Abstract
Aim:
The aim of this study was to examine and evaluate crucial variables in essential oils extraction process from Lavandula hybrida through static-dynamic and semi-continuous techniques using response surface method.
Materials and Methods:
Essential oil components were extracted from Lavandula hybrida (Lavandin) flowers using supercritical carbon dioxide via static-dynamic steps (SDS) procedure, and semi-continuous (SC) technique.
Results:
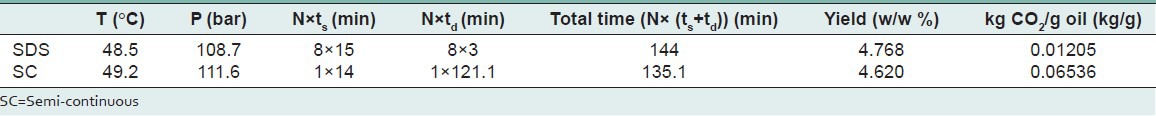
Using response surface method the optimum extraction yield (4.768%) was obtained via SDS at 108.7 bar, 48.5°C, 120 min (static: 8×15), 24 min (dynamic: 8×3 min) in contrast to the 4.620% extraction yield for the SC at 111.6 bar, 49.2°C, 14 min (static), 121.1 min (dynamic).
Conclusion:
The results indicated that a substantial reduction (81.56%) solvent usage (kg CO2/g oil) is observed in the SDS method versus the conventional SC method.
Keywords: Essential oil, lavandin, lavandula hybrida, response surface method, static-dynamic steps, supercritical carbon dioxide
INTRODUCTION
Lavandin is a sterile hybrid of Lavandula angustifolia P. Mill. × Lavandula latifolia Medikus (Lamiaceae).[1,2] The two main components of Lavandin essential oil are linalool and linalyl acetate. Linalyl acetate is highly appreciated as food additive because of its flavor, and Linalool is used as a natural insecticide or pesticide as well as in food and fragrance applications.[3] Essential oils, especially Lavandin essential oil, can be used as natural biocides to decrease the side effects of the synthetic chemical biocides. Lavandin essential oil is used as antinociceptive and gastroprotective effects of inhaled and orally administered.[4] Lavandin is also known as a highly antiseptic, antifungal and antibacterial agent.[5,6]
Essential oils are currently being extracted from natural products either by hydrodistillation or solvent extraction. Losses of some volatile compounds, low extraction efficiency, degradation of unsaturated compounds through thermal or hydrolytic effects and toxic solvent residue in the extract may be encountered using these extraction methods. These shortcomings have led to the consideration of the use of supercritical fluids in essential oil extraction process. Carbon dioxide is the most commonly used supercritical fluid because of its modest critical condition.[7,8,9] Recently, a few studies were performed in regard to separation of essential oil from different types of Lavender. Essential oil was extracted from Turkish Lavender flowers,[10,11] Italian lavender.[12,13] and Iranian lavender[14] using supercritical carbon dioxide. A new process design and operation for microwave accelerated steam distillation of essential oils from Italian lavender were developed and compared with steam distillation.[15] A total of 85 components were identified in the two dimensional GC-MS analyses of the nine samples of Australian Lavender essential oil using low-polarity and polar capillary columns.[16] Also other studies were started to separate essential oil from Lavandin flower using supercritical carbon dioxide.[17,18,19] Moreover, we have extracted essential oil from Lavandin utilizing pressurized fluid extraction technique.[20] This study aimed to extract essential oil from Lavandin flowers using supercritical carbon dioxide in static-dynamic steps (SDS) procedure and conventional semicontinuous (SC) method for the separation of 1,8-Cineole, linalool, linalyl acetate, and camphor. Moreover, in order to achieve the maximum extraction yield the operating conditions of both methods were optimized via the response surface method.[14,20]
MATERIALS AND METHODS
Experimental
Lavandin flowers samples were obtained from Isfahan Agricultural Research Center. Ethanol was utilized as the trap solvent for the collection of solute in the supercritical fluid and was used for washed up the whole system that it will dissolve any contamination remaining in SFE system. N-hexanol (99.6%, Merck) was used as the internal standard for the GC-FID calibration analysis. Pure 1,8-Cineole (470,826, ≥99%, Aldrich), linalool (51,782, ≥99%, Fluka), linalyl acetate (45,980, ≥95%, Fluka), and camphor (148,075, 96%, Aldrich) were used as the standards as the four important components of the Lavandin essential oil. Industrial grade carbon dioxide (≥ 99%, Zamzam) was used as the supercritical fluid.
Preparation of Lavandin flower
The Lavandin flower was dried at 40°C for a period of 3 h prior to extraction. In the end of the normal drying process of Lavandin the moisture content was around 10.2%. Following the extraction procedures flowers were finely grinded using laboratory equipments.[21,22] Since extraction kinetics in this study was controlled by the kernel particle size, an important sieving step was carried out to achieve reproducible extraction yield in which the samples were passed through a sieve with mesh sizes between 20 and 30 (particle diameters ranging over 0.60-0.85 mm). The dried samples were kept within sealed bag in the cold and dry place until they were used.
Supercritical fluid extraction: Apparatus and procedure
The supercritical extraction system shown in Figure 1 was used for SDS and SC methods.[20,23] In order to increase the purity of the CO2, which is stored in a CO2 tank (1), we passed it through a cell of molecular sieve filter (2) and stainless steel 2 μm pore size filter (3). Then, CO2 was charged by a feed carbon dioxide transfer pump (4). A two way needle valve (5) was placed at the effluent of the pump, thus the CO2 stream is easily controlled and saved properly for further use. Carbon dioxide was heated before entering the extraction cell (12) by using a preheating coil (10) that was placed in an oven (12). After reaching the corresponding supercritical fluid conditions inside the extraction cell, the static time (CO2 flow stopped) was provided for extraction process by closing the valve (13). In the end of the static time the dynamic extraction (CO2 flowing in the extractor) with constant volumetric flow rate of CO2 is started via opening the valve (13). At this stage, the system pressure was controlled and monitored by a back pressure regulator (14), and a carbon dioxide transfer pump. Lavandin flowers sample (~40 g) and glass bead (broken Pyrex laboratory glassware) with mesh sizes between 20 and 30 (particle diameters ranging over 0.60-0.85 mm) in a ratio of 40-60% (w/w) were loaded into a 100 mL cylindrical stainless steel extraction cell, respectively (11). Cotton wool was packed at the end of the cell to avoid solid samples being transferred into the tubing and clogging the system.[20] After set up the apparatus, carbon dioxide was charged into the extraction cell while the pump was set at the selected operating pressure and temperature was obtained by pump, oven, and preheating coil. After that the pump is turned off and isolated with a shut-off valve. Subsequently, a constant static extraction time is allowed for supercritical carbon dioxide to dissolve the essential oil. By passing the CO2 at a constant flow rate, the dissolved oil via static and dynamic extraction is discharged from the cell, trapped and collected with 10 mL of ethanol solvent. Collection vessel consists of stainless steel bead which was fixed in vessel for increasing trapping (15). After each extraction run, the whole system was washed up with ethanol (6) in which the valve (8) was opened and ethanol was pumped by high-pressure piston pump (7), and then purged with carbon dioxide (ethanol wash is not combined with the trap ethanol solvent). The essential oil contained in the extracted samples was kept in the refrigerator for further GC-FID analysis.
Figure 1.
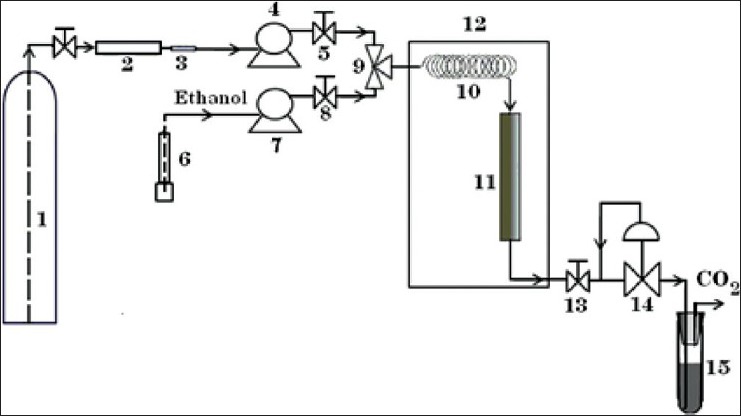
Schematic diagram of SFE system: (1) CO2 tank; (2) molecular sieve filter; (3) ss 2 μm pore size filter; (4) carbon dioxide transfer pump; (5, 8, 13) two-way needle valves; (6) Ethanol v; (7) high-pressure piston pump; (9) three ways valve; (10) preheating coil; (11) extraction cell; (12) thermostated oven; (14) back-pressure regulator; (15) sample collection vessel
The SDS procedure in this study was composed of a cyclic process in which static extraction, and dynamic extraction (constant extraction cell average residence time of 3 min with constant carbon dioxide volumetric flow rate of 5 mL/min) steps. For example the total 120 min static extraction time could be divided into 8 stages with 15 min for each stage. After each static stage the dynamic extraction begin. The total static extraction could be differing in time and number of stages. The SC method in supercritical fluid extraction is similar to an equilibrium-staged separation of SDS. Performing SDS procedures allows an efficient extraction, in which a substantial reduction solvent usage was observed in the method versus the conventional SC method. During the static time the system was allowed ample time to reach equilibrium, and then released during the dynamic interval (Extractant passed continuously through the extraction cell).[24] If enough essential oil is present in the Lavandin, it can be assumed that equal amounts of essential oil will be extracted.
GC-FID analysis
Four compounds were separated and determined using gas chromatography (GC). A gas chromatograph (Agilent Technologies, Model 6890N) was used with Helium (He) as the carrier gas, a HP-5 capillary column (30 m long, 0.25 mm I.D. and 0.25 μm film thick), and a flame ionization detector (FID). A sample injection volume of 0.2 μL in each analysis and the internal standard method was used to obtain the highest possible precision for quantitative GC measurements. The injection port and the detector temperatures were 230 and 250°C, respectively. Temperature programming was also used to separate the extracted components as follows: The initial oven temperature was 60°C for 1 min which was then increased to 120°C at a rate of 8°C/min where it was kept for 2 min to be subsequently increased to 220°C at a rate of 20°C/min. It was finally kept at 220°C for 1 min before terminating the program. The amounts of 1,8-Cineole, linalool, linalyl acetate, and camphor quantified by calculating the area under the chromatographic peaks divided by the area of n-hexanol (2300 ppm in sample solution) as an internal standard (As/Ais). In order to obtain the calibration curves, several solutions with different concentrations of 1,8-Cineole, linalool, linalyl acetate, and camphor in ethanol were injected into the GC-FID and the area under each peak was calculated, and the results were precisely obtained. The four linear calibration curves were fitted using a linear regression line with R2 ≥ 0.98. Finally, using the calibration curves, the extraction yield (Y) was determined using Eq. (1).
Y= (total mass of four components in extracted sample/mass of dried Lavandin flower) ×100 (1)
RESULTS AND DISCUSSION
Optimization of SDS operating conditions
A statistical experimental design based on “Box-Behnken (B-B) method” was planned[14] and the extraction yields were measured for different variables such as pressure, temperature, No. of stages × static time (N × ts) coded as x1, x2, and x3, respectively. These variables were investigated at three levels (−1, 0, 1) and the dependent variables were Yi and Y.
We used the Minitab (version 16.2) software package to design and evaluate these three independent variables at three levels on the responses according to Eq. (2).
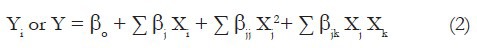
where Yi or Y = response, β0 = intercept, βj = linear coefficients, βjj = squared coefficients, βjk = interaction coefficients, Xi, Xj2, Xj, Xk = level of independent variables.
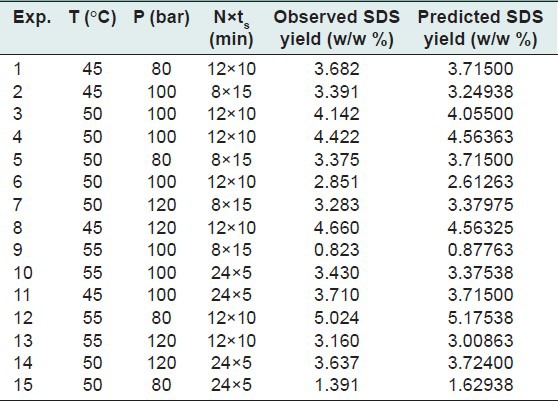
Table 1 shows the chosen ranges for three independent variables at three levels and the obtained experimental results of 15 runs are shown in Table 2.
Table 1.
Range of three independent variables in the SDS technique for the B-B method
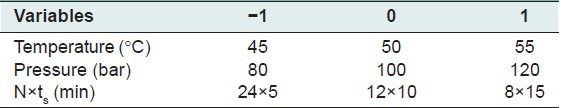
Table 2.
Responses of dependent variables using three levels-three factors B-B method to independent variables of SDS technique
A second-order polynomial equation is proposed for the prediction of SDS yield as a function of different variables as follows:
Y= -94.5180 + 3.2306 T + 0.4348 P – 0.8683 (N × ts) - 0.0332 T2-0.0019 P2 – 0.0418 (N × ts)2 (3)
Moreover theoretically predicted values of yield at different experimental conditions illustrated in Table 2.
The response surface model which was obtained from an experimental design was evaluated using ANOVA and analysis of residuals.[25] The results of the statistical analyses including the t-values (t-test) and P values of the extraction yield tabulated in Table 3. The R2 adjusted of the extraction yield was 96.07. This means that the developed models have been able to fully predict the extraction yield. The linear regression coefficients, R2, for the extraction yield was 98.59%, which shows good performance of the model as shown in Figure 2. The final extraction under the optimized conditions was performed and the calculations were verified. Based on the statistical results (ANOVA) with a confidence level of 95%, the effect of each term in the model could be significant provided that its P value be smaller than 0.05. Table 3 shows the degree of significance of different terms in the model which are based on the obtained results for the coefficients and P value.
Table 3.
The t-value and significance P value for the model of SDS extraction yield estimated by Minitab software
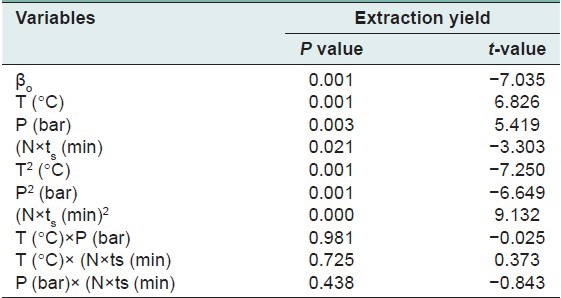
Figure 2.
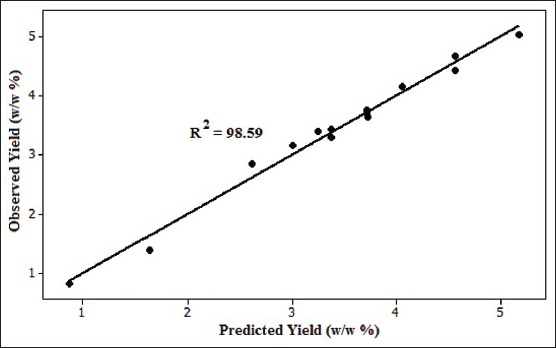
Observed SDS extraction yield versus SDS predicted extraction yield
Variables affecting SDS procedure
Figure 3 shows the effect of different operating temperature and pressure of supercritical CO2 on extraction yield at constant N × ts (8 × 15 min), and dynamic time (3 min). As temperature increases, two distinguished trends are observed in regard to the yield. In the range of 45-49°C, the yield increases with a mild slope until it reaches its maximum value (4.768%) and further increases up to 55°C causes a mild decrease in yield. The effect of temperature on solubility is complex, due to the combination of two variables, density and vapor pressure. The vapor pressure of the solute increases with temperature, causing an elevation in solubility. However, decreasing of solvent density may cause decreases of the solute solubility. The dominant effect will depend on the magnitude of each effect on the others for each system.[14] These two different regions of yield observation can be explained in terms of retrograde solubility in which the counter effect of solute vapor pressure and supercritical fluid density are affecting the extraction yield. In other words, in the first region (45-48.5°C) the extraction yield increases because of the increase in the solute solubility where the effect of the vapor pressure increase overcame the effect of the solvent density decrease. At 48.5°C, the retrograde solubility is reached in which the effect of solute vapor pressure becomes equivalent to the effect of CO2 density and in the second region (48.5-55°C) the extraction yield decrease because of the effect of the solvent density decrease overcame the effect of the increase in the solute solubility. The effect of supercritical fluid pressure is also demonstrated in Figure 3 for the operating range of 80-120 bar. The enhancement of extraction yield is observed as pressure is increased from 80 to 108.7 bar in which the maximum yield of 4.768% is reached and subsequent increase in pressure does not have any significant effect on yield. Beyond 108.7 bar, a decrease in yield is observed that is well expected due to the simultaneous counter effect of higher temperature which causes lower CO2 density with respect to density enhancement via pressure increase.[26,27]
Figure 3.
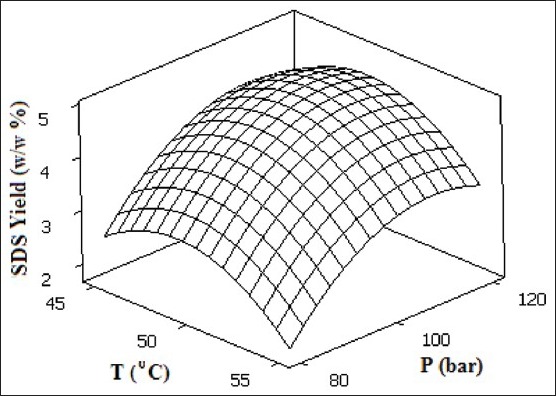
Contour plots and response surface of SDS extraction yield as a function of supercritical CO2 temperature and pressure at N × ts = 8 × 15 min
Figure 4 shows the effect of different operating pressure and (number of stages × static time) of supercritical CO2 on extraction yield at constant temperature (48.5°C), and dynamic time (3 min). Three sets of experimental runs in terms of N×ts were used: (1) 24 × 5, (2) 12 × 10, and (3) 8 × 15 min. The SDS extraction time of 8 × 15 min was selected as the most appropriate condition in view of high yield (4.768%), and lower solvent usage in which 67% and 33% in contrast to run number 1 and 2, respectively, and easier solute recovery, that was calculated is following:
Figure 4.
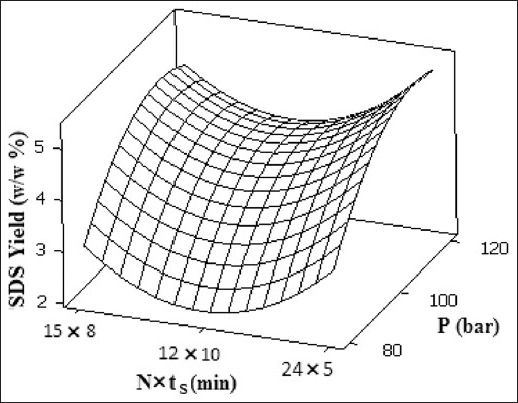
Response surface of SDS extraction yield as a function of supercritical CO2 pressure and No. of stages × static time at 48.5 °C
Solvent usage of state (1) = (24 stages) × (3 min (extraction cell average residue time)) ×f (5 mL/min) =360 mL (4)
Solvent usage of state (2) = (12 stages) × (3 min (extraction cell average residue time)) ×f (5 mL/min) =180 mL (5)
Solvent usage of state (3) = (8 stages) × (3 min (extraction cell average residue time)) ×f (5 mL/min) =120 mL (6)
It seems that the effect of dynamic extraction is prevailing in experiment number 1 and 2 and therefore not enough static extraction time is provided for the equilibrating of supercritical CO2/solute system. In other words, 15 min static time in experiment number 3 is enough for the system to reach its saturation solubility.[14] Whereas 5 and 10 min static times in experiment number 1 and 2 do not provide the saturation solubility of solutes (1,8-Cineole, linalool, linalyl acetate, and camphor) in supercritical CO2. As shown in Figure 4, the highest (5.024 as shown at run 12 in Table 2) yield was obtained at run number 1. In this regard, it is imperative to realize that the extraction time of static plus dynamic for experiment number 1 is 168 min in contrast to 144 min for the experiment number 3. In view of the aforementioned discussion, one can conclude that the optimum operating conditions for SDS technique are 108.7 bar, 48.5°C, and No. of stages × static time of 8 × 15 min at constant dynamic time of 3 min in order to obtain 4.768% yield.
Optimization of SC operating conditions
In order to optimize component and extraction yields (Y) in the SC process, a statistical experimental design based on the Central Composite Design (CCD) method[25] in conjunction with Minitab software using five level (-2, -1, 0, 1, 2) four variables (static time, dynamic time, supercritical fluid pressure and temperature) was planned with a fixed cell average residence time (3 min) utilizing a constant flow rate (5 mL/min). We used the Minitab software package to design and evaluate these four independent variables at five levels on the responses according to Eq. (3). The ranges for the selected levels of the four variables are shown in Table 4. The experimental extraction yield for different selected levels of variables is shown in Table 5 for 31 runs.
Table 4.
Range of four independent variables in the SC technique for the CCD
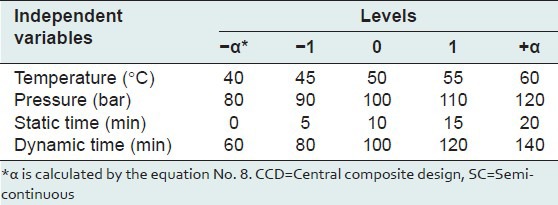
Table 5.
Response of dependent variables using five levels-four factors CCD method to independent variables of SC technique
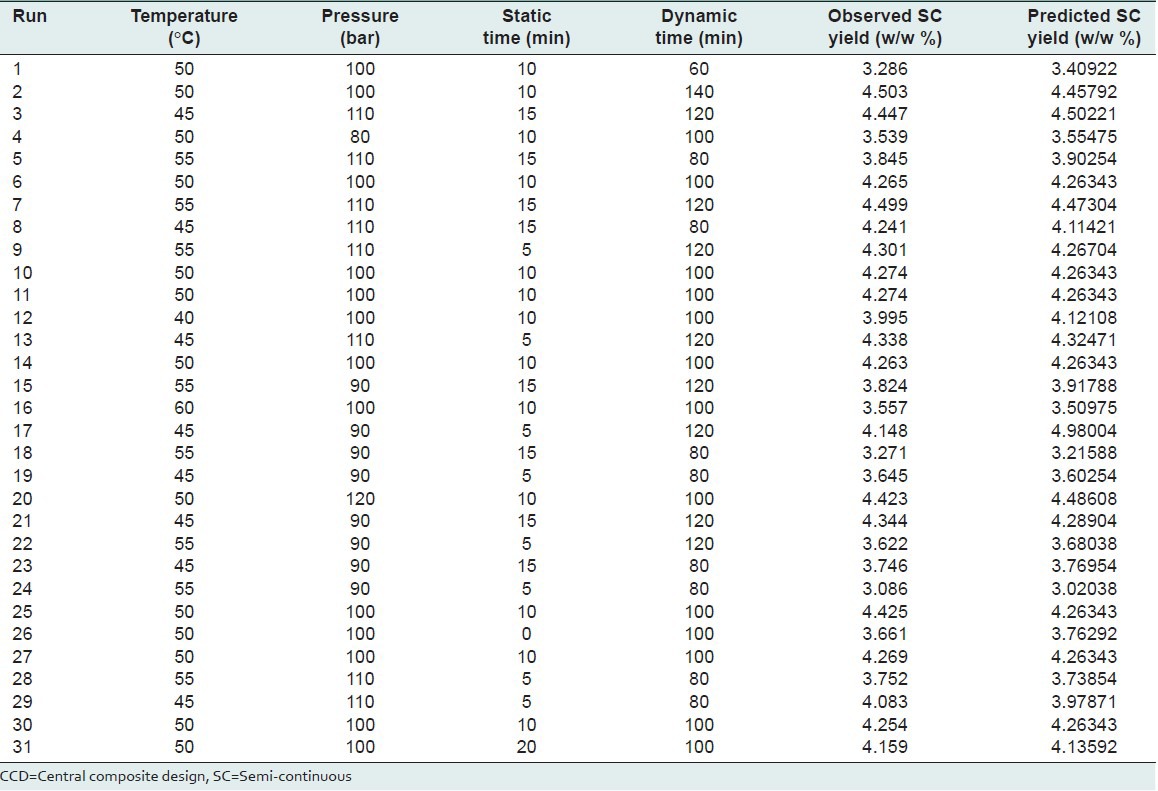

Moreover theoretically predicted values of yield at different experimental conditions illustrated in Table 5.
A second-order polynomial equation is proposed for the prediction of SC yield as a function of different variables as follows:
Y = -8.40463 + 0.19797 T + 0.07730 P + 0.07245 ts + 0.04686 td – 0.00448 T2 – 0.00061 P2 – 0.00314 ts2– 0.00021 td2 + 0.00171 T × P + 0.00046 T × td (8)
The response surface model which was obtained from an experimental design was evaluated using ANOVA and analysis of residuals. The results of the statistical analyses including the t-test and P values of the extraction yield were tabulated in Table 6. The R2 adjusted of the extraction yield was 95.31. This means that the developed models have been able to fully predict the extraction yield. The linear regression coefficients; R2 for the SC yield was also 97.50 as shown in Figure 5, which shows good performance of the model based on the observed and predicted yields. Based on the statistical results (ANOVA) with confidence level of 95%, the effect of each term in the models could be significant provided that its P value be smaller than 0.05 (P < 0.05) is shown in Table 6. It is imperative to realize that even though P value > 0.05 [Table 6] for the linear term of ts but due to Hierarchy rule in which the P value < 0.05 for the higher order (quadratic) of this variable.
Table 6.
The t-value and significance P value for the model of SC extraction yield estimated by Minitab software
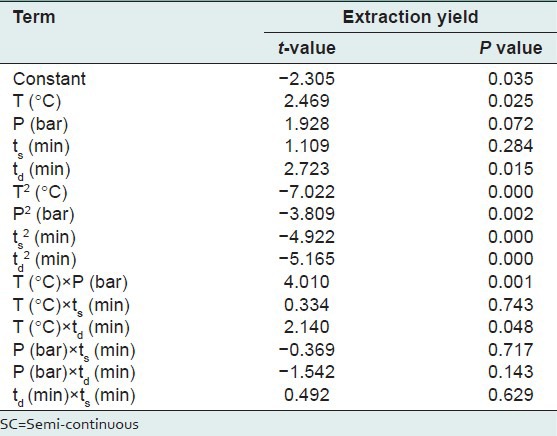
Figure 5.
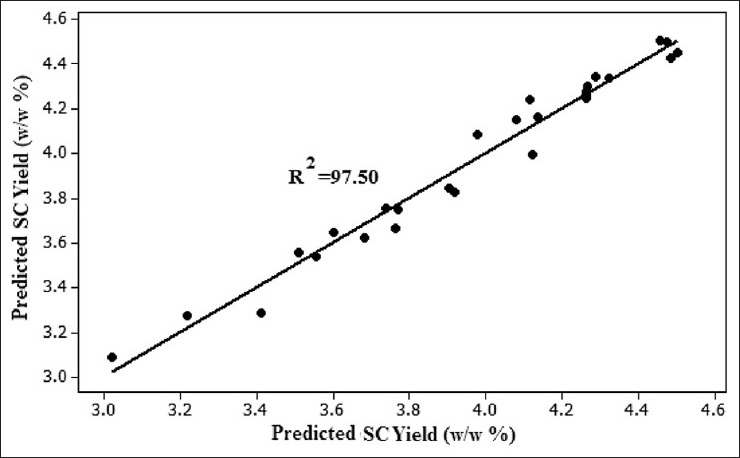
Observed SC extraction yield versus SC predicted extraction yield
Variables affecting SC procedure
The effect of temperature on the extraction yield is shown in Figure 6. Enhancement of extraction yield is observed via increasing temperature in the range of 40-49.2°C, in which higher solute solubility effect due to increased vapor pressure overcomes the effect of the solvent density decrease. Beyond 49.2°C, the retrograde solubility prevails and therefore, the effect of density decrease overcomes the influence of increased vapor pressure of solute. Thus, lower extraction yield is obtained in the range of 49.2-60°C. Figure 6 also shows the effect of pressure on the extraction yield in the range of 80-120 bar. It is observed that the extraction yield is enhanced by increasing pressure up to 111.6 bar due to higher density; in other words, better interaction between solvent and matrix, and solvation capabilities and also higher mass transfer driving force provide an appropriate medium for leaching process to take place. Beyond 111.6 bar, saturation limitation occurs and thus, a constant trend of extraction is obtained up to 120 bar.[26] It is important to optimize the contact of the supercritical fluid with the sample material in order to enhance the SFE efficiency. Several variables that influence the solvent contact time with sample material include flow rate, SFE time, and SFE mode (static with no flow-through or dynamic with flow-through). The static extraction prior to dynamic extraction usually improves the solute recoveries in SFE. The effect of static and dynamic extraction times on yield of SC method is illustrated in Figure 7. Samples were held in the static extraction mode in the range of 0-20 min, followed by a dynamic extraction in the range of 60-140 min at the constant flow rate of 5 mL/min. The enhancement of extraction yield is observed as static time is increased from 0 to 14 min in which the maximum yield of 4.620% is reached and subsequent increase in static time does not have any significant effect on yield. The observed extraction efficiency can be explained in terms of higher mass transfer driving force up to 14 min. Using dynamic extraction, higher mass transfer driving force at the beginning provides a suitable condition for extraction and this continues up to 121.1 min and after that a constant mode of extraction is observed due to the very low essential oil concentration of the matrix.[14] The SC optimum operating conditions to achieve maximum extraction yield (4.620%) for temperature, pressure, static time, and dynamic time were 49.2°C, 111.6 bar, 14 min, and 121.1 min, respectively.
Figure 6.
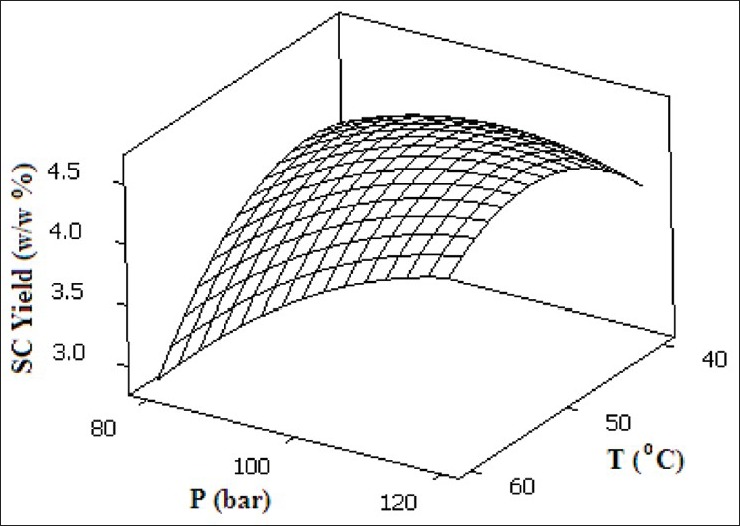
Response surface of SC extraction yield as a function of supercritical CO2 temperature and pressure at ts =14 min and td =121.1 min
Figure 7.
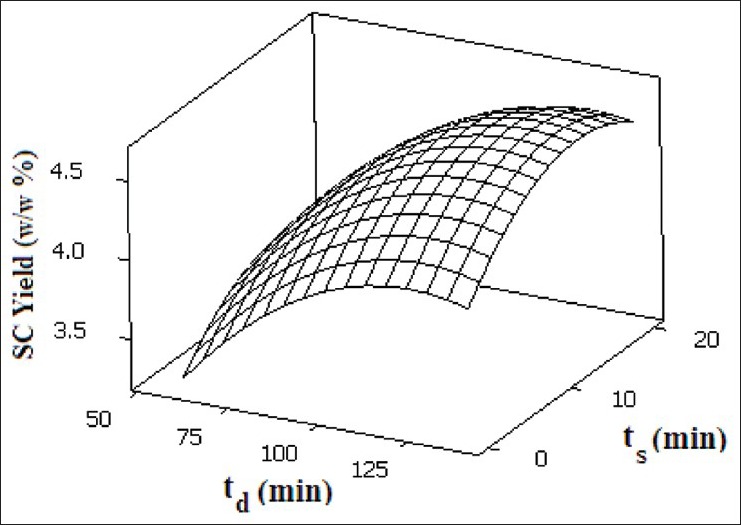
Response surface of SC extraction yield as a function of static and dynamic extraction time at 49.2 °C and 111.6 bar
Comparison of SDS and SC
These findings of this study are compatible with the results of other studies.[17,18,19] Lavandin flowers essential oil composition of this study is slightly difference from to other researches as the weight composition of four major components were 1,8-Cineole (8.1%), linalool (34.1%), linalyl acetate (30.5%), and camphor (7.3%). The total mass of four components (1,8-Cineole, linalool, linalyl acetate, and camphor) in Lavandin essential oil to be 80% of the total mass of essential oil (four components + waxes). Furthermore, the obtained optimum extraction yield (4.768%) via SDS method is slightly higher than the yield obtained by SC (4.620%) method performed in this study. Moreover, the SDS method is superior in terms of the major saving of solvent consumption (kg CO2/g oil) (81.56%) that was calculated is following:
Optimum SDS solvent usage (kg CO2/g oil) @ (T = 48.5°C, P = 108.7 bar, % Yield = 4.768%)
(ρ of CO2 (0.000479 kg/mL)) × (8 stages) × (3 min (extraction cell average residue time)) × f (5 mL/min)/(g oil by SDS (4.768)) =0.01205 kg CO2/g oil (9)
Optimum SC solvent usage (kg CO2/g oil) @ (T = 49.2°C, P = 111.6 bar, % Yield = 4.620%)
(ρ of CO2 (0.000491 kg/mL)) (1stages) × (123 min (extraction time)) × f (5 mL/min)/(g oil by SC (4.620)) =0.06536 kg CO2/g oil (10)
The summary of results SDS and SC at optimum conditions are tabulated in Table 7.
Table 7.
Comparison of SDS and SC at optimum condition
CONCLUSION
The experimental supercritical extraction of essential oil from Lavandin flowers was carried out via a SDS technique and conventional SC method and the results demonstrated that SC-CO2 extraction is a viable technique to be applied in food, fragrance, pharmaceutical and natural biocides industries. Utilizing a response surface method to optimize the operating conditions revealed in which maximum extraction yield of 4.768% and 4.620% can be achieved by SDS and SC procedure at optimum operating conditions. Essential oil extracted from Lavandin flowers contains up to 80% (w/w) 1,8-Cineole, linalool, linalyl acetate, and camphor. Comparison of two methods demonstrated that SDS method provides slightly higher yield. Moreover, the SDS method is superior in terms of the major saving of solvent consumption (81.56%).
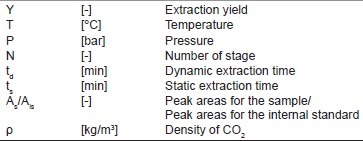
Notations.
ACKNOWLEDGEMENTS
The financial support by research center of natural products health in North Khorasan University of Medical Sciences is gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bombarda I, Dupuy N, Da JP, Gaydou EM. Comparative chemometric analyses of geographic origins and compositions of lavandin var. Grosso essential oils by mid infrared spectroscopy and gas chromatography. Anal Chim Acta. 2008;613:31–9. doi: 10.1016/j.aca.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Steltenkamp RJ, Casazza WT. Composition of the essential oil of lavandin. J Agric Food Chem. 1967;15:1063–9. [Google Scholar]
- 3.Varona S, Kareth S, Martin A, Cocero MJ. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J Supercrit Fluids. 2010;54:369–77. [Google Scholar]
- 4.Barocelli E, Calcina F, Chiavarini M, Impicciatore M, Bruni R, Bianchi A, et al. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sci. 2004;76:213–23. doi: 10.1016/j.lfs.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Varona S, Martin A, Cocero MJ. Formulation of a natural biocide based on lavandin essential oil by emulsification using modified starches. Chem Eng Process. 2009;48:1121–8. [Google Scholar]
- 6.Varona S, Rodriguez-Rojo S, Martin A, Cocero MJ, Duarte CM. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J Supercrit Fluids. 2011;58:313–9. [Google Scholar]
- 7.Yamini Y, Khajeh M, Ghasemi E, Mirza M, Javidnia K. Comparison of essential oil compositions of Salvia mirzayanii obtained by supercritical carbon dioxide extraction and hydro distillation methods. Food Chem. 2008;108:341–6. [Google Scholar]
- 8.Kamali H, Jalilvand MR, Aminimoghadamfarouj N. Pressurized fluid extraction of essential oil from Lavandula hybrida using a modified supercritical fluid extractor and a central composite design for optimization. J Sep Sci. 2012;35:1479–85. doi: 10.1002/jssc.201200043. [DOI] [PubMed] [Google Scholar]
- 9.Jalilvand M, Kamali H, Nematollahi A. Pressurized fluid extraction of rice bran oil using a modified supercritical fluid extractor and a central composite design for optimization. J Liq Chromatogr Relat Technol. 2013;36:1562–74. [Google Scholar]
- 10.Adasoglu N, Dincer S, Bolat E. Supercritical-fluid extraction of Essential oil from Turkish lavender flowers. J Supercrit Fluids. 1994;7:93–9. [Google Scholar]
- 11.Akgun M, Akgun NA, Dincer S. Extraction and modeling of lavender flower essential oil using supercritical carbon dioxide. Ind Eng Chem Res. 2000;39:473–7. [Google Scholar]
- 12.Reverchon E, Dellaporte G, Senatore F. Supercritical Co2 Extraction and Fractionation of Lavender Essential Oil and Waxes. J Agric Food Chem. 1995;43:1654–8. [Google Scholar]
- 13.Da Porto C, Decorti D, Kikic I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009;112:1072–8. [Google Scholar]
- 14.Ghoreishi SM, Kamali H, Ghaziaskar HS, Dadkhah AA. Optimization of Supercritical Extraction of Linalyl Acetate from Lavender via Box-Behnken Design. Chem Eng Technol. 2012;35:1641–8. [Google Scholar]
- 15.Chemat F, Lucchesi ME, Smadja J, Favretto L, Colnaghi G, Visinoni F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal Chim Acta. 2006;555:157–60. [Google Scholar]
- 16.Shellie R, Mondello L, Marriott P, Dugo G. Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J Chromatogr. 2002;970:225–34. doi: 10.1016/s0021-9673(02)00653-2. [DOI] [PubMed] [Google Scholar]
- 17.Flores G, Blanch GP, del Castillo ML, Herraiz M. Enantiomeric composition studies in Lavandula species using supercritical fluids. J Sep Sci. 2005;28:2333–8. doi: 10.1002/jssc.200500124. [DOI] [PubMed] [Google Scholar]
- 18.Varona S, Martin A, Cocero MJ, Gamse T. Supercritical carbon dioxide fractionation of Lavandin essential oil: Experiments and modeling. J Supercrit Fluids. 2008;45:181–8. [Google Scholar]
- 19.Oszagyán M, Simándi B, Sawinsky J, Kéry Á, Lemberkovics E, Fekete J. Supercritical Fluid Extraction of Volatile Compounds from Lavandin and Thyme. Flavour Fragr J. 1996;11:157–65. [Google Scholar]
- 20.Kamali H, Jalilvand MR, Aminimoghadamfarouj N. Pressurized fluid extraction of essential oil from Lavandula hybrida using a modified supercritical fluid extractor and a central composite design for optimization. J Sep Sci. 2012;35:1479–85. doi: 10.1002/jssc.201200043. [DOI] [PubMed] [Google Scholar]
- 21.Sahena F, Zaidul IS, Jinap S, Karim AA, Abbas KA, Norulaini NA, et al. Application of supercritical CO2 in lipid extraction-A review. J Food Eng. 2009;95:240–53. [Google Scholar]
- 22.Liu SC, Yang F, Zhang CH, Ji HW, Hong PZ, Deng CJ. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J Supercrit Fluids. 2009;48:9–14. [Google Scholar]
- 23.Kamali H, Ghaziaskar HS. Pressurized hot water extraction of benzoic acid and phthalic anhydride from petrochemical wastes using a modified supercritical fluid extractor and a central composite design for optimization. J Supercrit Fluids. 2010;54:16–21. [Google Scholar]
- 24.Yamini Y, Sefidkon F, Pourmortazavi SM. Comparison of essential oil composition of Iranian fennel (Foeniculum vulgare) obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Flavour Fragr J. 2002;17:345–8. [Google Scholar]
- 25.Shekarchizadeh H, Kadivar M, Ghaziaskar HS, Rezayat M. Optimization of enzymatic synthesis of cocoa butter analog from camel hump fat in supercritical carbon dioxide by response surface method (RSM) J Supercrit Fluids. 2009;49:209–15. [Google Scholar]
- 26.Huang Z, Chiew YC, Lu WD, Kawi S. Solubility of aspirin in supercritical carbon dioxide/alcohol mixtures. Fluid Phase Equilib. 2005;237:9–15. [Google Scholar]
- 27.Akgun M, Akgun NA, Dincer S. Phase behaviour of essential oil components in supercritical carbon dioxide. J Supercrit Fluids. 1999;15:117–25. [Google Scholar]


