Summary
Studies have demonstrated that the membrane attack complex (MAC) of complement can evoke seizures when injected directly into rodent brain. In the course of studies examining the role of complement in the development of experimental cerebral malaria (ECM), we observed fewer seizures in mice deficient in C5, a component required for MAC formation. To determine if the MAC contributed to the tonic-clonic seizures characteristic of ECM, we performed long-term video-electroencephalography (EEG) on C5−/− mice with Plasmodium berghei ANKA-induced cerebral malaria and observed significantly reduced spike and seizure frequency compared to wild type mice. Our data suggest a role for the MAC in malaria-induced seizures and that inhibition of the terminal complement pathway may reduce seizures and seizure-related neurocognitive deficits.
Keywords: Plasmodium berghei ANKA, terminal complement pathway
Malaria remains one of the most common and deadly parasitic diseases worldwide, despite effective chemotherapeutics and widespread distribution of insecticide-treated bednets. The majority of malaria cases are caused by Plasmodium falciparum or Plasmodium vivax, however, P. falciparum causes cerebral malaria (CM), the most severe and frequently fatal form of malaria. Cerebral malaria is distinguished by the development of unarousable coma and occurs primarily in early childhood in endemic regions, although travelers to endemic regions may also be affected.1 Children that develop CM have seizures of a generalized nature, although seizures with a focal origin are frequently observed. Status epilepticus is common in children with CM with concurrent increase in intracranial pressure. Children who survive CM often develop epilepsy and neurological sequelae, which include transient or prolonged neurocognitive deficits such as psychosis, ataxia and language and behavioral problems.2
In the course of studies designed to determine the role of the complement system in a well-established animal model for cerebral malaria (experimental cerebral malaria, ECM), we anecdotally observed that mice deficient in complement component C5 had few seizure-like events compared to wild type mice. In addition, we found that mice treated with anti-C9 antibodies, which prevents formation of the complement membrane attack complex (MAC), had significantly higher survival rates and lower clinical signs of disease,3,4 including seizures. These results suggested that the MAC (which is composed of C5b, a proteolytic fragment of C5, C6, C7, C8 and multiple C9 molecules) may directly or indirectly contribute to the development of malaria-related seizures. To address this possibility, we performed EEG studies in which we compared epileptic events between wild type and C5−/− mice, the latter of which lack a central component required for MAC formation.
For these experiments, mice (seven wild type and eight C5−/−, eight to twelve weeks in age, C57BL/6J appropriately backcrossed) were anesthetized and maintained with 2.5% isofluorane. Six small holes were drilled through the skull, bilaterally, ~2-3 mm posterior and lateral to Bregma, ~4 mm posterior to Bregma and 5 mm lateral to midline and ~ 6 mm posterior to Bregma and 3-4 mm lateral to midline, using a dental drill equipped with a 1.0 mm drill bit. Three 1.6 mm stainless steel screws (Small Parts, Inc.) were screwed halfway into the 2 holes closest to Bregma and in 1 hole farthest from Bregma. Then, an EEG electrode (Plastics One, Inc.) with 2 lead wires and a ground wire, cut to a length that would touch but not penetrate the dura (~1.5 mm), were inserted into the remaining three drill holes with the ground wire positioned in one of the holes farthest from Bregma. The lead wires were placed bilaterally over the cortical surface of the parietal hemispheres in the region over the underlying hippocampi. This 2 electrode system does not allow us identify the anatomical origin of epileptic activity. Once the electrode was positioned, dental acrylic was applied to form a stable cap on the skull that cements the electrode in place. When the acrylic had dried, the scalp was closed with skin glue (3M Vetbond). One week after electrode placement surgery, mice were individually housed in specially-constructed EEG monitoring cages. EEG data was acquired using Biopac Systems amplifiers (Biopac EEG100C) and AcqKnowlege 4.2 EEG Acquisition and Reader Software (BIOPAC Systems, Inc.). Data was stored and analyzed in digital format. Cages were also equipped with IR Digital Color CCD cameras (Digimerge Technologies) that recorded each animal concurrently with EEG monitoring; recordings were acquired for review using security system hardware and software (L20WD800 Series, Lorex Technology, Inc.). All collected data was manually analyzed for abnormalities by an experienced observer blinded to genotype. All EEG recordings were scored for the presence of isolated spikes, repetitive spiking, and seizures. Spikes were defined as having a duration of <200 ms with 5× baseline amplitude, whereas repetitive spiking activity was defined as ≥3 spikes lasting ≤ 5 s. Seizures were defined as high-frequency and high-amplitude repetitive spiking, or low frequency, high-amplitude spike-wave patterns for a duration of ≥ 10 s. Corresponding videos were analyzed for associated behavior during these abnormal events. We observed that mice during seizures froze, developed facial automatisms and forelimb clonus then fell onto their sides during hind limb clonus (tonic-clonic seizure behavior). This behavior corresponded with electrographic seizure onset, and continued throughout the duration of the electrographic seizure. Immediately following behavioral and epileptiform discharges, animals characteristically remained briefly frozen before resuming normal behavior. Epileptiform discharges correlated with brief freezing behavior, or if they occurred during sleep, was accompanied by a brief head twitch. All studies were performed with approval from the UAB IACUC.
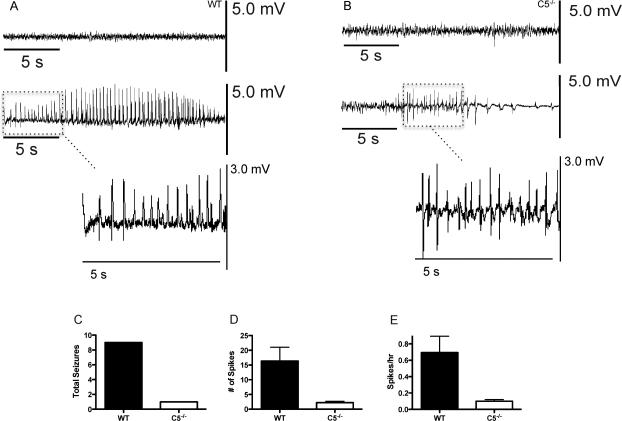
Continuous EEG and video monitoring of all mice commenced 24 h prior to induction of experimental cerebral malaria (ECM). Representative EEG recordings from uninfected wild type and C5−/− mice reveal no significant differences in basal EEG activity between the two genotypes (Fig. 1A and B, upper tracing, respectively). Experimental cerebral malaria (ECM) was then induced by injecting both groups of mice i.p. with 5 × 105 Plasmodium berghei ANKA-infected RBCs (PbA). Continuous EEG and video monitoring was conducted until 9 days post-infection, or until the animals became moribund and/or died. Mice were also visually scored twice daily for clinical signs of neurologic disease as previously described, and classified as having ECM if they displayed appropriate symptoms or succumbed to infection between days 5-9 post-infection as previously described.3 A representative EEG recording uninfected wild type and C5−/− mice demonstrates essentially no differences in baseline tracings (Fig. 1A and B, upper tracings). Shown in supplemental Fig. 1 is an expanded view of baseline tracings for both genotypes. In contrast, an infected wild type mouse on day five post-infection shows a spontaneous, high amplitude, 23 second average duration seizure accompanied by convulsive tonic-clonic behavior that would occur with increasing frequency as the infection progressed (Fig. 1A, middle tracing). EEG recordings from infected C5−/− mice showed occasional spike activity but the C5−/− mice rarely had seizures (Fig. 1B, middle tracing). Higher magnification views of epileptiform discharges for both genotypes during infection are shown in lower panels of Fig. 1A and B. Overall, wild type mice had higher seizure frequency, and higher spike frequency compared to C5−/− mice (Fig 1C-E).
Figure 1.
Representative EEG recordings from wild type (A) and C5−/− (B) mice at baseline and at day 5 post-infection with PbA. Baseline recordings for both mouse genotypes are essentially identical (upper recording in each panel). In contrast, PbA-infected wild type mice had recurring and unprovoked abnormal EEG activity for an extended duration (middle tracing, panel A). C5−/− mice frequently presented with a large number of spikes but markedly fewer seizures (middle tracing, panel B). Higher magnification EEG tracings comparing spikes between infected wild type and C5−/− mice are shown in the lower portions of panels A and B, respectively. Wild type mice had markedly more seizures (C), spikes (D) (p=0.012), and number of spikes/hour (E) (p=0.013) compared to C5−/− mice at the peak of clinical disease (n= 7 for both groups). The data shown are pooled from two separate experiments.
Discussion
In this report we show that deletion of C5, the complement component required for the initiation of MAC formation, significantly reduces the number of epileptiform discharges and seizures in ECM. These results support earlier studies demonstrating that interfering with the complement terminal pathway provides protection against ECM clinical disease manifestations.3-5 Deletion of C5 raises the question of which C5-derived fragment, C5a or C5b, makes the dominant contribution to seizure development in malaria. Inflammation as a seizure trigger is well established.6 C5a is a potent inflammatory mediator that contributes to the production of pro-inflammatory cytokines such as IL-1β and TNF-α, known to decrease seizure threshold. Previous studies have demonstrated that both C5a receptors are not critical for the development of ECM.3,7 Furthermore, deletion of carboxypeptidase N, which inactivates C5a, by removal of its carboxy-terminal arginine residue delays onset of, but does not alter susceptibility to ECM.8 Thus, it seems unlikely that C5a significantly drives seizure development in ECM. C5b, alternatively, is critical for the formation of the MAC and studies have demonstrated that MAC mediates inflammation and evokes seizures in rat brain. In these studies, purified terminal complement pathway components were sequentially injected into either the lateral ventricles or the hippocampus, proximal to the dentate gyrus.9,10 The formation of the MAC resulted in neutrophil and macrophage infiltration and increased expression of ICAM-1 and cytokines, CXCL1 and MCP-1. Furthermore, MAC injections resulted in behavioral and electrographic seizures and neuronal death in the dentate gyrus and CA1 and CA3 regions of the hippocampus, in a dose-dependent fashion. Together these results indicate that the MAC can serve as both a central nervous system inflammatory mediator and inducer of spikes and seizures, suggesting that C5b, and ultimately the MAC, contribute to seizures in ECM.
To our knowledge, this is the first time EEG recording has been performed in this disease model. Our data suggest that ECM may be useful in assessing the efficacy of seizure treatment modalities for human CM. For example, based on our studies in ECM using C5-deficient mice, we believe that inhibition of the complement terminal pathway using clinically available anti-C5 antibodies would have significant therapeutic value.11 Complement-based treatment of patients displaying early symptoms of CM may provide a dual benefit of preventing or reducing the severity of cerebral disease and minimizing the chance of developing long-term neurocognitive sequelae associated with CM.
Supplementary Material
Supplemental Figure 1. An expanded view of representative baseline EEG recordings from wild type and C5−/− mice.
Acknowledgement
This work was supported by NIH grants (T32 AI07051, F31 NS077811), and Eunice Kennedy Shriver NICHD grant (P30-HD38985). The authors thank Dr. Harold Sontheimer for his support during these studies.
Footnotes
Disclosures
The authors have no conflicts of interest. There is no financial relationship to disclose for all the authors. We confirm that we have read the Journal's position on issues involved in ethical publication and affairs and that this report is consistent with those guidelines.
References
- 1.White NJ, Pukrittayakamee S, Hien TT, et al. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 2.Mishra SK, Newton CR. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5:189–198. doi: 10.1038/nrneurol.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos TN, Darley MM, Hu X, et al. Cutting edge: the membrane attack complex of complement is required for the development of murine experimental cerebral malaria. J Immunol. 2011;186:6657–6660. doi: 10.4049/jimmunol.1100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos TN, Darley MM, Weckbach S, et al. The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria. J Biol Chem. 2012;287:24734–24738. doi: 10.1074/jbc.C112.378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SN, Berghout J, Lovegrove FE, et al. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J Exp Med. 2008;205:1133–1143. doi: 10.1084/jem.20072248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Erdman LK, Lu Z, et al. Functional roles for C5a and C5aR but not C5L2 in the pathogenesis of human and experimental cerebral malaria. Infect Immun. 2014;82:371–379. doi: 10.1128/IAI.01246-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darley MM, Ramos TN, Wetsel RA, et al. Deletion of carboxypeptidase N delays onset of experimental cerebral malaria. Parasite Immunol. 2102;34:444–447. doi: 10.1111/j.1365-3024.2012.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong ZQ, Qian W, Suzuki K, et al. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J Neurosci. 2003;23:955–960. doi: 10.1523/JNEUROSCI.23-03-00955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casarsa C, De Luigi A, Pausa M, et al. Intracerebroventricular injection of the terminal complement complex causes inflammatory reaction in the rat brain. Eur J Immunol. 2003;33:1260–1270. doi: 10.1002/eji.200323574. [DOI] [PubMed] [Google Scholar]
- 11.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. An expanded view of representative baseline EEG recordings from wild type and C5−/− mice.