Abstract
Background:
Bougainvillea spectabilis (BS) (family Nyctaginaceae) is said to possess hypoglycemic and anti-inflammatory activities in experimental animals. We had set forward to examine the potential anti-inflammatory activities of BS in experimental models of inflammation.
Materials and Methods:
Fresh dried leaves from the flowering plant of BS were collected from the local area during the flowering season and air dried (215.00 g). Methanol was extracted, and the solvent was removed on a rotary evaporator under reduced pressure. The extract was freeze-dried (lyophilized) and the yield was 8 g. This was used as an emulsion prepared in propylene glycol and orally administered (20 and 50 mg/kg). Acute anti-inflammatory activity of BS was evaluated using carrageenan and dextran whereas chronic anti-inflammatory (immunoregulatory) activity was evaluated by Freund's adjuvant-induced arthritis model.
Results:
BS (20 mg/kg and 50 mg/kg) had shown significant anti-inflammatory effects 20.6% and 67.6%, respectively, on carrageenan-induced acute inflammatory models. In dextran-induced edema, the effect was 30% and 66%, respectively. The standard drug indomethacin (87.3% and 91.5%, respectively) showed better inhibitory response in both models. In arthritic model 50 mg/kg of BS showed significant chronic anti-inflammatory effect (38.46%) in comparison to the standard drug dexamethasone (84.6%).
Conclusion:
Our data indicate that the methanol extract of BS (50 mg/kg) leaves has significant anti-inflammatory and immunoregulatory activity. Further studies involving isolation of active principles will help to pinpoint the mechanisms contributing to the observed activities of BS.
Keywords: Anti-inflammatory, Bougainvillea spectabilis, immunoregulatory
INTRODUCTION
Inflammation is generally considered as a protective response to tissue injury by a stimulus. Natural plant products are now emerging as important alternative therapeutic options to establish anti-inflammatory drugs as they are cheap, abundantly available, and relatively less toxic.
The plant contains biochemical defense agents, o-dihydrophenols, anthocyanin, lignin, and proline. The traditional medical practitioners of Kolli hills, Tamil Nadu, are using this plant to cure inflammation.[1] Bougainvillea spectabilis (BS) (family Nyctaginaceae) has also been reported to possess hypoglycemic activity in experimental animals. This property has been attributed to the presence of pinitol in the leaves.[2,3] The genus Bougainvillea, in the Nyctaginaceae family of plants, has 14 species. Three of them are horticulturally important.[4] BS is one of them. It is a large climber with characteristic thorns and hair on stems and leaves. BS leaves extract inhibited tomato spotted wilt tospovirus on capsicum annuum and ground water in laboratory tests.[5] Its antiviral protein was characterized by Balasaraswati et al. and anti-inflammatory activities were also observed by Joshi et al.[5,6] It is a very familiar plant commonly grown in Indian gardens. The traditional plant has the antidiabetic potential. The blood glucose lowering potential of BS wild leaf extract in streptozotocin-induced type I diabetic albino rats was reported. The ethanolic extract of the leaves has shown antihyperglycemic activity probably due to increased uptake of glucose by enhanced glycogenesis in the liver and also due to increase in insulin sensitivity.[7,8]
There are information's available regarding its phytochemistry, pharmacological, and toxicological activities. As part of a continuing search for plant derived anti-inflammatory agents, the aim of this study was to explore the application of a methanol extract of BS leaves as a potential anti-inflammatory agent in animal models of acute and chronic inflammation.
MATERIALS AND METHODS
Animals
Male albino Wistar rats (100–120 g) and Swiss albino mice (15–25 g) of either sex were used. All animals were housed under standard laboratory conditions of temperature (24°C ± 5°C), a 12 h light/dark cycle was maintained and animals were provided with free access to commercial pellet feed and water ad libitum. They were acclimatized for 1 week before starting the experiments and were subdivided for different experimental schedules. The study received prior approval from the Institutional Animal Ethics Committee and followed OECD guidelines. CPCSEA/544 is the Institutional Animal Ethics Committee of IPGMER, Kolkata.
PREPARATION OF PLANT PRODUCT
Fresh dried leaves from the flowering plant BS were collected from the local area during the flowering season and air dried (215.00 g). The identity of the plant was authenticated by the Botanical Survey of India. Methanol extraction was carried out, and solvent removed on a rotary evaporator under reduced pressure. The extract was freeze-dried (lyophilized) and the yield was 8 g. That was used as an emulsion prepared in propylene glycol and was orally administered (0–50 mg/kg). BS in an initial pilot study was initially administered up to a maximum of 200 mg/kg b.w. However, maximum inhibition of edema formation was observed at 50 mg/kg b.w. and accordingly, these experimental animals received 20 and 50 mg/kg kg b.w.
Accordingly, the animals were grouped (n = 12 per group) as follows: Group I: Control, Group II received a standard drug, indomethacin (2.0 mg/kg); Group III and IV received BS at a dose of 20 and 50 mg/kg b.w. respectively.
Carrageenan induced rat paw edema
A fresh solution of 1% carrageenan was prepared by dissolving 50 mg of carrageenan powder in 5 ml of normal saline (0.9% NaCl). All animals were starved for 18 h before starting the experiment. Animals of Group III and IV were pretreated with the test drug in the doses mentioned above 30 min prior to the carrageenin injection. All orally administered drugs were given via a rat feeding cannula. Subsequently, 30 min after the respective treatments, 0.1 ml of 1% carrageenan was injected subcutaneously into the subplantar region of right hind paw of all animals to induce edema, and the paw volume was measured plethysmographically.[9] The paw volume was measured initially at 0 h and subsequently at 1, 2, 3, and 4 h after carrageenan injection. The mean increase in paw volume was measured hourly and at every time point, the percentage inhibition of edema was calculated.[10]

Dextran induced rat paw edema
In this model, dextran was used as the phlogistic agent and the methodology of Rowley et al., (1956) was followed.[11] Indomethacin and BS were administered 30 min prior to the injection of dextran. A volume of 0.1 ml of 2% dextran solution in normal saline was injected into the subplantar tissue of right hind paw of each rat.[12] The paw volume was recorded by a plethysmometer initially at 0 h and subsequently hourly at 1, 2, and 3 h after dextran injection. The percentage inhibition of edema at every time point was calculated as per Vetrichelvan and Jegadeesan.
Adjuvant induced arthritis
The method of adjuvant arthritis in rats as described by Pearson et al. (1959) exhibits many similarities to human rheumatoid arthritis and was accordingly followed as a model of chronic or immunologically induced inflammation.[13] Male Sprague-Dawley rats (130–150 g) and the grouping was similar to that described above except that the standard drug (Group II) was dexamethasone (0.1 mg/kg).
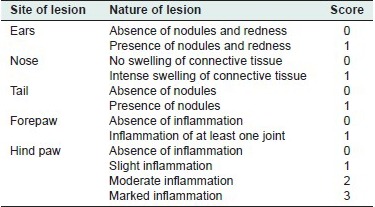
On day 1, 0.1 ml of complete Freund's adjuvant was injected into the subplantar region of the left hind paw of each rat. (Each ml of complete Freund's adjuvant (sigma) contains 1 mg Mycobacterium tuberculosis (H37RA, ATCC 25177) heat killed and dried 0.85 ml paraffin oil and 0.15 ml mannide monooleate). Dosing with a test compound or the standard was started on the same day and continued daily orally for 12 days. Paw volumes of both legs and body weight were recorded on the day of injection. On day 5, the volume of the injected paw was measured again, indicating the primary lesions and the influence of therapeutic agents on this paw. The severity of the adjuvant-induced disease was followed by measurement of the non-injected paw (secondary lesions) as follows:[14]
Toxicity studies
The potential toxicity of BS was evaluated in a sub-acute toxicity model in Swiss albino mice n = 12 (State OECD guidelines). Mice received BS orally in a stepwise fashion starting from 100 mg/Kg and increasing every 2 days up to a maximum of 1.5 g/kg b.w. They continued to receive the highest dose for an additional 2 weeks. Liver function tests (serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase, and renal function tests (urea and creatinine) were measured using commercially available kits.
Statistical analysis
The results were expressed as mean ± standard deviation, and the significance was evaluated by ANOVA, with P < 0.05 implying significance.
RESULTS
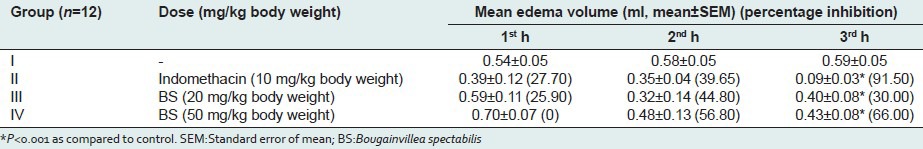
Bougainvillea spectabilis (20 mg/kg and 50 mg/kg) showed significant anti-inflammatory effects 20.6% and 67.6% and 30% and 66%, respectively, on carrageenan and dextran-induced acute inflammatory models. The standard drug indomethacin (87.3% and 91.5%, respectively) showed better inhibitory response in both models [Tables 1 and 2]. It suggests that BS possesses potent acute anti-inflammatory activity possibly due to the inhibition of the synthesis and release of mediators of inflammation, principally the prostaglandins. In the dextran-induced paw edema model, BS caused profound inhibition of edema volume both at 20 and 50 mg/kg b.w [Table 2]. The dextran-induced edema has been reported to be mediated mainly by histamine and serotonin released by the mast cells.[15]
Table 1.
Effects of methanolic extract of BS leaves in carrageenan-induced rat paw edema
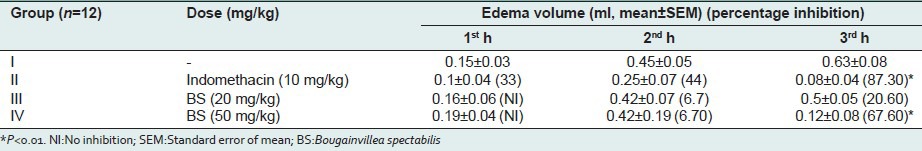
Table 2.
Effects of methanolic extract of BS in dextran-induced rat paw edema
In arthritic model, 50 mg/kg of BS showed significant chronic anti-inflammatory effect (38.46%) in comparison to the standard drug dexamethasone (84.6%) [Tables 3 and 4]. The severity of the adjuvant-induced disease was followed by measurement of the noninjected paw (secondary lesions) and arthritic index as mentioned in material and methods. Suppression of secondary lesions was best observed with dexamethasone. 50 mg/kg of methanolic extract of BS leaves showed significant suppression of secondary lesions (2.75 ± 0.20) but lesser in effect in comparison to dexamethasone (1.25 ± 0.50).
Table 3.
Effects of methanolic extract of Bougainvillea spectabilis leaves on Freund's adjuvant induced arthritis in rats
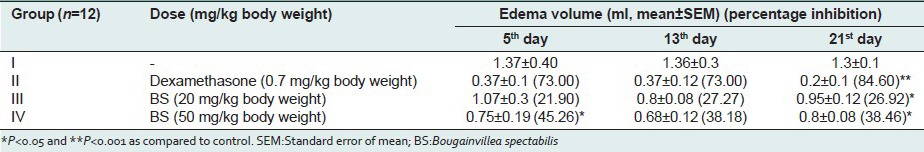
Table 4.
Effects of methanolic extract of BS leaves on arthritic index in an animal model of adjuvant-induced arthritis

DISCUSSION
The inhibition of carrageenan-induced inflammation in rats is an established model to screen compounds for potential anti-inflammatory activity. According to Vinegar et al., (1987) the development of carrageenan-induced edema is biphasic, the first phase occurs within 1 h of carrageenan administration and is attributed to the release of cytoplasmic enzymes, histamine, and serotonin, from the mast cells.[16] The second phase(>1.0 h) is mediated by an increased release of prostaglandins in the inflammatory area and continuity between the two phases is provided by Kinins. In this model, BS was initially administered up to a maximum of 200 mg/kg b.w. However, maximum inhibition of edema formation was observed at 50 mg/kg b.w. and accordingly, the experimental animals received 5, 20, and 50 kg/kg b.w. In all groups, the maximum inhibition was observed at the end of 3rd h [Tables 1 and 2]. Manivannan et al. showed that maximum inhibition was 59.5% at the end of 3rd h in carrageenan-induced model of acute inflammation. In cotton pellet-induced granuloma, inhibition was around 53.1%. Taken together, it suggests that BS possesses potent acute anti-inflammatory activity possibly due to the inhibition of the synthesis and/or release of inflammation, principally the prostaglandins.
The immune system has evolved to discriminate itself from nonself and is always on the alert to fight infectious invaders by keeping normal cells intact. These organisms have developed robust receptor-mediated sensing devices and effector mechanisms broadly described as innate and adaptive. Innate, or natural, immunity is primitive, does not require priming, is of relatively low affinity and is mediated mainly by complement, granulocytes, natural killer cells, macrophages, mast cells, and basophils. Adaptive or learned immunity is antigen specific, depends upon antigen exposure or priming and can be of very high affinity. This is mediated by T- and B-cells. B-cells make antibodies while T-cells function as helper, cytolytic, and regulatory cells. These cells are important components of the normal immune “orchestra” that harmoniously fights infection and tumors but also mediates transplant rejection and autoimmunity.[17] Immune responses can be pharmacologically manipulated in three ways namely immunosuppression, tolerance, and immunostimulation.
The immunologically mediated complete Freund's adjuvant arthritic model of chronic inflammation is considered as the best available experimental model of rheumatoid arthritis.[18] This method in rats was originally developed by Pearson et al., (1963) wherein an injection of complete Freund's adjuvant in the rat hind paw induces inflammation. In this method of immunologically mediated chronic synovial inflammation and arthritis, macrophages play a central role. After activation, they are capable of synthesizing mediators such as PGE2 and cytokines such as tumor necrosis factor-α and interleukin-1. In turn, these synthetic products induce the generation of a variety of enzymes which initiate cartilage and bone destruction.[19] Taken together, our data indicate that BS (50 mg/Kg of body weight) has a certain degree of both acute and chronic anti-inflammatory (immunoregulatory) activity as it was able to decrease the edema formation in all kind of models.
Absence of toxicity of Bougainvillea spectabilis in a subacute toxicity model
Subacute toxicity studies using the methanolic extract of BS leaves showed no major toxicity as evidenced by the absence of any hepatic or renal damage; the levels of SGOT, SGPT, alkaline phosphatase, urea, and creatinine of control and treated groups were not significantly different when tested statistically.
CONCLUSIONS
The results of the present study of methanolic extract of the leaves of BS revealed anti-inflammatory activity in both acute and chronic phases of inflammation. Further work is needed to locate the active principal from the methanolic extract of BS leaves and its phytopharmaceutical studies.
ACKNOWLEDGMENTS
The work received financial support from the Council of Scientific Research, Government of India and the University Grants Commission. Goutam Mandal was a Junior Research Fellow of Council of Scientific and Industrial Research, New Delhi. We express our thanks to Late Ramphal Mullick for his excellent technical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Manivannan E, Kothai R, Arul B, Rajaram S. Anti-inflammatory activity Bougainvillea spectabilis linn. Res J Pharm Biol Chem Sci. 2012;3:62. [Google Scholar]
- 2.Narayanan CR, Joshi DD, Mujumdar AM, Dhekne VV. Hypoglycemic action of Bougainvillea spectabilis leaves. Curr Sci. 1984;53:579–81. [Google Scholar]
- 3.Narayanan CR, Joshi DD, Mujumdar AM, Dhekne VV. Pinitol, a new anti-diabetic compound from leaves of Bougainvillea spectabilis. Curr Sci. 1987;56:139–41. [Google Scholar]
- 4.Umamaheswari A, Shreevidya R, Nuni A. In vitro antibacterial activity of Bougainvillea spectabilis leaves extracts. Adv Biol Res. 2008;2:01–05. [Google Scholar]
- 5.Balasaraswathi R, Sadasivam S, Ward M, Walker JM. An antiviral protein from Bougainvillea spectabilis roots; purification and characterisation. Phytochemistry. 1998;47:1561–5. doi: 10.1016/s0031-9422(97)00788-7. [DOI] [PubMed] [Google Scholar]
- 6.Joshi DD, Mujumdar AM, Narayanan CR. Anti-inflammatory activity of Bougainvillea spectabilis leaves. Indian J Pharm Med Plants Sci. 1984;46:187–8. [Google Scholar]
- 7.Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm Drug Res. 2010;67:113–8. [PubMed] [Google Scholar]
- 8.Purohit A, Sharma A: Blood glucose lowering potential of Bougainvillea spectabilis leaf extract in streptozotocin induced type-I diabetic albino rats. Indian Drugs. 2006;43:538. [Google Scholar]
- 9.Bhatt KR, Mehta RK, Shrivastava PN. A simple method for recording antiinflammatory effects on rat paw oedema. Indian J Physiol Pharmacol. 1977;21:399–400. [PubMed] [Google Scholar]
- 10.Vetrichelvan T, Jegadeesan M. Effect of alcoholic extract of Achyranthes Bidentata blume on acute and subacute inflammation. Indian J Pharmacol. 2002;34:115. [Google Scholar]
- 11.Rowley DA, Benditt EP. 5-Hydroxytryptamine and histamine as mediators of the vascular injury produced by agents which damage mast cells in rats. J Exp Med. 1956;103:399–412. doi: 10.1084/jem.103.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel HG, Vogel WH. Analgesic, anti-inflammatory and antipyretic activity. In: Vogel HG, Vogel WH, editors. Drug Discovery and Evaluation. Pharmacological Assays. New York: Berlin Heidelberg, Springer-Verlag; 1997. p. 382. [Google Scholar]
- 13.Pearson CM, Wood FD. Studies on polyarthritis and other lessions induced in rats by injection of mycobacterium adjuvant. I. General clinic and pathological characteristics and some modifying factors. Arthritis Rheumatol. 1959;2:440. [Google Scholar]
- 14.Schleyerbach R. Antiarthrotic and immunmodulatory activity. In: Vogel GH, editor. Drug Discovery and Evaluation. 2nd ed. Berlin, Germany: Springer; 2002. p. 802. [Google Scholar]
- 15.Lo TN, Almeida AP, Beaven MA. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther. 1982;221:261–7. [PubMed] [Google Scholar]
- 16.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc. 1987;46:118–26. [PubMed] [Google Scholar]
- 17.Janeway CA, Travers P, Walport M, Capra JD, editors. 4th ed. London: Current Biology Publications; 1999. Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- 18.Williams RO. Rodent models of arthritis: Relevance for human disease. Clin Exp Immunol. 1998;114:330–2. doi: 10.1046/j.1365-2249.1998.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins SJ. Cytokines and eicosanoids in rheumatic diseases. Ann Rheum Dis. 1990;49:207–10. doi: 10.1136/ard.49.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]


