-QS activity was carried out using C. violaceum CV026 biosensor strain. In C. violaceum CV026 plate assay, formation of halo zone indicates that A. pavonina is either inhibiting the C6-AHL competitively from binding to its transcriptional regulator, CviR; degrading the C6-AHL enzymatically, or removing the C6-AHL via active transport.[18,19] Inhibition of violacein production was quantified and from the results obtained in this study, it was proven that A. pavonina reduced violacein production significantly. In agreement to this finding, other plant extracts of Conocarpus erectus, Quercus virginiana and other higher plants have demonstrated the anti-QS activity against biosensor strain C. violaceum CV026.[20]
In P. aeruginosa, the production of pyocyanin is under the control of rhlI-rhlR QS system. Pyocyanin is highly permeable to the biological membrane and causes extensive cellular damage in the lungs of cystic fibrosis patients. Secretion of elastase and protease enzymes is also an important aspect of pathogenicity, which helps in combating adverse conditions and tissue colonization inside the host.[21] P. aeruginosa exhibits swarming motility, which helps in initial attachment and later in relocation of biofilm from one site to another.[22] In the present study, significant reduction in the pyocyanin production, proteolytic and elastolytic activities, swarming motility, and biofilm formation in P. aeruginosa PAO1were observed in the presence of AEF of A. pavonina.
The anti-QS compound present in the active fraction of A. pavonina was separated by TLC. Phytochemical analysis of A. pavonina plant is still in its primitive stages; exploration of its activity may invite further studies on the phytochemicals present. Tannin-rich fraction from Terminalia catappa showed inhibition of QS-mediated virulence factor production in C. violaceum and P. aeruginosa PAO1.[23] Flavonoids, catechin and naringin also been proved to inhibit QS in Gram-negative pathogens.[24] Some known mechanisms of QS inhibition include competitive binding of signal-like molecules to cognate receptors, as in the case of furanones, enzymatic degradation of QS signals, as in the case of AHL acylases. Further studies are needed to demonstrate the exact mechanism of QS inhibition by A. pavonina phytocompounds. It is interesting to study the important mechanism involved by the plants used in traditional phytomedicine and revealing the possible mechanism/mode of action shall aid in the invention of lead molecules for the antimicrobial drugs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rutherford ST, Bassler BL. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClean KH, Winson MK, Fish L, Taylor A, Chabra SR, Camara M, et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–11. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 3.Roger SS, Iglweski BH. Pseudomonas aeruginosa quorum sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 4.Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 2003;112:1300–7. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willcox MD, Zhu H, Conibear TC, Hume EB, Givskov M, Kjelleberg S, et al. Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology. 2008;154:2184–94. doi: 10.1099/mic.0.2008/019281-0. [DOI] [PubMed] [Google Scholar]
- 6.Williams P, Camara M, Hardman A, Swift S, Milton D, Hope VJ, et al. Quorum sensing and the population-dependent control of virulence. Philos Trans R Soc Lond Biol Sci. 2000;355:667–80. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster M, Greenberg EP. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Adedapo AD, Osude YO, Adedapo AA, Moody JO, Adeagbo AS, et al. Blood pressure lowering effect of Adenantherapavonina seed extract on normotensive rats. Records Nat Prod. 2009;3:82–9. [Google Scholar]
- 9.Su EN, Yu SS, Pei YH. Studies on chemical constituents from stems and leaves of Adenantherapavonina L. Zhongguo Zhongyao Zazhi. 2007;32:2135–8. [PubMed] [Google Scholar]
- 10.Kothale KV, Rothe SP. Phytochemical screening of Adenantherapavonina Linn. World J Sci Tech. 2012;2:19–22. [Google Scholar]
- 11.Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42:637–41. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 12.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Inter changeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vattem DA, Mihalik K, Crixell SH, McLean RJ. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78:302–10. doi: 10.1016/j.fitote.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Kessler E, Israel M, Landshman N, Chechik A, Blumberg S. In vitro inhibition of Pseudomonas aeruginosaelastase by metal-chelating peptide derivatives. Infect Immun. 1982;38:716–23. doi: 10.1128/iai.38.2.716-723.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihalik K, Chung DW, Crixell SH, McLean RJ, Vattem DA. Quorum sensing modulators of Pseudomonas aeruginosa characterized in Camellia sinensis. Asian J Trad Med. 2008;3:12–23. [Google Scholar]
- 16.Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, El Jaziri M, et al. Identification of catechin as one of the flavonoids from Combretumalbiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl Environ Microbiol. 2010;76:243–53. doi: 10.1128/AEM.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner VE, Li LL, Isabella VM, Iglewski BH. Analysis of the hierarchy of quorum-sensing regulation in Pseudomonas aeruginosa. Anal Bioanal Chem. 2007;387:469–79. doi: 10.1007/s00216-006-0964-6. [DOI] [PubMed] [Google Scholar]
- 18.Martinelli D, Grossmann G, Sequin U, Brandl H, Bachofen R. Effects of natural andchemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol. 2004;4:25. doi: 10.1186/1471-2180-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer WD, Teplitski M. Can plants manipulate bacterial quorum sensing? Aust J Plant Physiol. 2001;28:913–21. [Google Scholar]
- 20.Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol. 2006;105:427–35. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Siehnela R, Traxlerb B, An DD, Parsek MR, Schaeferb AL, Singh PK, et al. Unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2010;107:7916–21. doi: 10.1073/pnas.0908511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taganna JC, Quanico JP, Perono RM, Amor EC, Rivera WL. Tannin-rich fraction from Terminaliacatappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm formation and Las Astaphylolytic activity in Pseudomonas aeruginosa. J Ethnopharmacol. 2011;134:865–71. doi: 10.1016/j.jep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, et al. Evidence that halogenated furanones from Deliseapulchra inhibitacylatedhomoserine lactone (AHL)-mediated gene expression by displacingthe AHL signal from its receptor protein. Microbiology. 1999;145:283–91. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 2002;99:4638–43. doi: 10.1073/pnas.022056699. [DOI] [PMC free article] [PubMed] [Google Scholar]


