Abstract
Objective
Penetrating brain injury (PBI) has the highest risk for inducing post-traumatic epilepsy and retained foreign materials such as bullet fragments carry the greatest risk. This study examines the potential contribution of copper, a major component of bullets, to the development of epilepsy following PBI.
Methods
Anesthetized adult male rats received a penetrating injury from the dorsal cortex to the ventral hippocampus from a high speed small bit drill. In one group of animals, copper wire was inserted into the lesion. Control animals had only the lesion or the lesion plus stainless steel wire (biologically inert foreign body). From 6 to up to 11 months following the injury the rats were monitored intermittently for the development of epilepsy with video-EEG. A separate set of animals was examined for possible acute seizures in the week following the injury.
Results
22 of the 23 animals with copper wire developed chronic epilepsy compared to 3 of the 20 control rats (lesion and lesion with stainless steel). Copper was associated with more extensive injury. The control rats with epilepsy had larger lesions. In the acute injury group, there was no difference in the incidence of seizures (83% lesion plus stainless steel, 70% lesion plus copper).
Conclusions
Copper increases the risk for epilepsy and may increase damage over time, but there were no differences between the groups in the incidence of acute post-injury seizures. Lesion size may contribute to epilepsy development in lesion only animals. Copper maybe an independent risk factor for the development of epilepsy and possible secondary injury, but lesion size also contributes to the development of epilepsy. The consequences of prolonged exposure of the brain to copper observed in these animals may have clinical implications that require further evaluation.
Keywords: epilepsy, brain trauma, animal models
INTRODUCTION
Head trauma accounts for up to 20% of the acquired epilepsies and 6% of all causes for epilepsy1,2, but little is known about how the injuries lead to spontaneous seizures. Penetrating brain injuries (PBI) are commonly considered to be a problem for the military with more than 4000 American survivors from the recent wars3. However, the problem is much greater in the civilian population, as there are at least that many survivors of penetrating head injuries (largely gunshot wounds) annually in the United States4, many of whom will develop epilepsy.
PBI have the highest risk of developing post-traumatic epilepsy, and foreign fragments in the brain, including metal, are associated with the highest risk (about 60% will develop epilepsy)5,6. The risks may increase with more detectable metal fragments7, but it is not known if the fragments have a role in epileptogenesis or whether they are markers for a more extensive injury, which is also a risk factor5,6. Identifying remediable risk factors is essential for developing interventions to prevent epilepsy as well as for understanding the mechanisms of the disease.
Most current head injury models do not replicate penetrating ballistic brain injury. The fluid percussion model8,9, is similar to closed head injuries, and up to 50% eventually develop epilepsy, but penetrating injury pathology is not replicated9. A model based on the insertion of a balloon tipped microcatheter may replicate the effect of tissue shock waves but does not introduce foreign materials into the brain10, and chronic outcomes have not yet been reported11. A more recent model uses a piston driven 5mm into a rat’s brain, but only the acute pathology has been reported12. Neither of these models has included metal fragments. Copper is a component of most bullets and causes a significant inflammatory reaction in the brain13–15, which is also thought to contribute to epilepsy development 16.
In this study we wished to determine whether retained metal fragments, with a specific focus on copper, increased the risk for the development of epilepsy following a penetrating brain injury. If it is a risk factor the results could influence the clinical management of these injuries and also provide new directions for studying the mechanisms underlying this form of epilepsy.
MATERIALS AND METHODS
Adult male Sprague-Dawley (SD) rats (250–350 g) were used. Animals were maintained under standard laboratory conditions on a 12/12 hour light/dark cycle with free access to food and water. All animals were handled under protocols approved by the Animal Care and Use Committee (ACUC) of the University of Virginia which adheres to AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) international standards.
Trauma Induction
Rats were placed in a stereotaxic frame under 2% isoflurane anesthesia. Body temperature was maintained by a water blanket throughout the procedure. The scalp was prepped with a combination povidone iodine and isopropyl alcohol, and the skull was exposed. A high speed drill (Dremel 300m, Robert Bosch Tool Corporation, Illinois) was mounted vertically on the stereotactic arm with its extension cable. The hardened steel burr (1.5mm diameter) was pushed through the skull and dura into the brain at 1000 revolutions per minute using the stereotactic arm,. Bone and other tissue were not removed from the drill’s trajectory which was lowered 8mm below the brain surface (approimately 2mm from the ventral surface). The coordinates for the hippocampal lesion were based on a standard stereotaxic atlas17 from bregma −4.9mm AP and ±4.5mm lateral. In some of the animals we placed either copper wire (0.02 inches diameter, 5mm in length) or stainless steel wire (0.03 inches diameter, same length; an inert control for foreign material) into the lesion immediately after it had been created. The scalp was closed, and the rats were allowed to recover while housed singly.
All of our procedures are performed sterilely. The drill bit is always sterilized in a standard steam autoclave with other instruments before surgery. On a day in which several animals undergo surgery in sequence, the bit and other instruments are decontaminated in 70% alcohol between cases and then sterilized in a hot bead sterilizer immediately before use. The copper and stainless steel wires were decontaminated in 70% alcohol for at least 20 minutes and then placed in the hot glass bead sterilizer before insertion. We do not routinely give antibiotics after the procedure, but the rats do receive a long acting local anesthetic to the wound (bupivicaine) as well as oral analgesics (ketorolac) for 2 days following the surgery. The animals behaved normally the day following the injury, except for the animals with larger primary injuries in early days of procedure development. These rats had clear neurological impairments, and they were euthanized. We did not have such animals after the first several weeks of the project.
Recording Protocol
There were two clinically relevant questions that we wished to answer in this study. The first is whether the presence of the copper influenced the percentage of animals that became epileptic. The second question was whether there were early seizures and whether there was a relation between early seizures and chronic epilepsy. At five and a half months following the injury, electrodes were implanted into the contralateral hippocampus (bipolar twisted pair stainless steel) and the left and right frontal cortex (stainless steel) (Figure 1). The frontal electrodes were monopolar but linked to one another for the second channel. Seizure frequency, duration and behavior were recorded for each animal.
Figure 1. Experimental time line.
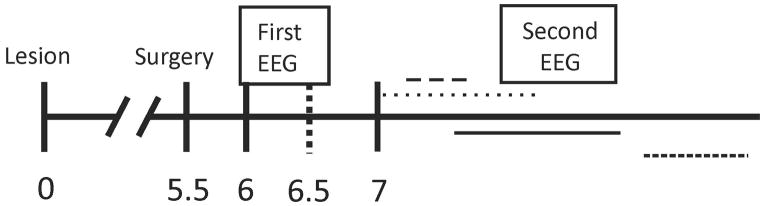
Numbers represent time after lesion in months. Electrodes were implanted 5.5 months after lesioning and the first EEG monitoring was performed for the two weeks starting 6 months after lesioning. The second EEG session was performed at variable times and for varying durations after 7 months. There was no difference between groups for total duration of monitoring or age at the completion of monitoring.
To identify chronic epilepsy we started video EEG monitoring 6 months following the lesion18, lesion (Figure 1). The first recordings lasted for 2 weeks, after which the animals were removed from monitoring. The next recording sessions started at least 7 months following the lesion. Recordings were performed intermittently as space on the recording system allowed (other studies were performed during this period). Any one recording session lasted a minimum of two weeks. The majority of recording time was obtained after the animals were 7 months post lesion, and a few animals were followed for as long as 11 months following the lesion. The emphasis on the longer followup was made to reduce the chance that failure to identify an animal with epilepsy did not occur because of an inadequate duration of monitoring and not waiting long enough for the epilepsy to develop. There were no differences between the groups with regard to time after injury at which the last recording was ended (8.9±0.42 months for copper animals and 9.0±0.38 months for lesion only, p=0.9) nor for the total duration of monitoring (6.0±1.1 weeks copper and 5.3±0.9 weeks lesion only, p=0.65). Following the final recording session, the animals were euthanized and examined for gross differences in injury.
To determine whether there were seizures in the first week following the injury, a separate set of rats had recordings performed immediately following lesioning. After the trauma while the rats were still under anesthesia, electrodes were placed in the contralateral hippocampus and the ipsilateral piriform cortex. We chose these recording sites because earlier kindling work had shown these areas were usually involved from the earliest stages when seizures were induced in the hippocampus19. The following day the animals were placed on video EEG monitoring for a week. Number of seizures, seizure behavior and duration were recorded.
Seizure Recording
The video EEGs were performed on a rodent epilepsy monitoring system that is a standard tool in our laboratory18. Each rat is housed individually in Plexiglass cages that allow full range of motion while connected to the amplifiers through a light weight flexible cable and low torque electrical swivel. The EEG video system consists of a Grass Model 12 amplifier and commercial EEG software (Harmonie, Stellate Systems). The EEG signal was digitized at 200Hz. The videos were recorded with an infrared sensitive camera under infrared lamps to allow visualization of behaviors in the dark. EEGs were reviewed daily offline.
Seizure patterns varied from animal to animal but the most common EEG patterns seen in these animals included either 1) a progressive build up in amplitude of rhythmic theta activity (5 Hz or greater) or spiking that evolved in frequency to clonic activity (regular spike and wave at a frequency of 2 Hz or less) followed by a postictal suppression or 2) an initial spike followed by attenuation of amplitude and a subsequent buildup of rhythmic activity19. These criteria have been developed over a number of years and are based on their similarities to human focal seizures recorded with intracranial recordings. Seizures were detected on manual review as we have found that automated detection criteria leads to many false positives and negatives. All suspicious patterns were correlated with the video to determine whether the change could represent recording artifact. Seizures were scored for behavioral accompaniment using the Racine 5 point scale.
Gross Pathology
After the monitoring, the rats were perfused with 4% paraformaldehyde under a lethal dose of pentobarbital anesthesia. The brains were examined grossly for overt damage, and cut into 4mm thick coronal slabs to obtain a qualitative estimate of the size of the associated lesion.
Statistics
The points for analysis were the proportion of each group with seizures (Fisher exact). For animals with seizures, seizure frequency (total number of seizures for the two acute groups), average seizure duration and median behavioral severity, standard t-tests were used, except for behavioral severity scores which were compared with a Mann-Whitney Rank Sum score. Results are presented as means ±SEM. Differences with p<0.05 or less were considered significant.
RESULTS
Thirty-one rats were lesioned and implanted with copper wire. Five died acutely following surgery and three lost their headsets prematurely so 23 rats with the lesion plus copper were included in the analysis. Seventeen rats received just the lesion, of which three died acutely and one lost its headset prematurely, so 13 rats completed the protocol. There were no differences in mortality between the copper and lesion only animals (p=1.00). Seven rats had stainless steel added to the lesion and there were no premature losses. Twenty two of the copper animals had at least two recorded seizures by the end of the study (Figure 2). Three of the lesion only and none of the stainless steel rats had seizures in the same period of time. There was no significant difference in the incidence of epilepsy between the lesion only and lesion plus stainless steel animals (p = 0.521, Fisher exact) so they were combined into a single control group. The difference in the development of epilepsy between the copper lesions and the controls was highly significant (p<0.001, Fisher’s exact) (Table 1). The copper animals had a significantly higher seizure frequency as well as a worse behavioral severity score, but there were no difference between the groups with regard to seizure duration (Table 1).
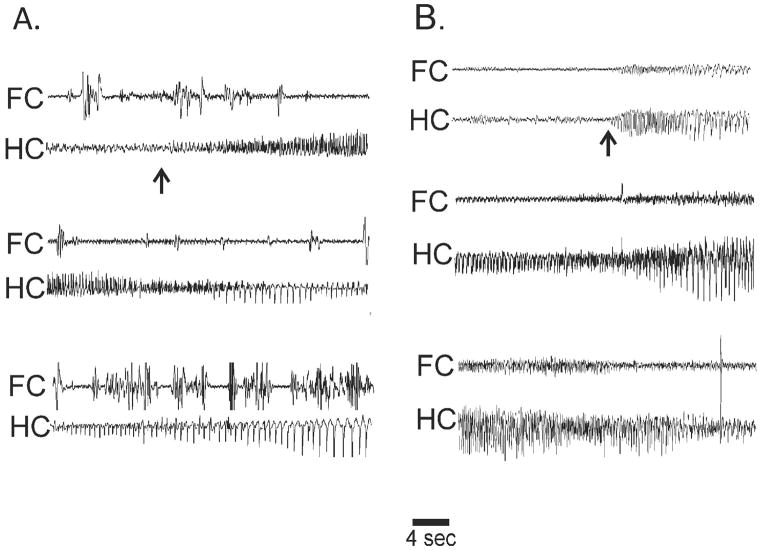
Figure 2. Spontaneous seizures in two rats with chronic epilepsy following lesions copper.
Recordings from linked bilateral prefrontal electrodes (FC) and a bipolar contralateral hippocampal (HC) electrode. A. Non-convulsive seizure involving only HC (arrow shows onset). High amplitude FC bursts were artifact from wet dog shakes. B. Convulsive seizure with first observed changes in HC (arrow) with subsequent recruitment of FC. FC involvement was shorter, over by the middle of the third row.
Table 1.
Long Term Epilepsy Outcomes of Different Lesion Types
| Group (n) | Number Epileptic (%) | Avg. seizures per day† | Avg duration (s) | Median behavioral severity |
|---|---|---|---|---|
| Copper (23) | 22 (96)* | 3.6±0.34** | 33.8±2.6 | 4*** |
| Control (20) | 3 (15) | 0.2±0.06 | 25.7±0.7 | 1 |
| Lesion only (13) | 3 (23) | 0.2±0.06 | 25.7±0.7 | 1 |
| Steel (7) | 0 (0) | -- | -- | -- |
p<0.001, Fisher exact
p<0.01, t-test
p<0.05 Mann-Whitney
Average seizures per day only includes animals with documented seizures
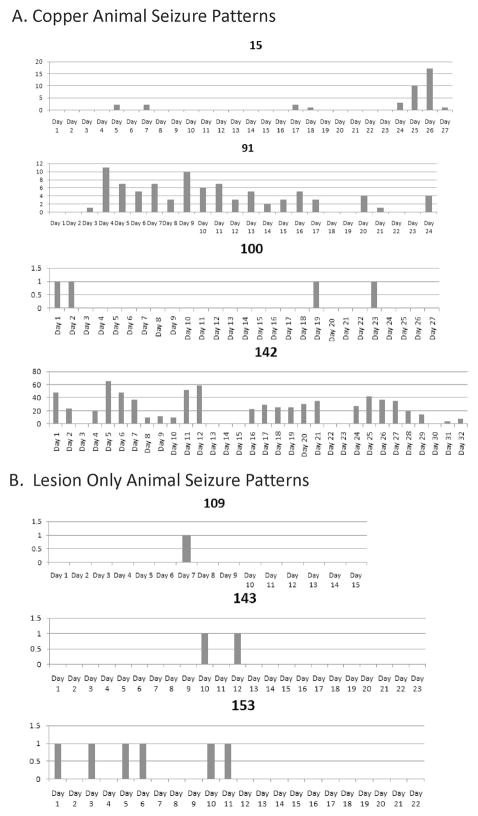
The pattern of seizure occurrence was different for each animal (Figure 3). Although many animals had a single seizure on the days they had one, others had more than 60. All rats had seizure free intervals lasting from a few days to two weeks or longer. The intermittent recording protocol probably resulted in a few animals with seizures being missed, there was no difference between the groups with regard to time monitored and the age at which they were monitored. Even if a few rats in the lesion group were misclassified, the overall frequency of seizures was much higher in the animals with the copper so the data suggest that both the risk for developing epilepsy and the severity of the epilepsy is much greater with the metal fragments.
Figure 3. Pattern of seizure occurrence in rats over consecutive monitoring days.
Figure demonstrates variable seizure frequency of seizures across the animals. These data were obtained at least 7 months after the trauma. A. Rats with copper and B. with only the lesion. Vertical axis is seizures per day. Note varying scales for each animal. Horizontal axis is consecutive monitoring days. Regardless of overall seizure frequency all rats had seizure free days, and seizure free intervals of 14 days or more were not unusual.
The acute deaths occurred predominantly during the startup phase of the project and with experience with the technique, deaths became rare. The remaining animals tolerated the trauma well and showed less than 10% of weight loss over the two consecutive weeks following the surgery. None of the animals needed extra care for feeding and cleaning.
Acute Seizures After Injury
There were 10 lesion plus copper animals and 6 lesion plus stainless steel rats that underwent one week of EEG recording following the lesion. Four other rats (two copper, two stainless steel) died in the first 24 hours and were not included in the outcome data. Seven of the copper (70%) and five of the stainless steel rats (83%) had acute seizures (NS, Fisher exact) (Figure 4). All of the seizures were non-convulsive, and there were no differences in total number of seizures or seizure duration between the two groups (Table 2). The majority of the seizures occurred between day 3 and 5 post lesion, although a few occured earlier and later.
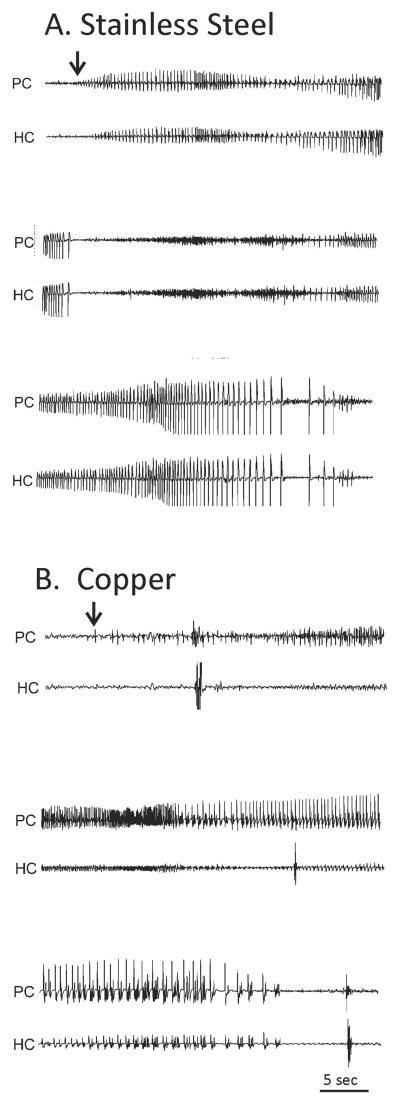
Figure 4. Spontaneous seizures in two rats in the first week following lesion.
Recordings were from ipsilateral piriform cortex (PC) and contralateral hippocampus (HC). Both seizures were non-convulsive. A. Seizure from rat with stainless steel wire in the lesion. Starts in PC (arrow) with build up of repetitive spiking and rapid involvement of HC. B. Seizure from rat with copper wire in the lesion. Starts in PC (arrow) with repetitive spiking and subsequent recruitment of HC.
Table 2.
Acute Post Lesion Seizure Outcomes
| Group (n) | Number with seizures (%) | Average number seizures† | Average seizure duration (s) | Median seizure severity |
|---|---|---|---|---|
| Copper (10) | 7 (70) | 11.9±4.9 | 52.7±6.3 | 1 |
| Steel (6) | 5 (83) | 4±0.632 | 50.4±17.7 | 1 |
Average number of seizures is total over the one week of recording for animals with recorded seizures.
Extent of Injury
For the majority of the chronic lesion only or lesion and stainless steel, the injury consisted of a single tract from the cortical surface approximately 2mm across (Figure 5A). There was no associated tissue discoloration. For the three lesion only animals that had documented seizures, the lesion was more extensive in breadth and depth, extending to the ventral surface involving the amygdala and piriform cortex (Figure 5C). For the rats that had copper wire added to the lesion, the size of the lesion was much larger, typically 5 or 6 mm in the anterior-posterior direction and 3–4 mm in the medio-lateral direction. In addition there was a consistent tissue discoloration with precipitated copper (Figure 5B). In an animal killed at 2 months post injury, there is clear necrosis around the copper wire and significant discoloration over the cortex (Figures 5D–F).
Figure 5.
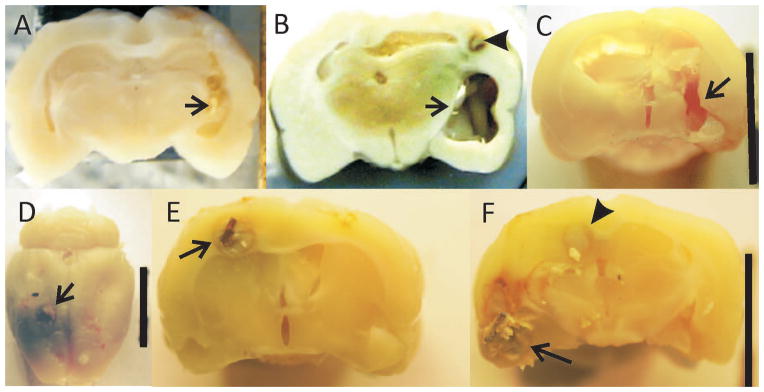
Gross sections showing the lesions from 3 rats. Arrows show lesion. A. Lesion only animal without epilepsy. Lesion consists of the drill tract with little addtional damage. B. Lesion plus copper after 7 months in rat with documented epilespy. There is significant tissue loss in the medial-lateral as well as anterior posterior directions extending far beyond the primary lesion. In addition, precipitated copper discolors the surrounding tissue. Arrowhead points to copper wire remnant surrounded by necrotic tissue. C. Lesion only animal with epilepsy. Lesion more extensive than in animal in (A) extending to the ventral surface as well as anterior and posterior to primary lesion in (A). D–F: Brain from an unmonitored rat with lesion plus copper two months after lesion. D. Arrow points to copper wire at cortical surface surrounded by precipitated copper. E. Copper wire surrounded by necrotic tissue. F. Same animal approximately 3mm posterior to (E) More extensive necrosis in region of amygdala and olfactory cortex (arrow) and area of necrosis (arrowhead) continuous with same area in (E). D–F demonstrate the early stages of the copper associated damage.
DISCUSSION
There were three findings of this study. First, the presence of copper significantly increased the risk of chronic epilepsy, as almost all of the animals with copper fragments developed epilepsy, whereas animals with the lesion alone or lesion and stainless steel rarely did, and only if there were large lesions. This latter finding suggests that lesion size may play a role in epileptogenesis as well. Second, acute seizures were common in the first week following any type of injury. The third and most surprising finding was the larger lesion associated with prolonged exposure to copper. This preliminary observation raises a number of possibilities about the pathophysiology of this type of epilepsy and may have implications for how penetrating injuries are treated, as this study suggests that prolonged copper exposure results in extensive secondary injury.
Two questions arise from these results. Is it the presence of the copper that causes the seizures or is it the larger secondary lesion that is the major factor in epilepsy development? These issues are key as we consider the pathophysiology of this form of epilepsy. These observations could have clinical implications, as the data suggest that copper may cause long term damage as well as epilepsy. The role of copper exposure in epileptogenesis can be addressed experimentally. If the copper itself causes the seizures, removal of the copper at any time could control the epilepsy. If it is the secondary damage, early removal of the copper could reduce the damage as well as the risk for epilepsy. A related question is whether there Is an amount of copper below which the damage and epilepsy do not occur? The clinical literature suggests that there may be7. Present guidelines for these injuries suggest a less aggressive approach with an emphasis on stopping bleeding, debriding overtly damaged tissue, removing readily accessible fragments and preventing infection 20–22. Removal of remote fragments is discouraged to avoid additional damage. However, if the fragments induce late secondary damage, it may be more important to remove them as early as possible. Although the studies have not specifically addressed the issue of functional deterioration following this type of injury, there is evidence that a significant number of individuals suffer a loss of cognitive or other function over time following an injury 23,24.
Copper is toxic to the brain. It is an oxidizing agent associated with neurodegeneration25–27. Copper induces significant inflammatory reactions compared to other studied metals 13–15. An older report associates gunshot wounds with copper pellets with sterile abscesses and seizures 28. The role of inflammation in epilepsy and seizures 29,30 is under active investigation, so the reported inflammatory reaction to the copper could play a role in the development of epilepsy in these animals. Long term serial studies are needed to determine whether foreign fragments are associated with progressive damage, as our data suggest.
The other finding of note from this study is the high incidence of seizures in the acute period. There is a clinical association between early seizures and the development of epilepsy 2. Although we did not follow this group of acute animals chronically, our long term data suggest that epilepsy risk is based on the nature of the injury: both groups had similar acute seizures, but the copper animals were more likely to develop chronic epilepsy. As we interpret the significance of findings from clinical and laboratory studies, this distinction between acute and chronic seizures may be important.
There are a number of models of post-traumatic or post-injury seizures, and each has its advantages and limitations, and the models should be viewed with how each relates to human post-traumatic seizures and epilepsy. The fluid percussion model of brain trauma may have associated hemorrhage, and the extent of injury can be variable. The incidence of epilepsy over the year following the injury ranges from 25–50% 9. In the post-natal hypoxia-ischemia model the seizures also develop over months following the injury 31,32. In both there is a relationship between the extent of the injury and the development of epilepsy 8. The fluid percussion model has many similarities to closed head injuries, in which there is a relation between the severity of the initial injury and the development of epilepsy 33,34. There have been reports that minimal injury from fluid percussion can result in brief absence seizures 35, but the claim has been controversial 36. The parallels with this model and the human condition are not clear.
More recently there have also been reports of new models of penetrating brain injuries10–12 which have focused solely on physiology and pathology in the several weeks following the injury. There are also reports about the effects of foreign materials on the development of seizures, including cobalt, alumina gel and iron chloride. Cobalt can induce seizures for about a month, but the seizures resolve, so it is not considered a model of chronic epilepsy37,38. Alumina gel can induce chronic epilepsy in primates and perhaps rabbits, but it has not worked in rats. However, its parallels to human epilepsy are less clear 39,40. Intracortical iron chloride has been reported as a model of epilepsy following an intracerebral hemorrhage, but the results across laboratories have been inconsistent 41,42. In contrast the model in this report has many parallels with chronic human epilepsy following a penetrating injury.
A question may be raised about the relation of this model to true gunshot wounds, which can cause massive damage to single or multiple lobes and follow many trajectories. The overwhelming majority of these injuries are fatal or cause severe impairments. The survivable injuries cause less damage and have relatively good outcomes. This model may replicate these simpler injuries with no overt neurological impairment to the animal. Attempts to create larger penetrating injuries had essentially 100% mortality from acute hemorrhage. The copper trauma model is adequate in comparing lesion only versus lesion plus metal. Keeping the primary lesion simple also made it easier to observe the secondary injury from the prolonged exposure to copper. Based on our observation that the three lesion only rats with seizures had qualitatively larger lesions, and the other reports suggesting that the development of epilepsy was related to the size of the injury it is likely that lesion size influences the development of epilepsy following a penetrating injury. Whether it was the copper itself or the size of the secondary injury from the copper that was the main contributor to the development of epilepsy requires further study.
The number of lesion only animals with epilepsy is likely an underestimate. With the recording protocols and the observed interseizure interval of up to 16 days, some animals could have been misclassified. Still, with similar periods of monitoring, the lesion only animals had a lower seizure burden and lower incidence of epilepsy. Outside of monitoring animals continuously for a number of months, we will never exclude the presence of epilepsy. As we examine the pathophysiology of the disease, a more certain determination will be necessary.
In our model copper has an impact on the development of epilepsy and may cause secondary injury. Copper may increase the risk for epilepsy in a number of ways but two possibilities include a direct effect of the metal on the environment in the brain or throught the extensive secondary injury from prolonged exposure. The early literature has consistently reported that copper induces a significant inflammatory reaction in the brain, and there is an extensive literature demonstrating that a number of components of neuroinflammation lower the seizure threshold and are potentially epileptogenic 16,29. The apparent secondary damage associated with the presence of the copper could also be the primary cause of the epilepsy, and the three lesion only rats had larger lesions. The other acquired epilepsies (fluid percussion and post hypoxia ischemia) show a clear relation between the extent of the damage and the development of epilepsy. The basis for this association is entirely unclear, but larger lesions could result in a critical mass of neurons with a lowered threshold for seizures.
In summary, this study has found that the continued presence of copper following a penetrating head injury increases the risk for the development of epilepsy and more extensive brain injury. These findings suggest that there may be factors that can be modified early after the injury that may improve long term outcomes, including the prevention or amelioration of epilepsy. Clinical studies addressing these questions are needed.
Acknowledgments
This study was supported in part by grants NS25605 and NS64438 from the NINDS.
Footnotes
Adherence to the Journal’s position on ethical publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures
Edward Bertram has received support from Epilepsia as an honorarium and from the International League Against Epilepsy and the American Academy of Neurology for travel. The remaining authors have no conflicts of interest.
References
- 1.Agrawal A, Timothy J, Pandit L, et al. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. 2006;108:433–439. doi: 10.1016/j.clineuro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein DH. Epilepsy after head injury. Epilepsia. 2009;50:4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 3.Fisher H Congressional Research Service U.S. Military Casualty Statistics. Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. 2013 http://www.fas.org/sgp/crs/natsec/RS22452.pdf.
- 4.Beaman V, Annest JL, Mercy JA, et al. Lethality of firearm-related injuries in the United States Population. Ann Emerge Med. 2000;35:258–266. doi: 10.1016/s0196-0644(00)70077-1. [DOI] [PubMed] [Google Scholar]
- 5.Salazar AM, Jabbari B, Vance SC, et al. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- 6.Raymont V, Salazar AM, Lipsky R, et al. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010;75:224–229. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eftekhar B, Sahraian MA, Nouralishahi B, et al. Prognostic factors in the persistence of posttraumatic epilepsy after penetrating head injuries sustained in war. J Neurosurg. 2009;110:319–326. doi: 10.3171/2008.4.17519. [DOI] [PubMed] [Google Scholar]
- 8.Kharatishvili I, Pitkänen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Pitkänen A, Immonen RJ, Gröhn OHJ, et al. From traumatic brain injury to posttraumatic epilepsy: What animal models tell us about the process and treatment options. Epilepsia. 2009;50:21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 10.Williams AJ, Hartings JA, Lu XC, et al. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J Neurotrauma. 2006;23:1828–1846. doi: 10.1089/neu.2006.23.1828. [DOI] [PubMed] [Google Scholar]
- 11.Williams AJ, Ling GS, Tortella FC. Severity level and injury track determine outcome following a penetrating ballistic-like brain injury in the rat. Neuroscience Letters. 2006;408:183–188. doi: 10.1016/j.neulet.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 12.Plantman S, Ng KC, Lu J, et al. Characterization of a novel rat model of penetrating traumatic brain injury. J Neurotrauma. 2012;29:1219–1232. doi: 10.1089/neu.2011.2182. [DOI] [PubMed] [Google Scholar]
- 13.Robinson RR, Johnson MT. Histopathological studies of tissue reactions to various metals implanted in cat brains. Aeronautical Systems Division Technical Report. 1962;61–397:1–13. U.S.A.F. [PubMed] [Google Scholar]
- 14.Dymond AM, Kaechele LE, Jurist JM, et al. Brain tissue reaction to some chronically implanted metals. J Neurosurg. 1970;33:574–580. doi: 10.3171/jns.1970.33.5.0574. [DOI] [PubMed] [Google Scholar]
- 15.Babb TL, Kupfer W. Phagocytic and Metabolic Reactions to Chronically Implanted Metal Brain Electrodes. Experimental Neurology. 1984;86:171–182. doi: 10.1016/0014-4886(84)90179-1. [DOI] [PubMed] [Google Scholar]
- 16.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxinos G. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 1986. [Google Scholar]
- 18.Bertram EH, Williamson JM, Cornett JC, et al. Design and Construction of a Long-term Continuous Video-EEG Monitoring Unit for Simultaneous Recording of Multiple Small Animals. Brain Research Protocols. 1997;2:85–97. doi: 10.1016/s1385-299x(97)00033-0. [DOI] [PubMed] [Google Scholar]
- 19.Bertram EH. The functional anatomy of spontaneous seizures in a rat model of chronic limbic epilepsy. Epilepsia. 1997;38:95–105. doi: 10.1111/j.1528-1157.1997.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines for the Surgical Management of Penetrating Brain Injury. Jour of Trauma. 2001;51:S16–S25. [PubMed] [Google Scholar]
- 21.Nagib MG, Rockswold GL, Sherman RS, et al. Civilian gunshot wounds to the brain: prognosis and management. Neurosurgery. 1986;18:533–537. doi: 10.1227/00006123-198605000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Cosar A, Gönül E, Kurt E, et al. Craniocerebral Gunshot Wounds: Results of Less Aggressive Surgery and Complications. Minim Invas Neurosurg. 2005;48:113–118. doi: 10.1055/s-2004-830222. [DOI] [PubMed] [Google Scholar]
- 23.Whitnall L, McMillan TM, Murray GD, et al. Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. J Neurol Neurosur Psych. 2006;77:640–645. doi: 10.1136/jnnp.2005.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 25.Gaggelli E, Kozlowski H, Valensin D, et al. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 26.Bush AI. Metals and neuroscience. Current Opinion in Chemical Biology. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Liu L, Zhang L, et al. Redox reactions of the R-Synuclein-Cu 2+ complex and their effects on neuronal cell viability. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sights WP, Bye RJ. The fate of retained intracerebral shotgun pellets An experimental study. J Neurosurg. 1970;33:646–653. doi: 10.3171/jns.1970.33.6.0646. [DOI] [PubMed] [Google Scholar]
- 29.Vezzani A, Balosso S, Ravizza T. Inflammation and epilepsy. Handbook of Clinical Neurology. 2012;107:163–175. doi: 10.1016/B978-0-444-52898-8.00010-0. [DOI] [PubMed] [Google Scholar]
- 30.Friedman A, Dingledine R. Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia. 2011;52 (Suppl 3):33–39. doi: 10.1111/j.1528-1167.2011.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadam SD, White AM, Staley KJ, et al. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 2010;30:404–415. doi: 10.1523/JNEUROSCI.4093-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annegers JF, Grabow JD, Groover RV, et al. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- 34.Annegers JF, Hauser WA, Coan SP, et al. A Population-Based Study of Seizures after Traumatic Brain Injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 35.D’Ambrosio R, Fender JS, Fairbanks JP, et al. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudek FE, Bertram EH. Counterpoint to “what is an epileptic seizure?” by D’ambrosio and Miller. Epilepsy Currents. 2010;10:91–94. doi: 10.1111/j.1535-7511.2010.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velisek L. Models of Chemically induced seizures. In: Pitkänen A, Schwartkroin PA, Moshé SL, editors. Models of seizures and epilepsy. Elsevier Academic Press; 2006. pp. 127–152. [Google Scholar]
- 38.Craig CR. Models of focal epilepsy in rodents. In: Peterson SL, Albertson TE, editors. From: Neuropharmacology methods in epilepsy research. 494. CRC Press; 1998. pp. 155–169. [Google Scholar]
- 39.Lockard JS, Uhlir V, DuCharme LL, et al. Efficacy of standard anticonvulsants in monkey model with spontaneous motor seizures. Epilepsia. 1975;16:301–317. doi: 10.1111/j.1528-1157.1975.tb06061.x. [DOI] [PubMed] [Google Scholar]
- 40.Bostantjopoulou S, Katsarou Z, Milonas I, et al. Focal experimental epilepsy in rabbits. Funct Neurol. 1990;5:127–133. [PubMed] [Google Scholar]
- 41.Ueda Y, Triggs WJ, Willmore LJ. Head Trauma: hermorrhage-iron deposition. In: Pitkänen A, Schwartkroin PA, Moshé SL, editors. Models of seizures and epilepsy. Elsevier Academic Press; 2006. pp. 495–500. [Google Scholar]
- 42.Lange SC, Neafsey EJ, Wyler AR. Neuronal activity in chronic ferric chloride epileptic foci in cats and monkey. Epilepsia. 1980;21:251–254. doi: 10.1111/j.1528-1157.1980.tb04070.x. [DOI] [PubMed] [Google Scholar]