Abstract
Background:
Natural products have continually played an important role in drug discovery because it serves as active principles in drugs as well as templates for synthesis of new drugs. Cayratia trifolia (L.) is a medicinal plant, which has been reported to have antiviral, antibacterial, antiprotozoal, hypoglycemic, anticancer and diuretic activities.
Objective:
Therefore, the objective of this study is to isolate and identify the natural compound from the ethanolic extract of Cayratia trifolia (L.) and to predict the Absorption, Distribution, Metabolism and Excretion (ADME) properties of isolated natural compound.
Materials and Methods:
Column chromatography and thin layer chromatography were used to isolate the natural compound and Fourier-transform infrared (FTIR) spectroscopy was used to predict the functional groups present in the isolated natural compound. The structural characterization studies were functionally carried out using 1H, 13C, two-dimensional nuclear magnetic resonance (NMR) and mass spectrometry methods.
Results:
FTIR showed that, the groups of OH, C-H, C = C may be present in the isolated natural compound. 1H, 13C, two-dimensional NMR and mass spectrometry data suggests that the isolated natural compound probably like linoleic acid. In silico ADME properties, prediction of the compound was under acceptable range.
Conclusion:
Based on the results, it can be concluded that, the isolated natural compound of linoleic acid that has been exhibited good medicinal properties.
Keywords: Cayratia trifolia (L.), Chromatography techniques, Spectroscopy methods, Linoleic acid and ADME properties
INTRODUCTION
Medicinal plants provide an inexhaustible resource of raw materials for the pharmaceutical, cosmetics and food industries and more recently in agriculture for pest control. People have learned to increase the power of medicinal plants by preparing medicinal compounds from them.[1,2] In traditional societies, nutrition and health care are strongly interconnected and many plants have been consumed both as food and for medicinal purposes.[3] Natural products from medicinal plants provide ultimate opportunities for new drug leads because of the unmatched availability of chemical diversity.[4,5,6] Due to the demand of chemical diversity in screening programs, seeking therapeutic drugs from natural products has grown throughout the world. In addition, a good proportion of drugs that have been approved for clinical trials, are either Natural products or their analogues.[7,8] The active compounds do not play an important role in the metabolism of plants and hence it is often referred to as secondary metabolites.[9] Finding new secondary metabolites is a prerequisite for the development of novel pharmaceuticals. This thematic series on the biosynthesis and function of secondary metabolites deals with the discovery of new biologically active compounds from all kinds of sources such as medicinal plants.[10]
Cayratia trifolia (L.) is the medicinal plant of the family Vitaceae. It is commonly known as Fox grape in English, it's a native to India, Asia and Australia. Whole plant of Cayratia trifolia (L.) has been reported to contain yellow waxy oil, steroids, terpenoids, flavonoids and tannins by preliminary phytochemical screening.[11,12] Leaves contain stilbenes, piceid, reveratrol, viniferin and ampelopsin.[13] Stem, leaves and roots are reported to possess hydrocyanic acid and delphinidin. Several flavonoids such as cyanidins are reported in the leaves.[14] Root paste mixed with coconut oil can be used as a decoction. Roots grounded with black pepper can be used as a poultice on boils. Infusion of seeds along with an extract of tubers is traditionally given orally to diabetic patients to check sugar level of blood.[15] Paste of tubers is applied on the affected part in the treatment of snake bite. Whole plant is used as diuretic, in tumors, neuralgia and splenopathy.[16] The bark extract has been reported to have antiviral, antibacterial, antiprotozoal, hypoglycemic, anticancer and diuretic activities in animal models.[17] Therefore, the main aim of the present study is to isolate and identify the natural compound from ethanolic extract of Cayratia trifolila (L.) and to analyze their drug like properties.
MATERIALS AND METHODS
Collection of plant material
Cayratia trifolila (L.) was collected from in and around the area of Kumbakonam, Thanjavur District, Tamil Nadu, India. The plant was authenticated by
Dr. P. Sathyanarayanan, Botanical Survey of India, Tamil Nadu Agricultural University Campus, Coimbatore. The voucher number is BSI/SRC/5/23/2010-2011/Tech. 1527.[18] Fresh whole plant material was washed under running tap water, air-dried and powdered.
Preparation of extract
Based on the previous studies, 300 g of plant powder was extracted with 1500 ml of ethanol for 72 h in occasional shaker at room temperature. The extract was collected and concentrated at 40°C under reduced pressure using a rotary evaporator. The dried extract was stored at 4°C until further compound isolation process.
Compound isolation
The ethanolic extract of Cayratia trifolia (L.) 5 g was fractionated on silica gel column (3 × 30 cm) and successfully eluted with petroleum ether (100%) followed by petroleum ether: Chloroform (8:2, 6:4, 4:4, 2:8 v/v). The column fractions were collected in 20 ml of test tubes. Totally 72 fractions were collected and each fraction was analyzed by thin layer chromatography (TLC) plates for a single spot.
Functional group analysis and structure elucidation
The Shimadzu FTIR-8400S Fourier Transform Infrared Spectrometer instrument used for analyzes the presence of functional groups in the isolated compound. The spectrometer works under purged condition. Solid sample of an isolated compound was dispersed in polyethylene pellets depending on the region of interest. This instrument has a typical resolution of 1.0/cm. Signal averaging, signal enhancement, baseline correction and other spectral manipulations were possible. Structure elucidations of the isolated compound were carried out using spectroscopic techniques: Mass spectrometry, 1H and 13C NMR together with two-dimensional experiments (correlation spectroscopy [COSY] and heteronuclear single quantum coherence [HSQC]). Optical rotation was determined with a Perkin-Elmer polarimeter (model 341). NMR spectra on solutions in CDCl3 were recorded on a Bruker DRX-500 NMR Spectrometer at 500 MHz (1H) and 125 MHz (13C); the signals of the deuterated solvent were taken as a reference. The molecular mass of the isolated compound was analyzed by mass spectrometry.
Absorption, distribution, metabolism and excretion properties prediction (ADME)
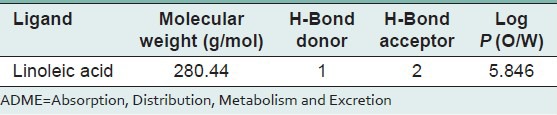
ADME properties prediction were carried out using QikeProp 2.3 module (Schrodinger Suite 2012).[19] QikProp helps in analyzing the pharmacokinetics and pharmacodynamics of the ligand by accessing the drug like properties. Significant ADME properties such as molecular weight (MW), H-bond donor, H-bond acceptor and log P (O/W) were predicted.
RESULTS
The ethanolic extract of Cayratia trifolia (L.) was subjected to column chromatography by using different solvents such as petroleum ether and chloroform in the increasing order of polarity and the fractions were collected. Totally, 72 fractions were collected which is shown in Table 1. Out of these, 10th fraction identified as a single spot [Figure 1] with the Rf value (0.72 cm) using TLC analysis; it may indicate the presence of a single compound in this fraction. About 20 mg of the pure compound was obtained from the single fraction and it was used for further studies.
Table 1.
Collected fractions details by column chromatography
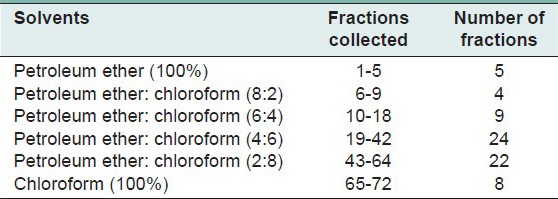
Figure 1.

Thin layer chromatography (TLC) analysis of the isolated natural compound
FTIR analysis of identified functional groups were broad band at 3412/cm for the hydroxyl group, 2928/cm for C–H group, 1728/cm and 1623/cm to show the presence of the carbonyl group and C = C presence in the single faction were shown in Table 2 and Figure 2.
Table 2.
Functional group analysis of 10th fraction using FTIR
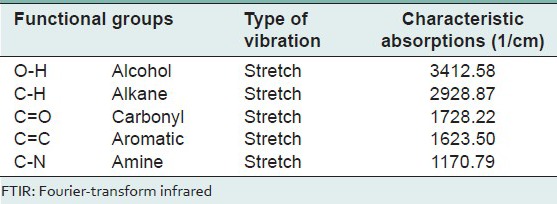
Figure 2.
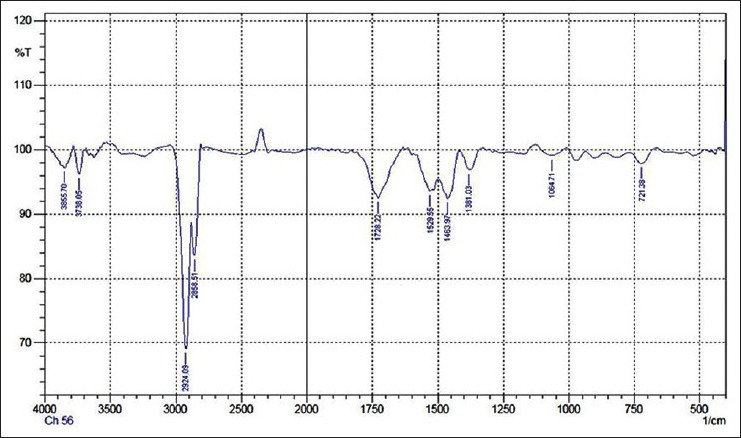
Fourier Transform infra-red spectroscopy analysis of the isolated natural compound
The absorptions of the NMR spectrometry have made a tremendous impact in many areas of chemistry, biology and medicine. In the 1H NMR [Figure 3] spectrum the triplet at δ 0.81 is due to a terminal methyl group, the strong singlet at δ 1.28 is due to long chain methylene groups. The strong signals at δ 1.59, 1.79 and 1.98 are due to methylene protons attached to unsaturated systems and the signal at δ 2.30 are due to two bis allylic protons. The signals at δ 4.56 (1H), 5.12 (2H) and at 5.36 (1H) suggest that the compound contains two double bonds. In the 1H–1H COSY NMR [Figure 4] spectrums cross peaks are observed between double bonded protons and the allylic methylene protons. The protons of one double bond are not coupled with the protons of the other double bond. The coupling between the methyl group at δ 0.81 and methylene protons at δ 1.59, 1.79 and 1.98 were observed. In the 13C NMR [Figure 5] spectrum the signal at δ 15.9 is due to the methyl group, a single at δ 39.4 is due to the α-carbon atom to the carbonyl group, the bunch of signals between 17.6 and 34.4 are due to long chain methylene carbons. The signals at 124.6, 129.3, 134.8 and 135.6 are due to four unsaturated carbon atoms. In the HSQC [Figure 6] spectrum the unsaturated protons and the unsaturated carbons are correlated. Being a long chain fatty acid, mostly it will be a mixture with its homologues fatty acids. The weak signal at δ 173.0 is due to a quaternary carbon (carbonyl carbon). Mass spectrometry analysis revealed [Figure 7] that, molecular weight of the isolated compound was 280.44 (g/mol). All the data suggests that, the compound may be an unsaturated long chain fatty acid probably like linoleic acid [Figure 8 and Table 3]. ADME properties of the isolated natural compound of linoleic acid shown in Table 4 and it was under acceptable range.
Figure 3.
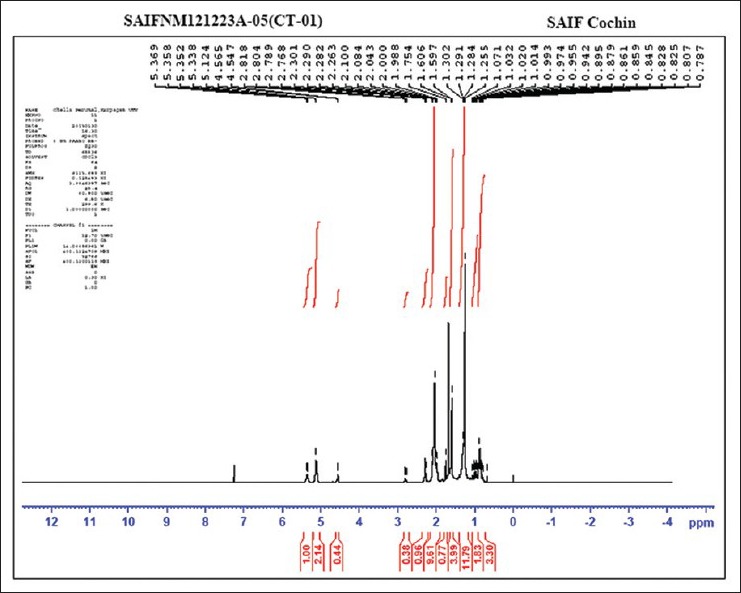
1H NMR spectrum analysis of the isolated natural compound
Figure 4.
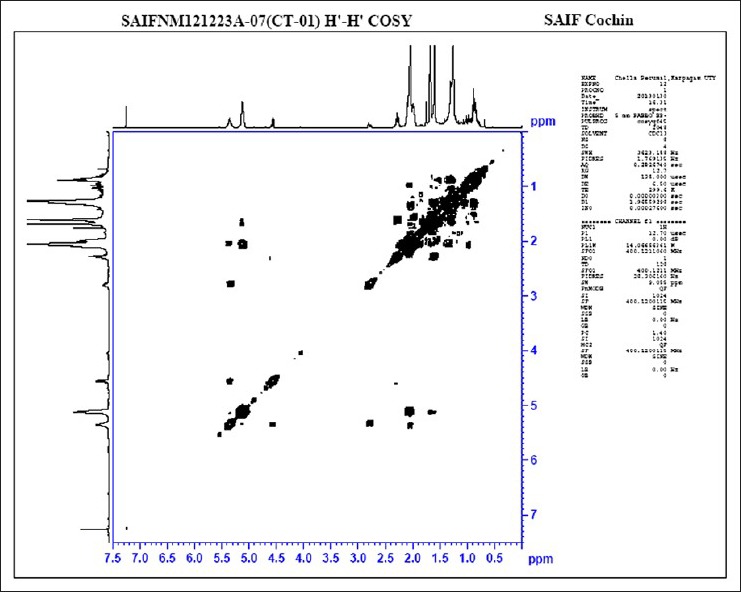
H–H COSY spectrum analysis of the isolated natural compound
Figure 5.
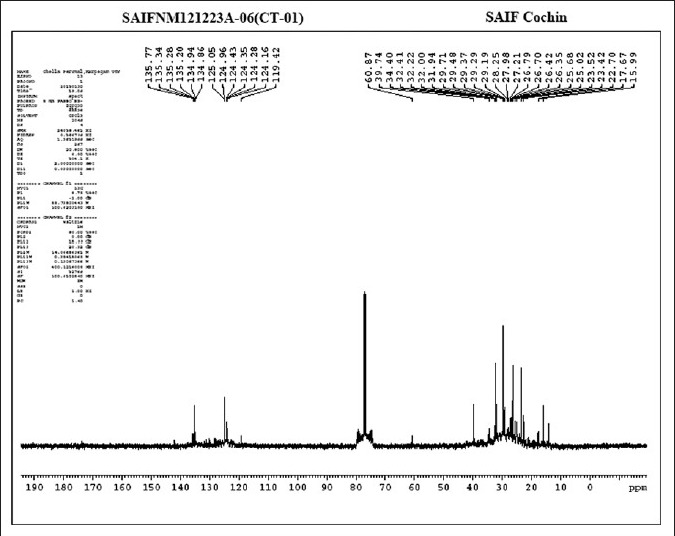
13C NMR spectrum analysis of the isolated natural compound
Figure 6.
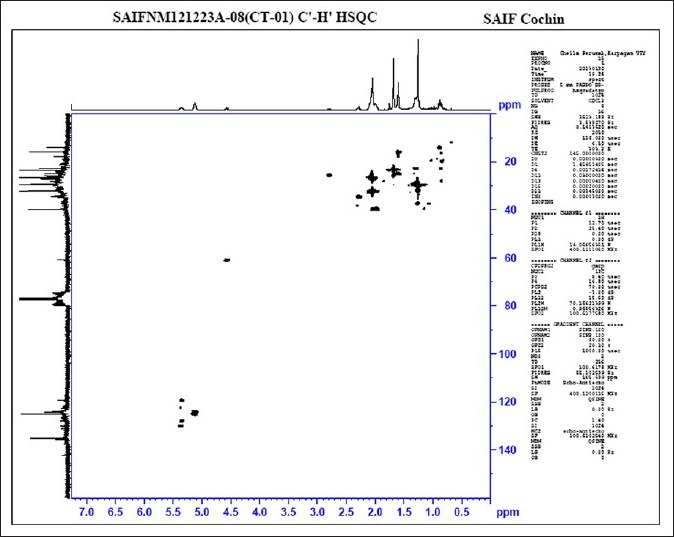
C–H HSQC spectrum analysis of the isolated natural compound
Figure 7.
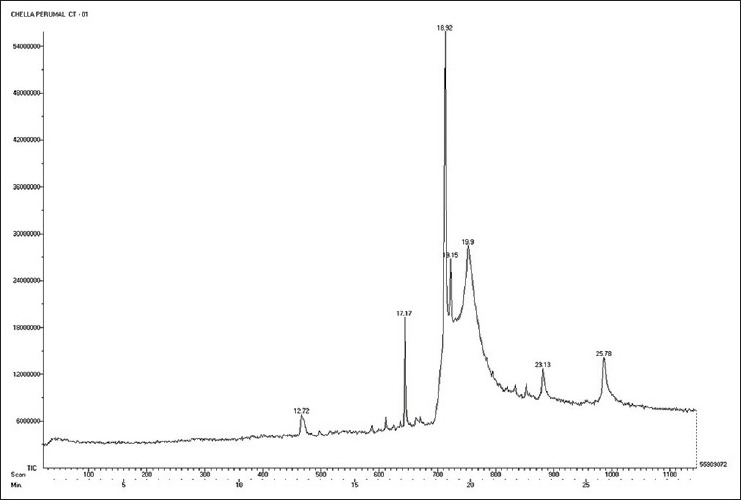
Mass spectrometry result of isolated natural compound
Figure 8.
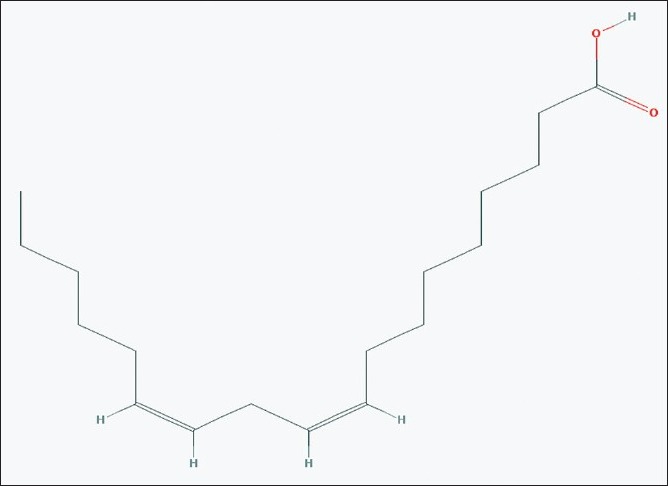
Structure of linoleic acid
Table 3.
Isolated natural compound of linoleic acid details
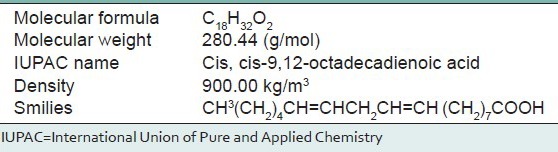
Table 4.
ADME properties of linoleic acid
DISCUSSION
Natural compounds are present in crude plant extract, but they might not be extracted using a single solvent. Different compounds according to their polarity elute out in different solvents.[20] The vital role of these natural constituents of medicinal plants is alkaloids, tannins, flavonoids, steroid, terpenoid, carbohydrate and phenolic compounds.[21] The identification of natural compounds using chromatography and spectroscopic techniques may provide efficient information regarding qualitative and quantitative composition of herbal medicines.[22]
In the present study, column chromatography and TLC eluted a natural compound of linoleic acid is an essential fatty acid that must be consumed for proper health. A diet only deficient in linoleate causes mild skin scaling, hair loss and poor wound healing in rats.[23] Linoleic acid has become increasingly popular in the beauty products industry because of its beneficial properties on the skin. Research points to linoleic acid's anti-inflammatory, acne reductive and moisture retentive properties when applied topically on the skin.[24,25,26,27]
CONCLUSION
In the present study, the natural compound of linoleic acid was isolated and identified from the ethanolic extract of Cayratia trifolia (L.). The ADME properties of the linoleic acid were under acceptable range. Therefore, it can be concluded that this natural compound possess many biological activity. In future, this compound may lead to drug design and development for curing various illness and disorder.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jacob SJ, Shenbagaraman S. Evaluation of antioxidant and antimicrobial activities of the selected green leafy vegetables. Int J PharmTech Res. 2011;3:148–52. [Google Scholar]
- 2.Deshpande HA, Bhalsing SR. Phytochemical analysis of Cassia obtusifolia, Cassia auriculata, Tephrosia purpurea, Helictres isora and Centella asiatica. Int J Pharma Bio Sci. 2011;2:363–7. [Google Scholar]
- 3.Chhetri HP, Yogol NS, Sherchan J, Anupa KC, Mansoor S, Thapa P. Phytochemical and antimicrobial evaluations of some medicinal plants of Nepal. Kathmandu Univ J Sci Eng Technol. 2008;1:49–54. [Google Scholar]
- 4.Stepp JR, Moerman DE. The importance of weeds in ethnopharmacology. J Ethnopharmacol. 2001;75:19–23. doi: 10.1016/s0378-8741(00)00385-8. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari AK, Rao M. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr Sci. 2002;83:30–8. [Google Scholar]
- 6.Dickschat JS. Biosynthesis and function of secondary metabolites. Beilstein J Org Chem. 2011;7:1620–1. doi: 10.3762/bjoc.7.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obi RK, Nwanebu FC, Nnaji UU, Onuoha LN, Chiegboka N. Ethanolic extraction and phytochemical screening of two Nigerian herbs on pathogens isolated from wound infections. Int J Compr Pharm. 2011;10:1–5. [Google Scholar]
- 8.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tutul E, Uddin Z, Rahman O, Hassan A. Angiospermic flora of Runctia sal forest (Bangladesh) II. Magnoliopsida (Dicots) Bangladesh J Plant Taxon. 2010;17:33–45. [Google Scholar]
- 10.Gupta J, Kumar D, Gupta A. Evaluation of gastric anti-ulcer activity of methanolic extract of Cayratia trifolia in experimental animals. Asian Pac J Trop Dis. 2012;2:99–102. [Google Scholar]
- 11.Kumar D, Gupta J, Kumar S, Arya R, Kumar T, Gupta A. Pharmacognostic evaluation of Cayratia trifolia (Linn.) leaf. Asian Pac J Trop Biomed. 2012;2:6–10. doi: 10.1016/S2221-1691(11)60180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perumal PC, Sophia D, Raj CA, Ragavendran P, Starlin T, Gopalakrishnan VK. In vitro antioxidant activities and HPTLC analysis of ethanolic extract of Cayratia trifolia (L.) Asian Pac J Trop Dis. 2012;2:S952–6. [Google Scholar]
- 13.Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar V, Guha G, Kumar RA. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol. 2011;49:363–9. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi AM, Khan MA, Ahmad M, Zafar M, Jahan S, Sultana S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J Ethnopharmacol. 2010;128:322–35. doi: 10.1016/j.jep.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 16.Guha G, Rajkumar V, Kumar RA, Mathew L. Antioxidant activity of Lawsonia inermis extracts inhibits chromium (VI)-induced cellular and DNA toxicity. Evid Based Complement Alternat Med. 2011;2011:576456. doi: 10.1093/ecam/nep205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojewole JA. Hypoglycaemic effect of Clausena anisata (Willd) Hook methanolic root extract in rats. J Ethnopharmacol. 2002;81:231–7. doi: 10.1016/s0378-8741(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 18.Owolabi J, Omogbai EK, Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigelia africana (Bignoniaceae) stem bark. Afr J Biotechnol. 2006;6:882–5. [Google Scholar]
- 19.Pascaline J, Charles M, Lukhoba C, George O. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district Kenya. J Anim Plant Sci. 2011;9:1201–10. [Google Scholar]
- 20.Ahmed FA, Ali RF. Natural compounds and antioxidant activity of fresh and processed white cauliflower. Biomed Res Int. 2013;2013:1–9. doi: 10.1155/2013/367819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa-Pereira L, Pocheville A, Angulo I, Paseiro-Losada P, Cruz JM. Fractionation and purification of bioactive compounds obtained from a brewery waste stream. Biomed Res Int. 2013;2013:408491. doi: 10.1155/2013/408491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender DA. The promise of metabolomics. J Sci Food Agric. 2005;85:7–9. [Google Scholar]
- 23.Maloney V. Plant metabolomics. BioTeach J. 2004;2:92–9. [Google Scholar]
- 24.Ryczkowski J. IR Spectroscopy in catalysis. Catal Today. 2001;68:263–381. [Google Scholar]
- 25.Verma A, Rizvi SM, Shaikh S, Ansari MA, Shakil S, Ghazal F, et al. Compounds isolated from Ageratum houstonianum inhibit the activity of matrix metalloproteinases (MMP-2 and MMP-9): An oncoinformatics study. Pharmacogn Mag. 2014;10:18–26. doi: 10.4103/0973-1296.126653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahon K, Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacognosy Res. 2011;3:13–8. doi: 10.4103/0974-8490.79110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JA, Son JH, Song SB, Yang SY, Kim YH. Sterols isolated from seeds of Panax ginseng and their antiinflammatory activities. Pharmacogn Mag. 2013;9:182–5. doi: 10.4103/0973-1296.111288. [DOI] [PMC free article] [PubMed] [Google Scholar]


