Abstract
Phytochemical study and research on acute toxicity were performed on the aerial parts (leaves and stems) of Euphorbia hirta Linn. The phytochemical screening and chromatography revealed the presence of saponin, sterol, terpene, alkaloids, polyphenols, tannins and flavonoids and especially mucilage. The evaluation of total polyphenols and total flavonoids gave 120.97 ± 7.07 gallic acid equivalents (GAE) mg/g (mg of GAE/g of extract) of dry extract and 41.4 ± 0.5 mg quercetin equivalent per gram (QE/g) (mg of QE/g of plant extract) of dry extract respectively. The physicochemical study revealed moisture content of 7.73% ± 0.00%, total ash 7.48% ± 0.03%. Sulfuric ash 9.05% ± 0.01%, hydrochloric acid insoluble ash of 0.8% ± 0.02%. The search for minerals salt revealed the presence of Cr, Zn, K, Ca and Mg having an important role in glucose metabolism. The acute toxicity study showed that the toxic dose may be above 3000 mg/kg. The results of these studies indicate that extracts from the leaves and stem of E. hirta Linn. contains trace elements and minerals salt and bioactive secondary metabolites which explain their therapeutic uses for treating diabetes mellitus.
Keywords: Euphorbia hirta Linn, mineral salt, phytochemical, trace elements
INTRODUCTION
The use of medicinal plants for the treatment of metabolic diseases is gaining ground worldwide.[1] Diabetes mellitus is a disease frequently encountered in all communities, especially those living in developing countries,[2] this disease is steadily posing a serious economic problem and decrease patients’ comfort.[3,4] Indeed World Health Organization (WHO) predicts that by 2030, diabetes will be the seventh leading causes of death worldwide.[5] Estimation of the global incidence of diabetes in 2013 and the projection for the year 2035 as given by the International Diabetes Federation is 592 million.[6] The WHO in its resolution AFR/RC50/R3 (2000)[7] recommended and encouraged research and the use of medicinal plants especially in countries where access to modern medicine and conventional treatment is difficult. Consequently, research on antidiabetic medicinal plants traditionally used and with less side effects or without any toxicic effect was undertaken by N’Guessan and Tra Bi et al.[8,9] They are, Allium sativum L. (Liliaceae), Cassia occidentalis L. (Caesalpiniaceae) Chrysophyllum cainito L. (Sapotaceae), Smooth Clerodendrum (L.) Gaertn. (Verbenaceae), Crescentia cujete L. (Bignoniaceae), Euphorbia hirta (L) (Euphorbiaceae) Jatropha curcas L. (Euphorbiaceae) Jatropha gossypiifolia L.(Euphorbiaceae) Mangifera indica L.(Anacardiaceae). E. hirta was selected. Because it is ubiquitous and easily accessible especially in developing, tropical and subtropical countries.[10,11] Traditionally, this plant species is used in the treatment of gastrointestinal disorders[12,13,14] bronchial and respiratory diseases,[15] kidney stones, diabetes, conjunctivitis and many other diseases.[10] Numerous properties of this plant have already been demonstrated, among other activities antipyretic, analgesic, anti-diabetic and bactericidal activity.[10]
This study aim at searching for the chemical constituents responsible for the antihyperglycemic activity of this plant in other to use the extracts of this plant in the management of diabetic patients.
MATERIALS AND METHODS
Materials
Chemicals and equipment
Godin reagent, 10% ferric chloride, anisaldehyde and ultraviolet (UV) were used for thin layer chromatography (TLC).
Plant material
It consists of the aerial part (stem and leaves) of E. hirta Linn., collected in February 2012 in Adzopé, in the region of Agnéby northern part of Abidjan (Côte d’ Ivoire). The identification was done at the Department of Botany, University of Félix Houphouët Boigny Abidjan. A specimen was deposited at the Herbarium of the National Centre of Floristic at the University under N°. F.Y. 01, Février 2012. The plant, was collected, washed with water and then dried in the shade on racks at ambient temperature in the laboratory.
Methods
Preparation of extracts
Dried plant was coarsely pulverized. An aliquot of powdered drug has been used for the physicochemical analysis and for the determination of minerals salt. Maceration of 100 g in 1000 mL of distilled water for 24 h was made. The filtrate corresponding to the form of traditional use was aliquoted. An aliquot of the filtrate or totum or extemporaneous extract, concentrated in a rotary evaporator and then lyophilized was used for assay of the total polyphenols, total flavonoids and evaluation of acute toxicity. The other aliquot exhausted successively with different solvents of increasing polarity gave the petroleum ether fractions, chloroform, ethyl acetate fractions, the butanol fraction and the fraction of water remaining. All extracts were used for phytochemical study.
Characterization of the chemical constituents
Characterization of secondary metabolites of drugs of E. hirta, used the phytochemical screening and TLC as described in the work of Godin 1954; Georgievskii et al., 1990; Dawson et al., 1991.; Wagner and Bladt, 1996; Dekker, 2002; Kouri, 2004; Lagnika, 2005.[16,17,18,19,20,21,22]
The conventional characterization reagents had been used to highlight the following chemical groups: Alkaloids (Dragendorff reagent), polyphenols and tannins (ferric chloride), flavonoids (reaction of cyanidin), sterols and terpenes (Lieberman reaction) the saponins (foaming Index) reducing compounds (Félhing reagent) and mucilage (ethanol 60°). For TLC, the test solutions were prepared by dissolving 10 mg of each dried extract in 1 ml of a mixture of methanol-water (1:1). Ten microliters were deposited on a plate of silica gel 60 GF254, 0.25 mm thick using micropipettes. The visualizing reagent used are: petroleum ether: ethyl acetate (1:2, v/v); nButanol:acetic acid:water (60:15:25, v/v/v) and (40:10:50, v/v/v); ethyl acetate:methyl ethyl ketone:formic acid:water (50:30:10:10, v/v/v/v). The plates were dried and observed under UV lamp at 254 nm and 366 nm. The 10% ferric chloride in methanol and Godin reagent followed by heating anisaldehyde, have allowed the constituents compounds to be visible.
Determination of total polyphenols and total flavonoids
The amount of total phenols in the totum was determined by the colorimetric method of Singleton et al.[23] modified par Lamien-Meda et al.[24] using the Folin-Ciocalteu reagent the quantity of flavonoids totaux was determined by the method of Arvouet-Grand et al.[25]
After 2 h and 10 min of reaction, for total phenols and total flavonoids respectively, the absorbance was determined at 760 nm and 415 nm in a spectrophotometer (Epoch 251465, Biotek Instruments, USA). The amount of total phenolic compounds was determined against a standard curve (Y = 0.005 × X + 0.0885; R2= 1) performed with different concentrations of gallic acid. The total phenol concentration was expressed in mg gallic acid equivalent (GAE)/gram of dry matter GAE mg/g. As for total flavonoids, the standard curve was made with different concentration of quercetin (y = 0.0289 × X + 0.0036; R2= 0.99). The results were expressed in milligrams quercetin equivalent per gram of dry extracts (mg of QE/g). Each test was done in triplicate
Evaluation of acute toxicity
This assessment was carried out in accordance with the method of Ecobichon.[26] The mice were divided into two homogeneous groups according to their body weight. One lot was treated with the crude extract and the other served as the control lot. Animals fasted for 4 h with free access to water. The totum was administered at a dose of 3000 mg/kg body weight orally. Mortality was investigated in 14 days and abnormal behavior compared to the control lot. In case of death, the test will be repeated with reduced doses.
Physicochemical study
This study involved the determination of the water content and ash.
Water content
The determination of the water content was performed using the gravimetric method according to AOAC.[27] Five tests of 2 g each were introduced in five calibrated crucibles. The five samples were dried in an oven at 105°C for 24 h. The crucibles were cooled in a desiccator and weighed. The masses obtained were used to calculate the mass loss and calculate the water content of the powder as a percentage.
Ash contents
Total ash
The dried powder used during the determination of the water content was reduced to ashes in a furnace at 600°C for 6 h. After cooling in a desiccator, the ash was weighed. The masses obtained were used to calculate the masses of ash and calculate the total ash content, expressed as a percentage.
10% hydrochloric acid insoluble ash
Total ash obtained was mixed with 20 ml of 10% hydrochloric acid. The whole mixture was boiled in a water bath for 15 min. The solution obtained was filtered through watt man paper. The residue was collected in a calibrated crucible and calcinated in an oven at 600°C for 6 h. After cooling in a desiccator, the mass of ash insoluble in hydrochloric acid was measured expressed as a percentage.
50% Sulfuric acid ash
Five tests of 5 g powder each were introduced in calibrated crucibles. It was added to the contents of each crucible 5 ml of 50% H2SO4. The solution was placed in the oven at the temperature of 600°C for 6 h. After calcination and cooling in a desiccator, the ashes were weighed and the mass of sulfuric ash was expressed as a percentage.
Detection and determination of minerals salt
The qualitative and quantitative analysis of minerals was made by microanalysis energy spectrometry coupled with scanning electron microscopy-energy dispersive spectrometry (SEM/EDS or SEM-EDS) = SEM-EDS). This microscopy, at variable pressure in D.C A.C. (MEB FEG Supra 40 VP zeiss) is equipped with an X-ray detector (Oxford Instruments) connected to a platform EDS microanalysis (Inca Dry Cool without liquid nitrogen).
Two g of sample were burnt to ash (ASTM D 482) and then cooled in a desiccator. Then 10 mg of ash were spread evenly with carbon double-sided adhesive on a primed and attached to the object holder of the SEM/EDS gate pad. Whole equipment was introduced into the chamber of the SEM-RX for microanalysis (EDS).
RESULTS
Major chemical constituents
The quantity of the aqueous extraction of E. hirta Linn., after lyophilization, was 18.17% in contrast to Kandalkar et al.[28] who found 24 ± 1.5%. The phytochemical screening has allowed to identify, reducing sugars, alkaloids, saponins, polyphenols, tannins and flavonoids, terpenes and sterols and especially abundant mucilage TLC was used to calculate the frontal reports and observe the colors of the different spots of the chromatograms. These parameters are reported in Tables 1–7 below.
Table 1.
Chromatogram of the crude aqueous extract of Euphorbia hirta Linn
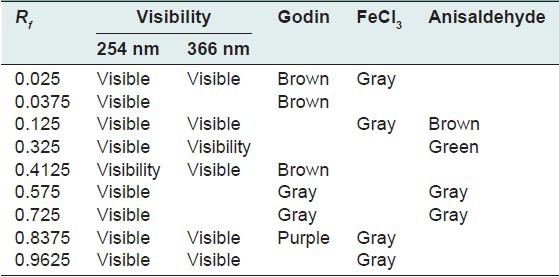
Table 7.
Chromatogram of the petroleum ether fraction of Euphorbia hirta Linn
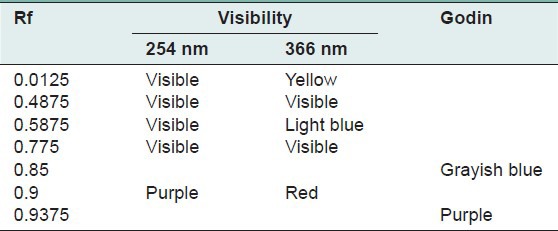
Table 2.
Chromatogram of dry lyophilized extract of Euphorbia hirta Linn
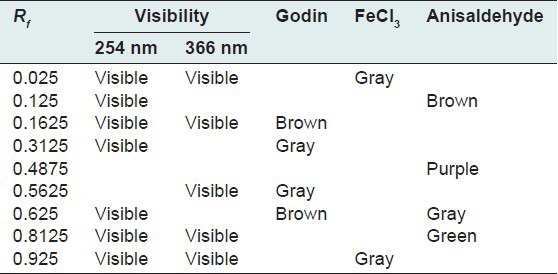
Table 3.
Chromatogram of the aqueous fraction of Euphorbia hirta Linn
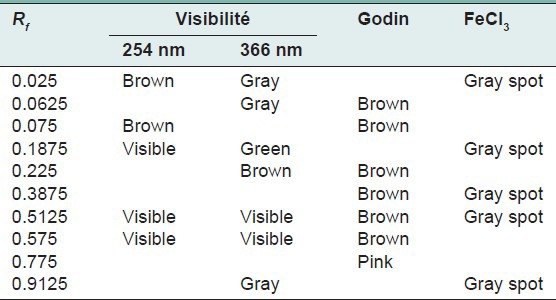
Table 4.
Chromatogram of the butanol fraction of Euphorbia hirta Linn
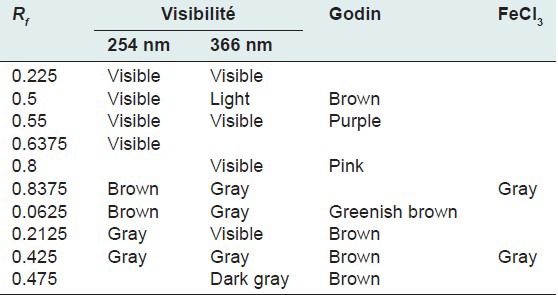
Table 5.
Chromatogram of the ethyl acetate fraction of Euphorbia hirta Linn

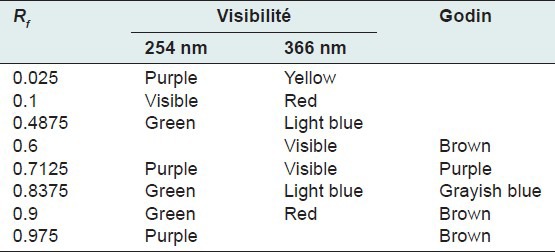
Table 6.
Chromatogram of the chloroform fraction of Euphorbia hirta Linn
Determination of total polyphenols and total flavonoids
The total polyphenol content in the aqueous totum is 120.97 ± 7.07 mg GAE/g of dry matter, while the total flavonoid content is 41.4 ± 0.5 mg QE/g of dry matter.
Toxicity
The aqueous maceration of E. hirta Linn., at a dose of 3000 mg/kg did not cause toxicity during 14 days of observation.
The physicochemical study
The physicochemical study revealed a moisture content of 7.73 ± 0.00%, total ash 7.48 ± 0.03%. Sulfuric ash 9.05 ± 0.01%, hydrochloric acid insoluble ash 0.8 ± 0.02%.
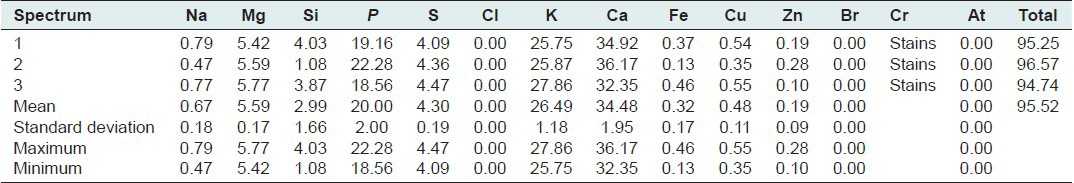
The mineral salt
Found are shown in Tables 8 and 9. Scanning of E. hirta Linn. ash has revealed many minerals salt and gases like magnesium, potassium, calcium, zinc and traces of chrome.
Table 8.
Euphorbia hirta Linn. (stem and leaves)'s salt; all results in weight%

Table 9.
Euphorbia hirta Linn. (stem and leaves)'s salt; all results in compound%
DISCUSSION AND CONCLUSION
This study have investigated the secondary metabolites and minerals in the aerial part of E. hirta Linn. responsible for the anti-hyperglycemic activity of this ubiquitous herb. It also assessed the toxicity of aqueous macerated.
About the composition of secondary metabolites of the plant collected in Côte d’Ivoire, the phytochemical screening showed the main chemical constituents, they are: Reducing sugars, alkaloids, saponins, polyphenols, tannins and flavonoids, sterols and terpenes, and the presence of mucilage. The presence of mucilage, in Abelmoschus esculentus Moench (Malvaceae) by a study conducted by Masashi et al.[29] was responsible for the anti-hyperglycemic activity on rats,[30] the anti-antidibeticactivity on rats or mice[31] and their traditional use as antidibetic.[18,19]
Thin layer chromatography visualized under UV 254 nm and 366 nm for each extract or fraction, presented clear or colored fluorescent spots. The observed colors are yellow, blue, red, purple, green, gray and brown or purple that may correspond to several classes of secondary metabolites: Yellow, green (coumarins);[17] any yellow (flavonols and/or aurones); blue and purple (flavonesmethylees).[18] To state the precise nature reagents that may reveal the different chemical groups were used.[20,21,32]
Godin reagent and anisaldehyde allowed to see spots of brown or purple and yellow which could reveal flavonoids and sapogenins. Violet colors, blue and pink could be sterols or tri terpenes and greenish gray spots, green, black or black-brown could be polyphenols or tannins.[20,21]
FeCl3 allow viewing gray spots on yellow background. These reveal polyphenols or tannins.[20] By applying these bibliographic data on all the results obtained, we conclude that all the revealed spots correspond to polyphenols, tannins and flavonoids, sterols and tri-terpenes and saponins.
Our results, showing the presence of chemical groups identified by the Phytochemical screening and TLC, are consistent with research conducted in Nigeria by Okoli et al.[33] by Tiwari et al., in India[34] and Basma et al., in Malaysia,[35] on the same species. Furthermore our investigations discover mucilage. This study confirms that the extracts and fractions are rich in secondary metabolites [Tables 1 and 7]. Also despite the diversity of climate and soil types where this species grows, E. hirta Linn. retains the same qualitative composition of secondary metabolites.
The dosage of the polyphenolic compounds and of total flavonoids shows a large quantity of these compounds. The amount of polyphenols content when extracted Basma et al.,[35] with methanol (206.17 ± 1.95 mg GAE/g) seems more important than in those extracted by water (120.97 ± 7.07 mg GAE/g). While the amount of flavonoid content when extracted with water (41.4 ± 0.5 mg QE/g) in E. hirta Linn. is substantially identical to that extracted with methanol (37.970 ± 0.003 mg QE/g). These extraction methods do not influence the availability of each secondary metabolite when the plant is consumed. All comparisons of these methods are useful for the implementation of the best method of extracting a group of metabolites. The presence of poly phenolic compounds and flavonoids involved in the management of metabolic diseases especially diabetes.[36,37,38]
Concerning the toxicity, E. hirta did not show any toxic effect at 3000 mg/kg bw in rats. These results are in agreement with those of one hand,[39] which showed a non-toxic effect of the extracts in doses of 3000, 6000 and 9000 mg/kg. Also, other authors reveal a protective effect of leaf extracts of E. hirta.[40] Contrary to that of Oyewale[41] found that toxicity of aqueous extract with an LD50 equal to 1412.5 ± 4.7 μg/mL that is 1412.5 ± 4.7 mg/kg to the safety limit on shrimp. Not with standing Adedapo[42] calls for caution in the use of ethanol extract of this plant because some chromatographic fractions of this extract have shown toxic effect on rats. The protective effect of E. hirta could be due to the large content of polyphenol and flavonoid.[41]
In view of the importance of E. hirta Linn., for its composition and secondary metabolites for its various pharmacological properties, especially against the installation of diabetes, analysis of physicochemical properties was also performed. The moisture content was 7.73 ± 0.00% whereas Kandalkar et al. reported 4.2 ± 2.00%.[28] Our results are higher, but <10% indicating a good preservation quality of the drug.[43] The total ash value, insoluble in hydrochloric and sulfuric acid were respectively 7.48% ±0.03%, 0.8% ±0.02% and 9.05 ± 0.01%. The values of total ash, ash insoluble in hydrochloric acid and sulfuric acid found by this author Kandalkar et al.,[28] are still weak and are respectively equal to 5.5 ± 0.3%, 2.5 ± 0.5% and 7.0 ± 0.4% except for ash insoluble in hydrochloric acid. The high total ash value found on our samples is certainly in favor of a heavy load of minerals in the plants.[44]
Therapeutically, it is preferable to associate the presence of minerals improving insulin sensitivity.[45] Minerals are essential elements involved in many mechanisms. They are supplied into the body through the diet or medication. The search for these trace elements has enabled us detect numerous minerals including chromium, mineral improving insulin sensitivity through modulation of insulin metabolism and stimulating insulin sensitivity.[46] Magnesium, enzyme activator, participates in major metabolisms, Potassium, Calcium, and Zinc are supporting a good insulin activity because their disability is due to insulin resistance.[45] Thus, E. hirta is evaluated as a source of polyphenols, mucilage and minerals support the antidiabetic activity.
CONCLUSION
The search for different components of E. hirta L. has enabled us to have a better understanding of anti-hyperglycemic activity of this plant. Indeed, the simultaneous presence of mucilage, secondary metabolites and mineral salt exerts a synergistic action to reduce hyperglycemia and at the same time undertakes remedial action due to the presence of polyphenolic compounds. It is also desirable to encourage people in developing countries to consume occasionally the stems with leaves of E. hirta in view of the increasing installation of diabetes mellitus in our populations worldwide.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.WHO. Enhancing the Role of Traditional Medicine in Health Systems: a Strategy for the African Region. Brazzaville: World Health Organization, Regional Office for Africa; 2013; 2013. Sep 2-6, Situation analysis and justification AFR/RC63/6 on Situation Analysis. WHO Regional Office for Africa. Sixty-Third Session of the WHO Regional Committee for Africa, Brazzaville, Republic of Congo; pp. 7–14. [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 4.Oga AS, Tebi A, Aka J, Adouéni KV, Malan KA, Kouadio LP, et al. Diabetes mellitus diagnosed in Côte d’Ivoire: Their epidemiological particularities. Med Trop. 2006;66:241–6. [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva: World Health Organization; 2011. Global Status report on Noncommunicable Diseases 2010. [Google Scholar]
- 6.International Diabetes Federation 2013. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Last accessed on 2013 Nov 17]. IDF Diabetes Atlas. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf . [Google Scholar]
- 7.World Health Organization Regional Office for Africa. Final Report of the fiftieth session of the WHO Regional committee for Africa. Brazzaville: Burkina Faso, 28 August-2 September 2000; 2000. Resolution AFR / RC50 / R3 entitled Promoting the Role of Traditional Medicine in Health care Systems: Strategy for the African Region. WHO Regional Office for Africa; pp. 10–12. [Google Scholar]
- 8.N’Guessan K. University of Cocody-Abidjan, Faculty of Biosciences, Laboratory of Botany; 2008. Medicinal plants and traditional medical practices of Abbey and Krobou people in the region of Agboville (Coted’ivoire). Doctorate Thesis of Natural Science; p. 235. [Google Scholar]
- 9.Tra Bi FH, Irié GM, N’Gaman KC, Mohou CH. Studies of some therapeutic plants used in the treatment of Hypertension and diabetes: Two diseases Emerging in Côte d’Ivoire. Nat Sci. 2008;5:39–48. [Google Scholar]
- 10.Kumar S, Malhotra R, Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 2010;4:58–61. doi: 10.4103/0973-7847.65327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood SK, Bhardwaj R, Lakhanpal TN. Jodhpur, New Delhi, India: Scientific Publishers; 2005. Ethnic Indian Plants in Cure of Diabetes; p. 164. [Google Scholar]
- 12.Tona L, Kambu K, Ngimbi N, Mesia K, Penge O, Lusakibanza M, et al. Antiamoebic and spasmolytic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa, Congo. Phytomedicine. 2000;7:31–8. doi: 10.1016/S0944-7113(00)80019-7. [DOI] [PubMed] [Google Scholar]
- 13.Galvez J, Zarzuelo A, Crespo ME, Lorente MD, Ocete MA, Jiménez J. Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med. 1993;59:333–6. doi: 10.1055/s-2006-959694. [DOI] [PubMed] [Google Scholar]
- 14.Gálvez J, Crespo ME, Jiménez J, Suárez A, Zarzuelo A. Antidiarrhoeic activity of quercitrin in mice and rats. J Pharm Pharmacol. 1993;45:157–9. doi: 10.1111/j.2042-7158.1993.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 15.Chopra RN, Chopra IC, Handa KL, Kapoor LD. Vol. 42. Calcutta, India: Academic Publishers; 1994. Indigenous Drugs of India 1994; pp. 1216–25. [Google Scholar]
- 16.Godin P. A new spray reagent for paper chromatography of polyols and cetoses. Nature. 1954;174:134. [Google Scholar]
- 17.Georgievskii VP, Komissarenko NF, Dmitrouk SE. éd Novosibirsk: Naouka; 1990. Medicinal Plants Bioactive substances. Translated from Russian; p. 336. [Google Scholar]
- 18.Dawson R, Elliott D, Elliott W, Jones K. ed. Moscow: Mir; 1991. Dictionnaire de Biochimiste. [Google Scholar]
- 19.Dekker M. Coumarins: analysis by TLC. Encyclopedia of Chromatography. 2002:1–3. [Google Scholar]
- 20.Wagner H, Bladt S. 2nd ed. Berlin: Springer Verlag; 1996. Plant Drug Analysis in Thin Layer Chromatography Atlas; p. 384. [Google Scholar]
- 21.Kouri FC. Thèse de doctorat. Suisse: Université de Lausanne. 2004:211. [Google Scholar]
- 22.Lagnika L. Strasbourg/France: Université Louis Pasteur; 2005. Thèse de doctorat; p. 267. [Google Scholar]
- 23.Singleton VL, Ortofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Packer L, editor. Methods in Enzymology. London: Orlando Academic Press; 1999. pp. 152–78. [Google Scholar]
- 24.L amien-Meda A, Lamien CE, Compaore MM, Meda RN, Kiendrebeogo M, Zeba B, et al. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13:581–94. doi: 10.3390/molecules13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of propolis extract and identification of principal constituents. J Pharm Belg. 1994;49:462–8. [PubMed] [Google Scholar]
- 26.Ecobichon DJ. The Basis of Toxicity Testing. 2nd ed. Boca Raton FL, New York: CRC Press; 1997. Acute toxicity studies; pp. 43–86. [Google Scholar]
- 27.AOAC. 16th ed. Arlington, VA: AOAC International; 1995. Official Methods of Analysis of AOAC International; p. 250. [Google Scholar]
- 28.Kandalkar AM, Manjunath KP, Sholapur HP, Patel AM, Darade SS. Phytochemical and pharmacognostic evaluation of Euphorbia hirta Linn Leaves. J Pharm Res. 2009;2:349–52. [Google Scholar]
- 29.Tomoda M, Shimizu N, Oshima Y, Takahashi M, Murakami M, Hikino H. Hypoglycemic activity of twenty plant mucilages and three modified products. Planta Med. 1987;53:8–12. doi: 10.1055/s-2006-962605. [DOI] [PubMed] [Google Scholar]
- 30.Fofie NB, Sanogo R, Diarra B, Kanadjigui F. Antioxidant and anti-hyperglycaemic activity of Euphorbia hirta L. on Wistar rats. Int J Biol Chem Sci. 2013;7:2558–67. [Google Scholar]
- 31.Kumar S, Malhotra R, Kumar D. Antihyperglycemic, antihyperlipidemic and antioxidant activities of Euphorbia hirta stem extract. Int Res J Pharm. 2010;1:150–6. [Google Scholar]
- 32.Agnika L. Phytochemical study and biological activity of natural substances isolated from plants in Republic of Benin, PhD Thesis, University of Louis Pasteur, Strasbourg Pasteur. de Strasbourg, mention Sciences Pharmaceutiques. 2005:268. [Google Scholar]
- 33.Okoli RI, Turay AA, Mensah JK, Aigbe AO. Phytochemical and Antimicrobial Properties of Four Herbs from Edo State, Nigeria. Rep Opin. 2009;1:67–73. [Google Scholar]
- 34.Tiwari N, Chaudhary A, Mishra A. Phytochemical screening and antioxidant activities of some Indian medicinal plants used for malaria therapy. Pharm Lett. 2010;2:335–40. [Google Scholar]
- 35.Basma AA, Zakaria Z, Latha LY, Sasidharan S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac J Trop Med. 2011;4:386–90. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Bhardwaj P, Sharma P. Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacognosy Res. 2013;5:225–32. doi: 10.4103/0974-8490.118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onawumi OOE, Adelowo FE, Ipadeola AO, Edewor TI, Ayoola PB, Odunola OA. Preliminary studies on phytochemical and antimicrobial investigation of plants (Irawo-Ile) Mitracarpus villosus, Euphorbia hirta and Spermacoce ocymoides. Int J Res Rev Appl Sci. 2012;10:78–81. [Google Scholar]
- 38.N’Guessan AH, Déliko CE, Mamyrbékova Bekro-JA, Bekro YA. Phenolic content of 10 medicinal plants used in the traditional treatment of hypertension, an emerging disease in Côte d’Ivoire. Ind engineering Rev. 2011;6:55–6. [Google Scholar]
- 39.Lanhers MC, Nicola JP, Fleurentin J, Weniger B. Euphorbia hirta L. Ethnopharmacologia. 2005;36:9–23. [Google Scholar]
- 40.Suganya S, Sophia D, Raj CA, Rathi MA, Thirumoorthi L, Meenakshi P, et al. Amelioration of nitrobenzene-induced nephrotoxicity by the ethanol extract of the herb Euphorbia hirta. Pharmacognosy Res. 2011;3:201–7. doi: 10.4103/0974-8490.85009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyewale AO, Mika A, Peters F. Phytochemical, cytotoxicityandmicrobial screening of Euphorbia hirta Linn. Glob J Pure Appl Sci. 2002;8:49–55. [Google Scholar]
- 42.Adedapo AA, Abatan MO, Idowu SO, Olorunsogo OO. Effects of chromatography fractions of Euphorbia hirta on the rat serum biochemistry. Afr J Biomed Res. 2005;8:185–9. [Google Scholar]
- 43.Vol. 2. Lagos: OUA; 1988. OUA. Méthodes Générales d’Analyses. Pharmacopée Africaine; p. 264. [Google Scholar]
- 44.Sambo MH. Study of the traditional treatment of diabetes using a recipe prepared from bark of the trunk of Manilkara multinervis Dub (Sapotaceae) Thèse de pharmacie. 2006:41. [Google Scholar]
- 45.L efèvre PJ, Scheen AJ. Improving the action of insulin. Clin Invest Med. 1995;1:340–7. [PubMed] [Google Scholar]
- 46.Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, Chi J, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–91. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]


