Abstract
Background:
Swietenia macrophylla King. (Meliaceae) seeds (SMS); commonly known as sky fruit and locally known in Malaysia as Tunjuk Langit; have been used in traditional Malay medicine for the treatment of diabetes and hypertension. The people eat only a tiny amount of raw seed, weighing not more than 5 mg.
Aim:
To evaluate the safety of Swietenia macrophylla seeds (SMS) at a single-dose oral administration of 2 g/kg body weight (bw) in sprague dawley (SD) rats.
Materials and Methods:
Eight-week old male and female SD rats were administered a single-oral dose of 2g/kg bw. The rats’ general behavior, and toxic signs were observed throughout the 14-day study period. The food and water intake by rats and their body weight were monitored during the study period. At the end of the study period, the relative weights of the organs (lung, liver, spleen, heart, kidney, testis, stomach); the hematological and biochemical parameters were measured; the architecture and histology of the organs (liver, kidney and lungs) were observed.
Results:
Oral administration of SMS to rats did not affect, either food or water intake; relative organ weight of vital organs; the hematological and biochemical parameters; did not show significant changes in the architecture and histology of vital organs. Overall, there were neither signs of toxicity nor deaths recorded during the study period.
Conclusion:
The rat dose of 2 g/kg bw is equivalent to the human dose of 325 mg/kg bw, which is well below the usual amount consumed by people, did not show any signs of toxicity in rats.
Keywords: Diabetes, sky fruit, Swietenia macrophylla, toxicity, traditional Malay medicine, tunjuk langit
INTRODUCTION
Swietenia macrophylla King (Meliaceae) is a tall, lofty, evergreen tree found in tropical vicinities of the world. Its fruit is colloquially known as “sky fruit” as it appears to point toward the sky and in Malaysia, is known as “Tunjuk Langit”. Various parts of Swietenia macrophylla have been used to treat a great array of ailments in various traditional and folk-lore systems of medicine. The seeds in particular are purported to have ethno medicinal significance against numerous diseases running the gamut from treatment of leishmaniasis and abortion by an Amazonian Bolivian group, through to folk medicine in Indonesia, Malaysia and India for the treatment of wounds, hypertension, diabetes and malaria.[1,2]
Swietenia macrophylla has been reported to possess hypoglycaemic,[3,4,5] antimicrobial,[3,6,7] antimalarial[8] and antiviral[9] activities. Swietenia macrophylla is a good source of bioactive tetranorterpenoids, phargmalin-type limonoids.[10,11,12,13,14,15,16,17,18,19,20]
In Malaysia, the raw seeds have been used as a folk-lore medicine for the treatment of hypertension and diabetes. Despite the wide use of raw Swietenia macrophylla seeds in folk-lore medicine, there were no data on the safety of consuming its raw seeds for these claims. This present study was undertaken to provide scientific data on the safety focusing on the acute toxicity of Swietenia macrophylla seeds powder given orally to Sprague Dawley (SD) rats. The usual way to determine whether the plants are safe for human consumption is by evaluating the toxic effects of respective extracts. However, in this study, we have attempted to evaluate the acute toxicity of raw Swietenia macrophylla seeds without any extraction, to mimic the way humans have consume them.
MATERIALS AND METHODS
Plant material and extraction
Swietenia macrophylla seeds (SMS) collected in the months of November and December, 2011 from Agricultural Conservatory Park, Universiti Putra Malaysia (UPM) were used in the study. The plant was authenticated by Dr. Shamsul Khamis, Coordinator, Biodiversity Unit, Institute of Bioscience (IBS), UPM. A voucher specimen (SK247/02) was deposited at Institute of Bioscience, UPM. SMS were grounded to a fine powder using a domestic blender and suspended in olive oil for evaluating its acute toxicity.
Experimental animals
Eight-week-old male and female SD rats with weight range of 150 to 220 g obtained from Universiti Putra Malaysia animal house were used in the study. All animal experimentations were in accordance to the guidelines of “Handling of Laboratory Animals” by Ministry of Health Malaysia.[21] The study protocol was approved by the Institute Research and Ethics Committee, International Medical University (IMU), with a project ID number BMS I-01/2012 (11). The animals were housed in the Animal House, IMU. The rats were housed individually per cage and acclimatised for a week prior to the start of the study. An environment with room temperature of 27°C and 12 h of alternate light and dark cycle was maintained throughout the experiments. The rats were given unlimited supply of water and standard diet.
Acute toxicity study
The rats were assigned randomly to the control and treatment groups. In each group there were 20 rats out of which 10 rats were male and the remaining 10 rats were female. The treatment group were given oral administration, using an intubation needle, of SMS at a single dose of 2 g/kg body weight (bw) suspended in olive oil while the control group received olive oil. The animals were observed for toxic effects and the body weight was recorded daily throughout the 14–day study period. The rats were observed twice daily for toxic signs, viz., changes in skin, fur, eyes; occurrence of secretions and excretions, autonomic activity, changes in gait, posture, response to handling, presence of tonic movements (e.g. excessive grooming, repetitive circling) and bizarre behavior (e.g. self-mutilation, walking backwards).
All the rats were weighed once prior to administration of the dosing and daily thereafter till the final day of the experiment. On Day 14, blood sample was collected from each rat under ether anaesthesia. Half the volume of blood was collected in a tube containing anticoagulant (EDTA) and remaining half the volume in another tube without anticoagulant. The blood samples with anticoagulant were analysed using Sysmex, KX-21N (Sysmex Cooperation, Japan) to obtain hematological parameters. The serum was taken from the blood samples without anticoagulant and were analyzed using Vitalab Selectra, E-series (Netherlands) to obtain biochemical parameters. The animals were sacrificed by an overdose of ether and the rats were dissected to obtain the organs viz., lungs, liver, spleen, heart, kidneys, testis, stomach; for further examination and each organ's weight (absolute organ weight in g) was recorded.
Using the following formula, the relative organ weight (ROW) of each organ was calculated:

Histopathological evaluation
Necropsy was performed on all animals at the end of the study. All rats were killed by ether anaesthesia after recording the terminal body weights. The organs (lungs, liver, spleen, heart, kidneys, testis, and stomach) were fixed and stored in a 10% neutral buffered formaldehyde solution at room temperature until further analysis. The tissues were sliced laterally and longitudinally in order to place them into the cassettes. The tissues were then processed in Leica rotary microtome (model, RM 2135) in which the tissues were run through formalin, followed by a series of graded ethanol, xylene and paraffin wax. After processing overnight, the tissues were embedded in molten paraffin. Paraffin sections were cut at 4 μm of thickness, mounted on glass slides and left to air-dry. Briefly, paraffin sections were heated at 60°C for 45 min. The paraffin sections were deparaffinised in xylene, and rehydrated in a graded series of alcohol (two changes of absolute ethanol to xylene, one change of 90% ethanol, one change of 80% ethanol and one change of 70% ethanol) with a final wash in running tap water for 10 min. Each change was approximately 5 min. The sections were then stained with hematoxylin for 30 min followed by eosin. After completion of the staining, sections were dehydrated by sequential immersion in water, through graded ascending alcohol solutions (70%, 80%, 90% and 100% ×2) and two changes of xylene. Then it was followed by mounting of the stained tissues using DPX, rendering them suitable for light microscopy examination. The sections were viewed and photographed on a Nikon Eclipse 80i Fluorescence microscope with attachment of Nikon DS 5MC-U2 camera using NIS-elements BR software. Histological changes in vital organs, viz., testis, liver, kidney, heart, stomach, lung and spleen; in rats were graded on a scale of 0-9, based on the nature and severity of histological changes. The scoring system employed for assessing the histological changes as follows: Score 0 indicates preservation of normal architecture and histology, A1-degeneration of architecture in ≤20% cells, A2-degeneration of architecture in 21-50% cells, A3-degeneration of architecture in >50% cells, I1-minimal inflammation, I2-small localized inflammation, I3-diffused inflammation, N1-necrotic foci in few cells at one location, N2-necrotic foci at different locations and N3-diffuse necrosis.
Statistical analysis
Statistical analysis for each parameter was performed to obtain the mean value and standard deviation. Statistical Package for Social Science (Version 18.0) was used to obtain ANOVA for repeated measurements to analyse the effect of SMS. Results with P < 0.05 was considered as statistically significant.
RESULTS
To assess the acute toxic effects of SMS, 2 g/kg bw dose was orally given to male and female rats. All animals in the treatment and control groups showed an increase in the body weight on day-7 and day-14 compared to day-0 [Table 1]; however the increase in body weight was not statistically significant. It was observed that the average water intake [Table 2] by rats in treated group is high during week-1 and restored back to normal levels during week-2 of the acute toxicity study as compared to control group. The food consumed [Table 2] by rats in treated group is low during week-1 and restored back to normal levels during week-2 of the acute toxicity study as compared to control group. Neither mortality nor alteration in the behavioral pattern of the rats was noted. Overall, the animals treated with SMS showed no significant changes in the food and water intake behavior. The ROW of the lungs, livers, spleens, hearts, testes and stomachs of the rats in the treatment group showed no significant changes as compared with the control group [Table 3]. The hematological [Table 4] and biochemical parameters [Table 5] were within the range of the control animals and the mean values between the groups were not statistically significant. These data collectively indicate that SMS did not affect the vital organs and hematological and biochemical parameters. Examinations of the architecture of the major organs such as lungs, liver, spleen, heart, kidneys, testis, and stomach were conducted. The macroscopic examination did not show any significant changes in the vital organs of treated rats compared with the control rats, indicating that SMS was not toxic to rats. To further assess the tissue toxicity, microscopic examination of histological preparations of selected vital organs (lungs, liver, spleen, heart, kidneys, testis, and stomach) was performed. Scoring of pathological changes revealed that the SMS did not induce significant changes compared with the control animals [Table 6]. Examples of photomicrographs of histology of liver, kidney and lung are shown in Figure 1. The liver showed minimal and mild inflammation with few scattered inflammatory cells and focal inflammatory infiltrates. However, this finding was not considered significant as this phenomenon was also observed in all rats of both control and treated groups. There were no histological changes observed in the kidneys and lungs, with both organs showing a preserved normal architecture. The SMS powder had no apparent acute toxicity effect on SD rats at selected dose, 2 g/kg bw and therefore can be classified as non-toxic.
Table 1.
Summary of rats’ body weights recorded during the acute toxicity study of SMS
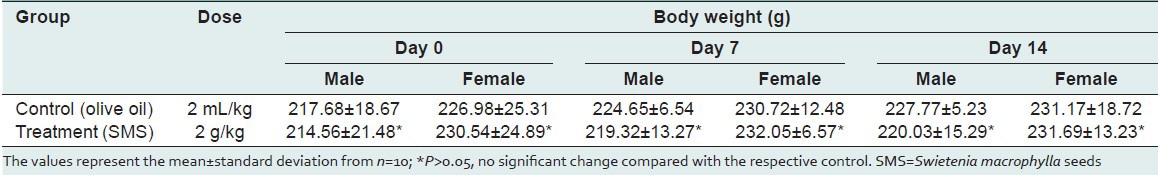
Table 2.
Summary of rats’ water intake (ml/rat.day) and feed consumed (g/rat.day) recorded during the acute toxicity study of SMS
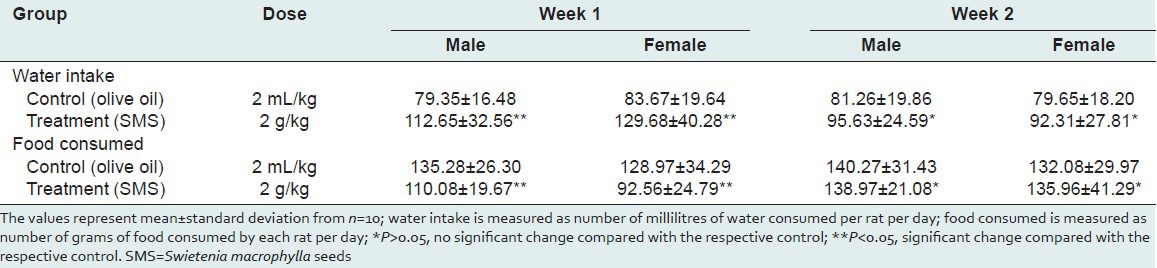
Table 3.
Summary of rats’ relative organ weights (per 100 g body weight) calculated at the end of acute toxicity study of SMS
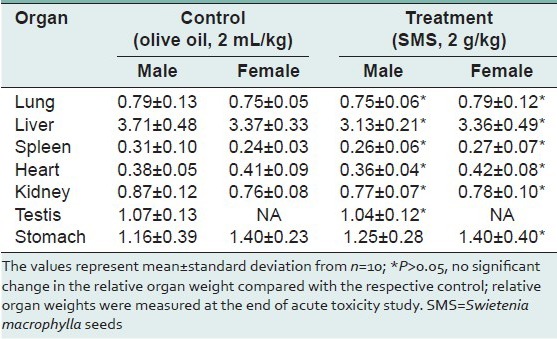
Table 4.
Summary of rats’ haematological parameters measured at the end of acute toxicity study of SMS
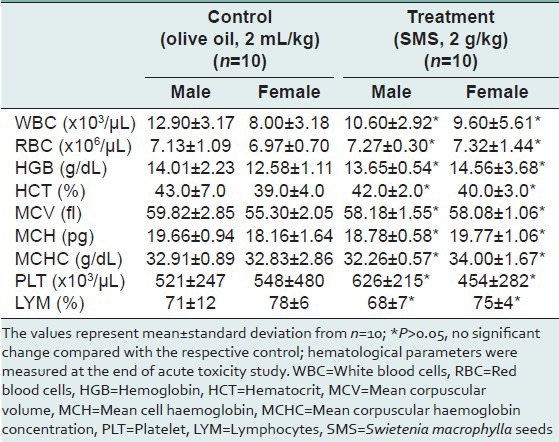
Table 5.
Summary of rats’ biochemical parameters measured at the end of acute toxicity study of SMS
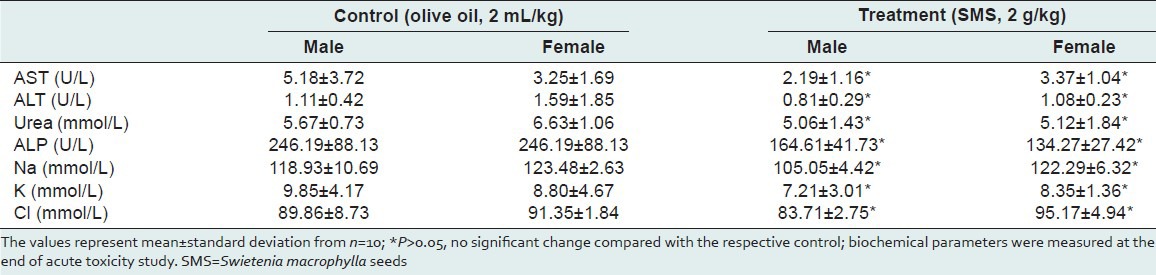
Table 6.
Summary of rats’ histological scores determined at the end of acute toxicity study of SMS
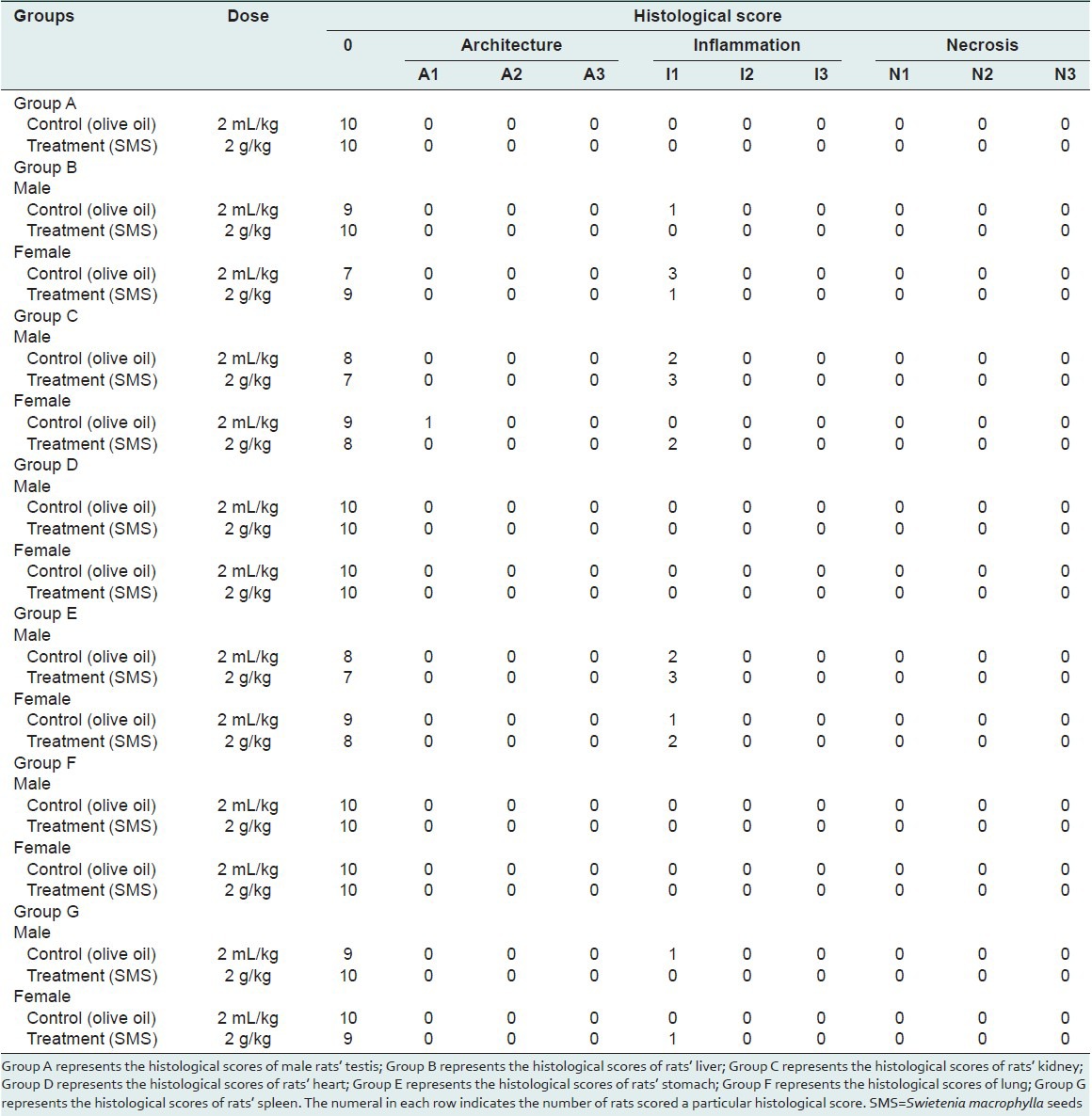
Figure 1.
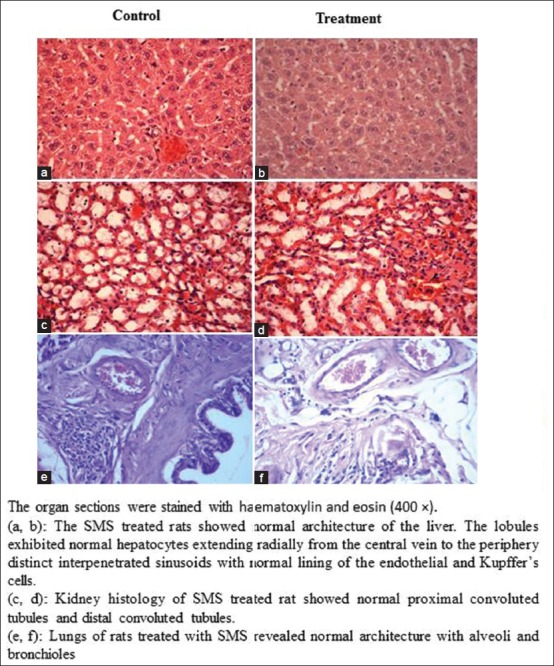
Representative photomicro graphs of the sections from the liver (a,b), kidney (c,d) and lungs (e,f) of control and SMS treated male rats
DISCUSSION AND CONCLUSION
Majority of the people in rural areas of Malaysia have been consuming medicinal plants for the treatment of various illnesses; especially chronic diseases like diabetes, hypertension, arthritis etc. However, evidence of safety of SMS reported in the literature is limited. Therefore, toxicology studies are important to determine the safe dose for human consumption.
In order to find the equivalent safe dose of SMS for human consumption, we have calculated the human equivalent dose (HED) using the following formula; which is developed based on the body surface area (BSA) normalization method; reported in the literature.[22]
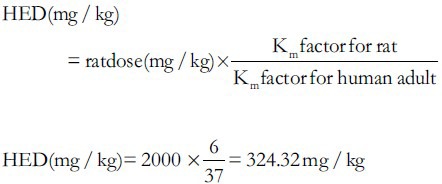
From the above calculations, the SMS may be safe until a dose of 325 mg/kg bw for human consumption.
In conclusion, the Swietenia macrophylla seeds (SMS) at a single dose of 2 g/kg bw did not show any signs of toxicity in Sprague Dawley rats. Based on these findings, we assume that consumption of SMS by humans is safe if the dose is less than 325 mg/kg body weight. The usual dose of SMS prescribed in Malaysian folk-lore medicine is one seed; weighing about 5 mg per day. Therefore, the prescribed dose of SMS in Malaysian folk-lore medicine is safe for human consumption.
ACKNOWLEDGMENTS
This study was supported by International Medical University (BMS I-01/2012 (11)); Ministry of Higher Education, Malaysia, FRGS grant (FRGS/1/2014/STWN10/IMU/02/1).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Burkill IH, editor. A Dictionary of the Economic Products of Malay Peninsular, Ministry of Agriculture, Malayasia: A Dictionary of the Economic Product of Malay Peninusular. 1935 [Google Scholar]
- 2.1st ed. Vol. 2. Malaysia: Compendium of Medicinal Plants Used in Malaysia; 2002. Herbal Medicinal Research Center, Institute for Medical Research. [Google Scholar]
- 3.Dewanjee S, Kundu M, Maiti A, Majumdar R, Majumdar A, Mandel S. In vitro evaluation of antimicrobial activity of crude extract from plants Diospyros peregrina, Cocciniagrandis and Swietenia macrophylla. Trop J Pharm Res. 2007;6:773–8. [Google Scholar]
- 4.Dewanjee S, Maiti A, Das AK, Mandal SC, Dey SP. Swietenine: A potential oral hypoglycemic from Swietenia macrophylla seed. Fitoterapia. 2009;80:249–51. doi: 10.1016/j.fitote.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Kalaivanan K, Pugalendi KV. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacogn Res. 2011;3:67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmalingam K, Tan BK, Mahmud MZ, Sedek SA, Majid MI, Kuah MK, et al. Swietenia macrophylla extract promotes the ability of Caenorhabditi selegans to survive Pseudomonas aeruginosa infection. J. Ethnopharmacol. 2012;139:657–63. doi: 10.1016/j.jep.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 7.El Zalabani SM, El-Askary HI, Mousa OM, Issa MY, Zaitoun AA, Abdel-Sattar E. Acaricidal activity of Swietenia mahogani and Swietenia macrophyllae thanolic extracts against Varroa destructor in honeybee colonies. Exp Parasitol. 2012;130:166–70. doi: 10.1016/j.exppara.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz V, Sauvain M, Bourdy G, Callapa J, Rojas I, Vargas L, et al. The search for natural bioactive compounds through a multidisciplinary approach in Bolivia. Part II. Antimalarial activity of some plants used by Moseteneindians. J Ethnopharmacol. 2000;69:139–55. doi: 10.1016/s0378-8741(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu SF, Lin CK, Chuang YS, Chang FR, Tseng CK, Wu YC, et al. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J Viral Hepat. 2012;19:364–70. doi: 10.1111/j.1365-2893.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan KC, Tang TS, Toh HT. Isolation of swietenolidediacetate from Swietenia macrophylla. Phytochemistry. 1976;15:429–30. [Google Scholar]
- 11.Mootoo BS, Ali A, Motilal R, Pingal R, Ramlal A, Khan A, et al. Limonoids from Swietenia macrophylla and S. aubrevilleana. J Nat Prod. 1999;62:1514–7. doi: 10.1021/np990199x. [DOI] [PubMed] [Google Scholar]
- 12.Da Silva MN, Arruda MS, Castro KC, da Silva MF, Fernandes JB, Vieira PC. Limonoids of the phragmalin type from Swietenia macrophylla and their chemotaxonomic significance. J Nat Prod. 2008;71:1983–7. doi: 10.1021/np800312h. [DOI] [PubMed] [Google Scholar]
- 13.Falah S, Suzuki T, Katayama T. Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pak J Biol Sci. 2008;11:2007–12. doi: 10.3923/pjbs.2008.2007.2012. [DOI] [PubMed] [Google Scholar]
- 14.Lin BD, Zhang CR, Yang SP, Zhang S, Wu Y, Yue JM. D-ring-opened phragmalin-type limonoidorthoesters from the twigs of Swietenia macrophylla. J Nat Prod. 2009;72:1305–13. doi: 10.1021/np900139c. [DOI] [PubMed] [Google Scholar]
- 15.Tan S, Osman H, Wong K, Boey P. New phragmalin-type limonoids from Swietenia macrophylla King. Food Chem. 2009;115:1279–85. [Google Scholar]
- 16.Maiti A, Dewanjee S, Sahu R. Isolation of hypoglycemic phytoconstituent from Swietenia macrophylla seeds. Phytother Res. 2009;23:1731–3. doi: 10.1002/ptr.2821. [DOI] [PubMed] [Google Scholar]
- 17.Goh BH, Abdul Kadir H, Abdul Malek SN, Ng SW. Swietenolidediacetate from the seeds of Swietenia macrophylla. Acta Crystallogr Sect E Struct Rep Online. 2010;66(Pt 6):o139. doi: 10.1107/S1600536810017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Huang S, Liao C, Wei D, Sung P, Wang T, et al. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010;120:379–84. [Google Scholar]
- 19.Lin BD, Zhang CR, Yang SP, Wu Y, Yue JM. Phragmalin-type limonoidorthoesters from the twigs of Swietenia macrophylla. Chem Pharm Bull. 2011;59:458–65. doi: 10.1248/cpb.59.458. [DOI] [PubMed] [Google Scholar]
- 20.Liu JQ, Wang CF, Chen JC, Qiu MH. Limonoids from the leaves of Swietenia macrophylla. Nat Prod Res. 2012;26:1887–91. doi: 10.1080/14786419.2011.625499. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Medical Research, Ministry of Health, Malaysia. Kuala Lumpur, Malaysi: Ministry of Haealth, Kuala Lumpur, Malaysia; 2000. Principles and guide to ethical use of laboratory animals. [Google Scholar]
- 22.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]


