Abstract
Background:
Stereospermum suaveolens DC. (Syn. S. chelonoides) belonging to family Bignoniaceae is an important medicinal plant in India. Traditionally, it is mainly used as analgesic, liver stimulant, astringent, wound healing and antidyspeptic. Roots of this plant are one of the ingredients of Dashamularishta. The plant has been studied for many pharmacological actions, only few were concerned with isolation of active compounds.
Objective:
The present work deals with the isolation and identification of phytochemical constituents present in the roots of Stereospermum suaveolens.
Material and Methods:
The compounds were isolated from the ethyl acetate-soluble fraction from the methanol extract of S. suaveolens by using open silica gel column chromatography and HPLC was carried out for all the fractions to target the major peaks in fractions.
Results and Conclusion:
The isolated compounds structures were elucidated by analysis of spectroscopic data (UV, IR, 1D-NMR, and MS) and characterized as Cycloolivil (1) reported for the first time from this plant species, Lapachol (2) and β-sitosterol (3), respectively.
Keywords: Cycloolivil, isolation, lapachol, stereospermum suaveolens, β-sitosterol
INTRODUCTION
Knowledge of chemical components of a plant is essential for quality control analysis of the plant, extract or any formulation containing them. A compound or group of compounds present in the plant is serving as “marker(s)”. The presence and concentration of markers will be considered to decide the quality of the herbal extracts/formulations. Knowledge of these compounds and their specific analytical methods will facilitate in preparation of specification for the marketed extracts/formulations in the regulated market and thus raise its standards.[1]
S. suaveolens DC. (Syn. S. Chelonoides) belonging to family Bignoniaceae, is a medium sized deciduous tree distributed in the sub-Himalayan tract and outer hills, central India, western Peninsula, Burma, Bangladesh and the English Forest. It is reported for its antipyretic property and also useful in excessive thirst, cough and asthma.[2] S. suaveolens is one of the ingredient of classical Ayurvedic preparation Dashamularishta.[3] The Bignoniaceae having about 100 genera with 800 species, are known for their antimicrobial, antiprotozoal, and anti-inflammatory properties.[4,5] Moreover, barks, flowers, roots and leaves of S. suaveolens are used by traditional healers, rural communities and pharmaceutical companies for treating vomiting, eructation, piles, acidity, diarrhoea, gonorrhoea, loss of taste, malaria and other fevers.[6] Decoction of roots used in intermittent and puerperal fevers. Stem bark is used as diuretic and tonic. Flowers with honey are used to stop cough.[7]
S. suaveolens contains naphthaquinone lapachol, root bark contains β-sitosterol, n-triacontanol, root heart wood contains lapachol, dehydro-α-lapachone and dehydrotectol. Leaves contain flavones glycoside 6-O-glucosylscutellarein,[8] dinatin, dinatin-7-glucuroniside,[9] dinatin 7-glucuronide,[8] quinones, stereochenols A and B, naphthoquinones, sterekunthal B and sterequinone C,[10] stereolensin,[11] p-coumaric acid, palmitic, stearic and oleic acids[12] have previously been reported from this plant. The Cycloolivil has not been reported previously from this species.
MATERIALS AND METHODS
General procedures
Melting points were obtained on a Thermonik apparatus. IR spectra were measured in KBr using IR Prestige-21 (FTIR Spectrophotometer, Shimadzu). 1H-NMR and[13] C-NMR spectra were recorded on Bruker 400 MHz spectrometers. MS spectra were measured with a LCQ Fleet- Thermo Fisher Scientific instrument. TLC and column chromatography were carried out on pre-coated silica gel 60 F254 plates (0.25 mm thick, Merck, Darmstadt, Germany) and silica gel (60-120 mesh, Swambe chemicals, India), respectively. HPLC study was carried out using Shimadzu hplc system LC 2010HT with UV and PDA detector in combination with Class LC solution software and Kromasil C-18 (250 × 4.6 × 5μ) column.
Plant material
The roots of S. suaveolens were mainly collected from Kukkae Subramanya, Karnataka State, India, in July 2012 and identified by Dr. Santhan, Plant taxonomist, Natural Remedies Pvt. Ltd. Bangalore, India. A voucher specimen (PCN/SC/254/2012) has been deposited in the Herbarium of the Natural Remedies Pvt. Ltd, Bangalore, India.
Extraction and isolation
The dried roots of S. suaveolens (1.0 kg) were powdered and extracted with methanol three times (4.0 L × 3, 60°C) in a static extractor for 2 h. The combined extracts were concentrated under vacuum at 60°C and dried in vacuum tray dryer to obtain crude extract (yield-8%). The methanolic extract was dissolved in water and partitioned successively with ethyl acetate, n-butanol affording ethyl acetate (yield-12%), n-butanol (yield-20%) and water (yield-66%) soluble fractions. Ethyl acetate fraction was chromatographed over silica gel (60-120 mesh, 1:3 ratio of extract) and the column was eluted with petroleum ether first followed by mixtures containing increasing amounts of ethyl acetate and methanol. The fraction eluted with 10% methanol in ethyl acetate was collected, kept aside to allow the precipitate settled at the bottom and precipitate was washed with petroleum ether repeatedly to obtain compound 1 (yield-0.043%) in pure form. The fractions eluted with 30% and 40% ethyl acetate in petroleum ether was collected, concentrated and washed with petroleum ether and acetone respectively to obtain compound 2 (yield-0.004%) and compound 3 (yield-0.00017%).
Compound 1: White crystals; mp 166-168°C; UV λmax (MeOH) nm: 204, 284; 1H NMR (400 MHz, CD3OD) δ 6.77 (1H, d, J = 8.0 Hz, H-5′), 6.70 (1H, d, J = 8.0 Hz, H-2′), 6.67 (1H, dd, J = 8.0, 2.0, H-6′), 6.63 (1H, s, H-8), 6.18 (1H, s, H-5), 4.03 (1H, d, J = 11.6 Hz, H-4), 3.81 (1H, d, J = 2.4 Hz, H-3aHb), 3.80 (3H, s, -OCH3), 3.79 (1H, d, J = 11.2 Hz, H-2aHb), 3.75 (3H, s, -OCH3), 3.58 (1H, d, J = 4.4 Hz, H-2aHa), 3.55 (1H, d, J = 4.4 Hz, H-3aHa), 3.26 (1H, d, J = 16.8, H-1b), 2.63 (1H, d, J = 16.8, H-1a), 2.05 (1H, d, J = 2.8, H-3); 13C NMR (100 MHz, CD3OD) δ 149.28 (C-3′), 147.65 (C-7), 146.26 (C-4′), 145.46 (C-6), 138.63 (C-1′), 133.71, (C-10), 126.59 (C-9), 123.72 (C-6′), 117.50 (C-5), 116.17 (C-5′), 114.10 (C-2′), 113.13 (C-8), 75.11 (C-2), 69.57 (C-2a), 61.00 (C-3a), 56.55 (-OCH3), 56.52 (-OCH3), 47.73 (C-3), 45.05 (C-4), 40.08 (C-1).
Compound 2: Yellow amorphous powder; mp 133–135°C; UV λmax (MeOH) nm: 253, 278, 335; 1H NMR (400 MHz, CDCl3) δ δ 8.13 (1H, dd, J = 8.0, 1.2, H-5), 8.07 (1H, dd, J = 7.6, 1.2, H-8), 7.76 (1H, ddd, J = 7.6, 1.6, 1.2, H-6), 7.69 (1H, ddd, J = 7.6, 1.6, 1.2, H-7), 5.21 (1H, t, J = 7.2, H-2′), 3.31 (2H, d, J = 7.6, H-1′), 1.75 (3H, s, H-4′), 1.68 (3H, s, H-5′); 13C NMR (100 MHz, CDCl3) δ 184.54 (C-4), 181.7 (C-1), 152.67 (C-2), 134.83 (C-6), 133.83 (C-3′), 132.94, (C-10), 132.84 (C-7), 129.44 (C-9), 126.77 (C-5), 126.04 (C-8), 123.48 (C-3), 119.64 (C-2′), 25.73 (C-5′), 22.61 (C-1′), 17.88 (C-4′).
RESULT AND DISCUSSION
Compound 1 [Figure 1] shows a molecular ion peak at m/z 375.13 M-H]- consistent with molecular formula C20H24O7 (calculated 376.39316). The IR spectrum showed the bands at 1027.14 cm-1, (C-O stretch), 1261.50 cm-1 1463.07 cm-1 (aromatic C = C stretch), 2905.89 cm-1 (C-H stretch) and 3488.41 cm-1 (O-H stretch). The 1H NMR spectrum revealed the presence of two methoxy groups at δ 3.75 and δ 3.80. Two –CH2OH groups at δ 3.55 (d, J = 4.4), δ 3.81 (d, J = 2.4), δ 3.58 (d, J = 4.4) and δ 3.79 (d, J = 11.4). One naphthalene ring at δ 2.63 (d, J = 16.8), δ 3.26 (d, J = 16.8), δ 2.05 (d, J = 2.8), 4.03 (d, J = 11.6), δ 6.18 (s), δ 6.63 (s), δ 6.7 (d, J = 2.0). One benzene ring at δ 6.67 (d, J = 8.0, 2.0), δ 6.77 (d, J = 8.0) and δ 6.7 (d, J = 2.0). 13C NMR spectra of this sample further confirmed the compound by showing peak at δ 56.4 and δ 56.55 which correspond to two –OCH3 groups. The signals at δ 69.57 and 61.0 confirm the two –CH2OH groups. The signals at δ 40.08, 75.11, 47.73, 45.05, 117.5, 145.46, 147.65, 113.13, 126.59 and 133.71 confirm the presence of one naphthalene ring. The signals at δ 138.63, 114.10, 149.28, 146.26, 116.17 and 123.72 confirm the one benzene ring. Also, on the basis of spectroscopic data and previously reported literature values, compound 1 was confirmed as Cycloolivil.[13]
Figure 1.
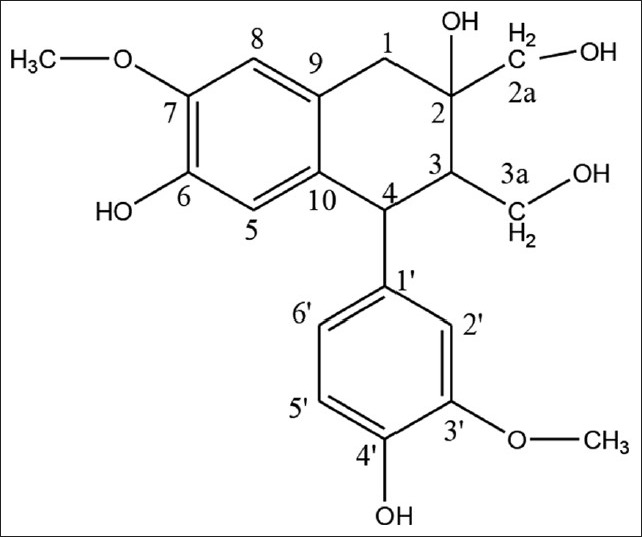
Structure of isolated Compound 1 (Cycloolivil)
Compound 2 [Figure 2] shows a molecular ion peak at m/z 241.09 [M-H] –consistent with molecular formula C15H14O3 (calculated 242.27). IR spectrum showed bands at 3332.43 cm -1 (stretching, O-H), 1661.75 cm -1 (stretching, C=O), 1642.46 cm -1 (stretching, C=O), 1592.31 cm -1 (aromatic ring) and 1273.07cm-1 (C-O). On the basis of spectroscopic data and previously reported literature values, compound 2 was determined as Lapachol.[14]
Figure 2.
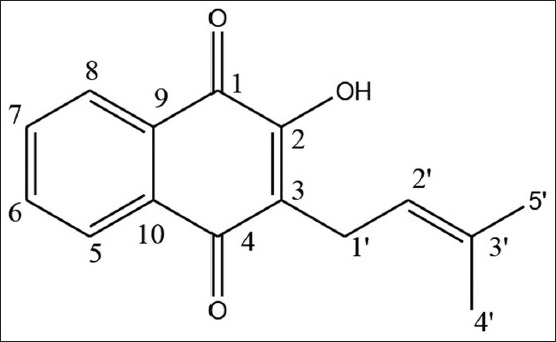
Structure of isolated Compound 2 (Lapachol)
Compound 3 [Figure 3] was obtained as white powder. TLC study was carried out to identify compound 3 with reference compound β-sitosterol using mobile phase- ethyl acetate: Petroleum ether (3:7). A purple colored spot (Rf 0.5) was observed after spraying with ANS (Anisaldehyde in sulfuric acid reagent).
Figure 3.
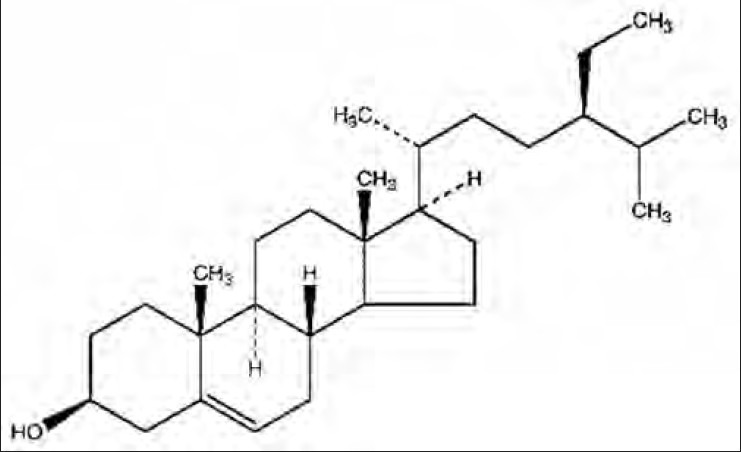
Structure of isolated Compound 3 (β-sitosterol)
Quantification of cycloolivil and lapachol in extract
The compounds Cycloolivil and Lapachol were quantified by using HPLC [Figure 4]. It is found that 3.67% w/w and 0.066%w/w present in root methanol extract of Stereospermum suaveolens, respectively.
Figure 4.
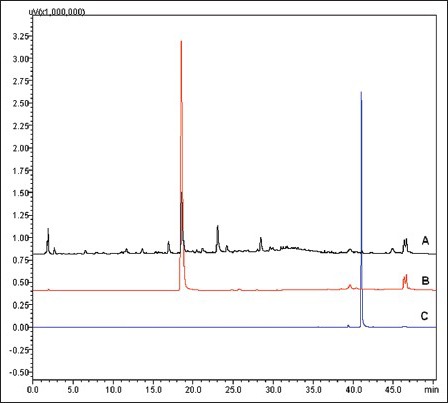
Overlay of HPLC chromatogram of Compounds 1 and 2 with methanol extract
CONCLUSIONS
The present study aims to isolate constituents toward chemical standardization of extract. There are few literature found on quantification of chemical constituents. This is the first time reporting the isolation of Cycloolivil from the root methanol extract of S. suaveolens. Cycloolivil is a lignan derivative present as major compound (yield-0.043%) when compared with Lapachol which is indicated in the HPLC chromatogram. Further studies may be conducted on the method validation by HPLC/HPTLC to quantify in formulations, extracts and raw materials for quality control purpose.
S. suaveolens is one of ingredient in Ayurvedic formulation called Dashamoolarishta; hence the chemical constituents of S. suaveolens can be used for quantification of formulation.
ACKNOWLEDGMENTS
The authors are thankful to Staff of Phytochemistry, M/s. Natural Remedies Pvt. Ltd., Bangalore, India, for providing all necessary facilities to carry out the research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Daniel M. New Hampshire: Enfield, Science Publishers; 2006. Medicinal plants chemistry and properties [M] pp. 3–4. [Google Scholar]
- 2.Ghani A. 1st ed. Dhaka: Asiatic Society of Bangladesh; 1998. Medicinal Plants of Bangladesh: Chemical Constituents and Uses. [Google Scholar]
- 3.Anonymous. Preparation of kwath and Dashmulakwath, in Bangladesh National Formulary of Ayurvedic Medicine, (Approved by the Govt. of Bangladesh vide Ministry of Health and Family Welfare, Memo No. Health-1/Unani-2/89/(Part-I) 116, dated 3-6-i1. 1992:20–32. [Google Scholar]
- 4.Binutu OA, Adesogan KE, Okogun JI. Antibacterial and antifungal compounds from Kigelia pinnata. Planta Med. 1996;62:352–3. doi: 10.1055/s-2006-957900. [DOI] [PubMed] [Google Scholar]
- 5.Onegi B, Kraft C, Kohler I, Freund M, Jenett-Siems K, Siems K, et al. Antiplasmodial activity of naphthoquinones and one anthraquinone from Stereospermum kunthianum. Phytochemistry. 2002;60:39–44. doi: 10.1016/s0031-9422(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 6.Troup RS. Dehradun, India: India International Book Distributors; 1986. Silviculture of Indian Trees Volume 2. Leguminosae (Caesalpinieae) to Verbenaceae. [Google Scholar]
- 7.Yoganarasimhan SN. Vol. 2. Tamilnadu, India: Cybermedia; 2000. Medicinal plants of India. [Google Scholar]
- 8.Gupta AK, Neeraj T, Madhu S. New Delhi: Medicinal Plants Unit Indian Council Medical Research; 2008. Quality Standards of Indian medicinal plants; pp. 290–7. [Google Scholar]
- 9.Subramanian SS, Nagarajan S, Sulochana MN. Flavonoids of the leaves of Stererospermum suaveolens. Curr Sci. 1972;41:102–3. [Google Scholar]
- 10.Haque MR, Rahman KM, Iskander MN, Hasan CM, Rashid MA. Stereochenols A and B, two quinones from Stereospermum chelonoides. Phytochemistry. 2006;67:2663–5. doi: 10.1016/j.phytochem.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran AG, Kotiyal JP. Stereolensin: A new flavone glucoside from Stereospermum suaveolens. Indian J Chem. 1979;18B:188–9. [Google Scholar]
- 12.Chatarjee A, Pakrashi SC. Vol. 2. New Delhi, India: National Institute of science Communication; 2000. The treatise on Indian medicinal plants; p. 10. [Google Scholar]
- 13.Masataka S, Eiko N, Masao K. Lignan and phenylpropanoid glycosides from Osmanthus asiatica. Phytochemistry. 1993;5:1215–9. [Google Scholar]
- 14.Oliveira MF, Lemos TG, De Mattos MC, Segundo TA, Santiago GM, Braz-Filho R. New enamine derivatives of lapachol and biological activity. An Acad Bras Cienc. 2002;74:211–21. doi: 10.1590/s0001-37652002000200004. [DOI] [PubMed] [Google Scholar]


