Abstract
Objective:
To assess the anthelmintic acivity of Bacillus cereus and Bacillus pumilus metabolites.
Materials and Methods:
The successive solvent extractions with petroleum ether, ethyl acetate and methanol. The solvent extracts were tested for anthelmintic activity against Pheretima posthuma at 20 mg/ml concentration. The time of paralysis and time of death of the worms was determined for all the extracts. Albendazole was taken as a standard reference and sterile water as a control.
Results:
All the sample extracts showed significant anthelmintic activity in paralyzing the worms comparable with that of the standard drug. The time of death exhibited by BP metabolites was close to the time exhibited by standard.
Conclusion:
The study indicates both bacteria Bacillus cereus and Bacillus pumilus have anthelmintic activity indicating potential metabolites in them.
Keywords: Anthelmintic, Bacillus cereus, Bacillus pumilus, Pheretima posthuma
INTRODUCTION
Helminthes infections are among the most common infections in humans, affecting a large population of the world. About 1/3rd of world's population harbors helminthes of one species, or another.[1] The intestinal roundworms, including hookworms and whipworms, harm the host by depriving him of food, causing blood loss, injury to organs, intestinal or lymphatic obstruction and by secreting the toxins.[2,3] These infections have led to lowering of immune systems for HIV, malaria and tuberculosis and debilitating the individual both physically and cognitively.[4] Majority of infections are generally limited to tropical regions and contribute to the prevalence of malnutrition, anemia, eosinophilia and pneumonia.[5]
Internal parasitic infection is a great threat not only to humans but also to the productivity of the sheep and goat industry.[6] It has a great impact on the economy of domesticated livestock throughout the world due to their adverse effect on productivity.[7]
Tremendous progress has been made in the development of anthelmintic drugs in the past 50 years. During this period, the current classes of synthetic drugs were developed including the benzimidazoles and imidazothiazoles. Another major step was achieved with the introduction of the avermectin class of macrolactones in the early 1980's. The discovery of this compound class led to anthelmintics drugs, such as ivermectin and doramectin, which have excellent broad spectrum activity and superior potency.[6]
Though many drugs are currently available in the market, still nematodes represent a serious threat to both plants and animals. Resistance to all of these classes of drugs has been observed. New methods of control of these parasites are being sought since a number of soil applied commercial nematocides are being phased out and resistance to anthelmintics is an increasing problem.[8] The resistance to nematodes has been reported from several countries.[9,10] Hookworms and some other parasitic nematodes have shown signs of resistance to albendzole, the current treatment approved by the World Health Organization.[4] The increasing prevalence of helminthes parasites that are resistant to convectional anthelmintics has been the spur for different research programs exploring alternative approaches to control the parasites and to discover new classes of anthelmintics, especially those with a novel mode of action.[6,11,12]
For much of our past history forages, plant parts or extracts have been used to combat parasitism and in many part of the world such natural products are still in use for this purpose. Many potential phytotherapeutics have been revealed that some plant species, when grazed or fed as conserved material may reduce the degree of internal parasite infection in sheep.[13,14,15]
Though, the development of the avermectin class of compounds obtained from a soil bacterium Streptomyces avermitilis initiated more research to look for microbial metabolites for potent anthelmintic compounds, less literature are available regarding microbial metabolites as anthelmintics. Hence in the present study, the metabolic extracts of two bacteria Bacillus cereus (BC) and Bacillus pumilus (BP) were tested for anthelmintic activity in vitro.
MATERIALS AND METHODS
Solvent extraction and preparation of samples
The two bacteria BC and BP were grown separately in large quantity in nutrient broth medium and incubated for 3 days at 35°C. The broth was centrifuged to separate the cells at 10,000 rpm for 20 min. The clear supernatant containing the metabolites was collected. The metabolites of both organisms were subjected to successive solvent extraction with petroleum ether, ethyl acetate and methanol (1:1) in a separating funnel. All the three solvent extracts were dried in separate plates. The BC petroleum ether extract was labeled as sample BC-1, ethyl acetate extract as BC-2 and methanol extract as BC-3. Similarly the BP extracts were labeled as BP-1, BP-2 and BP-3 respectively.
The adult Indian earthworms were used for the initial screening of anthelmintic compounds in vitro.[16,17] The assay was performed on adult earthworm Pheretima posthuma, due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings and also because of its easy availability.[18,19,20] The worms were collected from moist soil and washed with normal saline to remove all dirt and fecal matter. The earthworm of 8-12 cm in length and 0.3-0.5 cm in width were selected for study. The worms were divided into eight groups of six worms each. The worms of groups 1-3 were released into separate plates containing a solution of 50 ml of the sample extracts of BC, BC-1, BC-2 and BC-3 respectively. Similarly, the worms of groups 4-6 were released into the sample extracts of BP, BP-1, BP-2 and BP-3 respectively. All the samples were prepared in sterile water at a dose of 20 mg/ml. The group 7 worms were released into 50 ml of standard drug albendazole solution of 20 mg/ml concentration. The control group was placed in plain sterile water of 50 ml (group 8). The assay was carried as per the method of Ajaiyeoba et al.[21]
Observations were made for the time taken to paralysis and death of individual worms. Time for paralysis was noted when no movement of any sort could be observed except when the worms were shaken vigorously and failed to revive even after transfer to normal saline. Death was concluded when the worms lost their motility completely and failed to respond even after a touch with the needle followed by fading away of their body colors.
RESULTS
Bacillus cereus
The sample extracts BC-1, BC-2 and BC-3 paralyzed the worms in 15 min, 17 min and 15 min respectively at 20 mg/ml concentration. The time exhibited by sample extracts for paralysis was better when compared to the standard drug albendazole, which took 30 min for paralysis at 20 mg/ml.
The time taken for death of worms by sample extracts BC-1, BC-2 and BC-3 was 120 min, 130 min and 150 min, respectively. The time taken for death by all the sample extracts was more when compared with standard drug which brought the death in 70 min.
Bacillus pumilus
The sample extracts BP-1, BP-2 and BP-3 paralyzed the worms in 13 min, 16 min and 10 min respectively at 20 mg/ml concentration. This time response by sample extracts for paralysis was much better than the standard drug Albendazole, which took 30 min for paralysis at 20 mg/ml.
The time taken for death of worms by the sample extracts BP-1, BP-2 and BP-3 was 90 min, 85 min and 95 min, respectively. The time taken for death by all the sample extracts was more when compared to standard drug, which killed the worms in 70 min.
The time taken for paralysis of worms and subsequent death in the presence of different sample extracts, and standard drug are shown in Table 1. The histogram showing the paralysis of worms in minutes is shown in Figure 1. The histogram showing the death of worms in minutes is shown in Figure 2.
Table 1.
Anthelmintics activity of BC and BP metabolites
Figure 1.
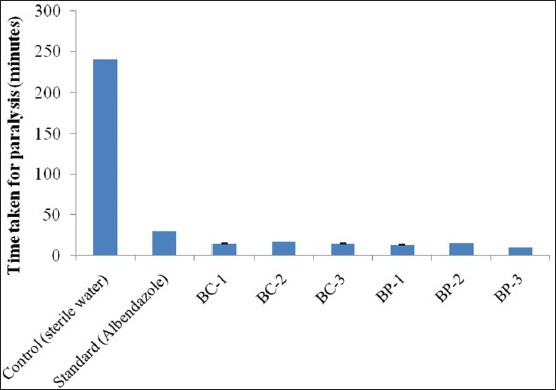
Paralysis of worms in minutes in presence of Bacillus cereus and Bacillus pumilus metabolites in comparison with standard. BC: Bacillus cereus; BC-1 Petroleum ether extract; BC-2 Ethyl acetate extract; BC-3 Methanol extract. BP: Bacillus pumilus; BP-1 Petroleum ether extract; BP-2 Ethyl acetate extract; BP-3 Methanol extract
Figure 2.
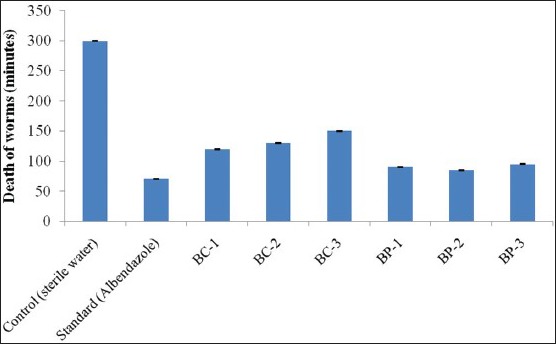
Death of worms in minutes in presence of Bacillus cereus and Bacillus pumilus metabolites in comparison with standard. BC: Bacillus cereus; BC-1 Petroleum ether extract; BC-2 Ethyl acetate extract; BC-3 Methanol extract. BP: Bacillus pumilus; BP-1 Petroleum ether extract; BP-2 Ethyl acetate extract; BP-3 Methanol extract
DISCUSSION
The aim of studies reported here was to test the potential of BC and BP metabolites for anthelmintic activity. The motive behind to carryout anthelmintic activity was the toxicity shown by the extracts during cytotoxicity studies on various cell lines. The toxicity on cancer cell lines of certain sample extracts was more when compared to toxicity on normal cell lines.
Many bacteria and fungi have been reported to have antinematode activity. Among bacteria the metabolites of genera Pasteuria, Pseudomonas, Serratia, and among fungi Arthrobotrys, Catenaria and Cephalosporium are reported to have antinematode activity.[22] “Lachnumon B1 and B2”, “mycorrhizin B1 and B2”, both brominated metabolites obtained from ascomycete Lachnum papyraceum with nematicidal and antimicrobial activities have been reported by Stadler et al.[23] “3-hydroxypropionic acid” obtained from endophytic fungi Phomopsis phaseoli and Melanconium betulinum with nematicidal activity against Caenorhabditis elegans and Meloidogyne incognita has been reported by Schwarz et al.[24]
“Avermectins”, a series of highly potent anthelmintic and insecticidal compounds of the macrolide structural class produced by the soil bacterium “S. avermitilis” was reported by Burg et al.[25] Avermectin A, a macrolide, with a molecular weight of 873 doltons is a commercially available compound with anthelmintic and insecticides activity. Since the discovery of the avermectins, members of the structurally and functionally similar milbemycin family have been isolated from Streptomyces hygroscopicus subsp. aureolacrinosus[26] Streptomyces cyaneogriseus subsp. moncyogenus[27] and Streptomyces sp. Strain E225.[28]
A solvent-extractable metabolite with a small molecular weight, <10,000 Dolton, produced by a novel strain of BP with pesticidal activity against rootworm, beet armyworm and nematodes have been reported by Heins et al.[29] A crystal protein isolated from soil bacterium Bacillus thuringiensis has been reported to kill the nematode worms and it is said to be safe for vertebrates.[30]
The present investigations have shown that both the bacterial extracts have significant anthelmintic activity. The paralyzing capacities of the sample extracts are comparatively better than the standard drug albendazole. Among the entire sample extracts the methanol extract of BP was good enough to paralyze the worms in 10 min when compared to standard drug time of 30 min. However, regarding the time taken for death of worms, the sample extracts of BC took more time when compared to sample extracts of BP. The time taken by BP extracts for death of worms (between 85 and 95 min) was close to that of the standard drug time of 70 min.
Since the sample extracts of BC showed early paralysis, but delayed death time of the worms, the effect of the extracts may be due to chloride ion conductance of worm muscle membrane, producing hyperpolarization and reduced excitability that leads to muscle relaxation and flaccid paralysis. The BP sample extracts showed not only early paralysis, but also the early death of worms, which was close to the time of death exhibited by standard drug. Hence, the metabolic compounds of BP responsible for anthelmintic activity appears to be different and also with a different mechanism of action from that of BC metabolites.
Though the BP strain (15.1), with antinematodal activity has been reported by Heins et al.[29] The BC strain with anthelmintic activity has not been reported in the past. Hence, as per the available literature and to the best of our knowledge, this may be the first report on BC bacteria with anthelmintic activity.
CONCLUSION
The present study concludes that the petroleum ether extract, ethyl acetate extract and methanol extract of both bacteria BC and BP have exhibited anthelmintic activity indicating potential metabolites in them. The future scope involves the need of isolation of active constituents responsible for the activity. Further studies are required to establish a mechanism of action.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bundy DA. Immunoepidemiology of intestinal helminthic infections. The global burden of intestinal nematode disease. Trans R Soc Trop Med Hyg. 1994;88:259–61. doi: 10.1016/0035-9203(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 2.Rang HP, Dale MM, Ritter JM. 4th ed. London: Churchil-Livingstone; 1999. Pharmacology; pp. 739–43. [Google Scholar]
- 3.Tripati KD. 5th ed. New Delhi: JP Publishers; 2003. Essentials of Medical Pharmacology. [Google Scholar]
- 4.Fang J. Soil bacteria could yield drug to treat roundworm. Nature. 2010:101. [Google Scholar]
- 5.Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Makonnen W, et al. Anti-malarial activity of withania somnifera L. Dunal extracts in mice. Ethiop Med J. 2006;44:279–85. [PubMed] [Google Scholar]
- 6.Ayers S, Zink DL, Mohn K, Powell JS, Brown CM, Bills G, et al. Anthelmintic constituents of Clonostachys candelabrum. J Antibiot (Tokyo) 2010;63:119–22. doi: 10.1038/ja.2009.131. [DOI] [PubMed] [Google Scholar]
- 7.Jabbar A, Iqbal Z, Kerboeuf D, Muhammad G, Khan MN, Afaq M. Anthelmintic resistance: The state of play revisited. Life Sci. 2006;79:2413–31. doi: 10.1016/j.lfs.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Ghisalberti EL. Secondary metabolites with antinematodal activity. Stud Nat Prod Chem. 2002;26:425–506. [Google Scholar]
- 9.Jackson F. Anthelmintic resistance – the state of play. Br Vet J. 1993;149:123–38. doi: 10.1016/S0007-1935(05)80083-1. [DOI] [PubMed] [Google Scholar]
- 10.Rolfe PF. Anthelmintic resistance in Australia, its development and management. Proceedings 4th International Congress for Sheep Veterinarians, Armidale, Australia. 1997:51–8. [Google Scholar]
- 11.Jackson F, Coop RL. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:S95–107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- 12.McKellar QA, Jackson F. Veterinary anthelmintics: Old and new. Trends Parasitol. 2004;20:456–61. doi: 10.1016/j.pt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Niezen JH, Waghorn TS, Charleston WA, Waghorn GC. Growth and gastrointestinal nematode parasitism in lambs grazing either lucerne (Medicago sativa) or sulla (Hedysarum coronarium) which contains condensed tannins. J Agric Sci. 1995;125:281–9. [Google Scholar]
- 14.Marley CL, Cook R, Barrett J, Keatinge R, Lampkin NH, McBride SD. The effect of dietary forage on the development and survival of helminth parasites in ovine faeces. Vet Parasitol. 2003;118:93–107. doi: 10.1016/j.vetpar.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Paolini V, Dorchies P, Hoste H. Effects of sainfoin hay on gastrointestinal nematode infections in goats. Vet Rec. 2003;152:600–1. doi: 10.1136/vr.152.19.600-b. [DOI] [PubMed] [Google Scholar]
- 16.Sollmann T. Anthelmintics their efficiency as tested on earthworms. J Pharmacol Exp Ther. 1918;12:129–70. [Google Scholar]
- 17.Dash GK, Suresh P, Sahu SK, Kar DM, Ganapaty S, Panda SB. Evaluation of Evolvulus alsinoides Linn. For anthelmintic and antimicrobial activities. J Nat Red. 2002;2:19–22. [Google Scholar]
- 18.Thorn GW, Adams RD, Brawnwald E, Isselbacher KT, Peterdorf RG. New York: McGraw Hill Co; 1977. Harrison's Principles of Internal Medicine; pp. 1088–90. [Google Scholar]
- 19.Vigar Z. 2nd ed. Singapore: P. G. Publishing House; 1984. Atlas of Medical Parasitology; pp. 216–8. [Google Scholar]
- 20.Chatterjee KD. Parasitology. In: Guha RA, editor. Protozoology and Helminthology. 6th ed. Calcutta: Sree Saraswaty Press Ltd; 1967. [Google Scholar]
- 21.Ajaiyeoba EO, Onocha PA, Olarenwaju OT. In-vitro anthelmintic properties of Buchnolzia coriaceae and Gynandropsis gynandra extract. Pharm Biol. 2001;39:217–20. [Google Scholar]
- 22.Rodgers PB. Potential of biological control organisms as a source of antifungal compounds for agrochemical and pharmaceutical product development. Pestic Sci. 1989;27:155–64. [Google Scholar]
- 23.Stadler M, Anke H, Sterner O. New metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. VII Structure determination of brominated lachnumon and mycorrhizin A derivatives. J Antibiot (Tokyo) 1995;48:158–61. doi: 10.7164/antibiotics.48.158. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz M, Köpcke B, Weber RW, Sterner O, Anke H. 3-hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry. 2004;65:2239–45. doi: 10.1016/j.phytochem.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, et al. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob Agents Chemother. 1979;15:361–7. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takiguchi Y, Mishima H, Okuda M, Terao M, Aoki A, Fukuda R. Milbemycins, a new family of macrolide antibiotics: Fermentation, isolation and physico-chemical properties. J Antibiot (Tokyo) 1980;33:1120–7. doi: 10.7164/antibiotics.33.1120. [DOI] [PubMed] [Google Scholar]
- 27.Carter GT, Nietsche JA, Hertz MR, Williams DR, Siegel MM, Morton GO, et al. LL-F28249 antibiotic complex: A new family of antiparasitic macrocyclic lactones. Isolation, characterization and structures of LL-F28249 alpha, beta, gamma, lambda. J Antibiot (Tokyo) 1988;41:519–29. doi: 10.7164/antibiotics.41.519. [DOI] [PubMed] [Google Scholar]
- 28.Hood JD, Banks RM, Brewer MD, Fish JP, Manger BR, Poulton ME. A novel series of milbemycin antibiotics from Streptomyces strain E225. I. Discovery, fermentation and anthelmintic activity. J Antibiot (Tokyo) 1989;42:1593–8. doi: 10.7164/antibiotics.42.1593. [DOI] [PubMed] [Google Scholar]
- 29.Heins SD, Manker DC, Jimenez DR, Marrone PG. Bacillus pumilus strain for controlling corn rootworm, nematode and armyworm infestations. US Patent: 6291426 [Google Scholar]
- 30.Hu Y, Georghiou SB, Kelleher AJ, Aroian RV. Bacillus thuringiensis Cry5B protein is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS Negl Trop Dis. 2010;4:e614. doi: 10.1371/journal.pntd.0000614. [DOI] [PMC free article] [PubMed] [Google Scholar]


