Abstract
Changes in technology, with decreases in the cost of molecular profiling, has expanded the interest in creating institution-wide personalized medicine platforms to allow selective assessment of a given patient’s tumor in real time, with the ability to utilize that information for patient care decisions. In order for this approach to function adequately, a multidisciplinary team must be created in an environment with dedicated support at an institutional level.
Keywords: Personalized medicine, Precision medicine, Next-generation sequencing, Genomics
Introduction
1. Principles of personalized therapy
In the late 1990s, the availability of testing for Her2 overexpression and trastuzumab, a monoclonal antibody targeting Her2, demonstrated the efficacy and elegance of a targeted therapeutic approach to cancer [1]. There are now several other tumor types that are routinely profiled molecularly given the availability of targeted agents specific to these known mutations, such as the use of vemurafenib in BRAF V600E in melanoma [2], crizotinib in non-small cell lung cancers (NSCLC) with EML4-ALK translocations [3], and cetuximab in RAS-wild type colorectal cancers [4].
Changes in available technology, with associated decreases in the cost of molecular profiling, has expanded the interest in creating institution-wide personalized medicine platforms to allow selective assessment of a given patient’s tumor in real time, with the ability to utilize that information for patient care decisions. In order for this approach to function adequately, a multidisciplinary team must be created in an environment with dedicated support from the hospital/university system. The ultimate application(s) need to be considered in order to build pathways for both research and clinical advancements in parallel. Requirements for implementation of a personalized cancer medicine program require adequate tumor tissue available for analysis and a standardized, high-quality laboratory for profiling to assure accuracy and reliability of the results. Once the data has been analyzed, identification of actionable mutations must be identified and paired with available targeted agents [5]. A multidisciplinary clinical team should be created to review the data and to make consensus decisions as to the application of new technologies and new tests in a clinical setting, as well as work together to sort meaningful and actionable findings from those that are incidental. These issues are not inconsequential and the role they play in patient counseling and management are part of a constantly evolving process.
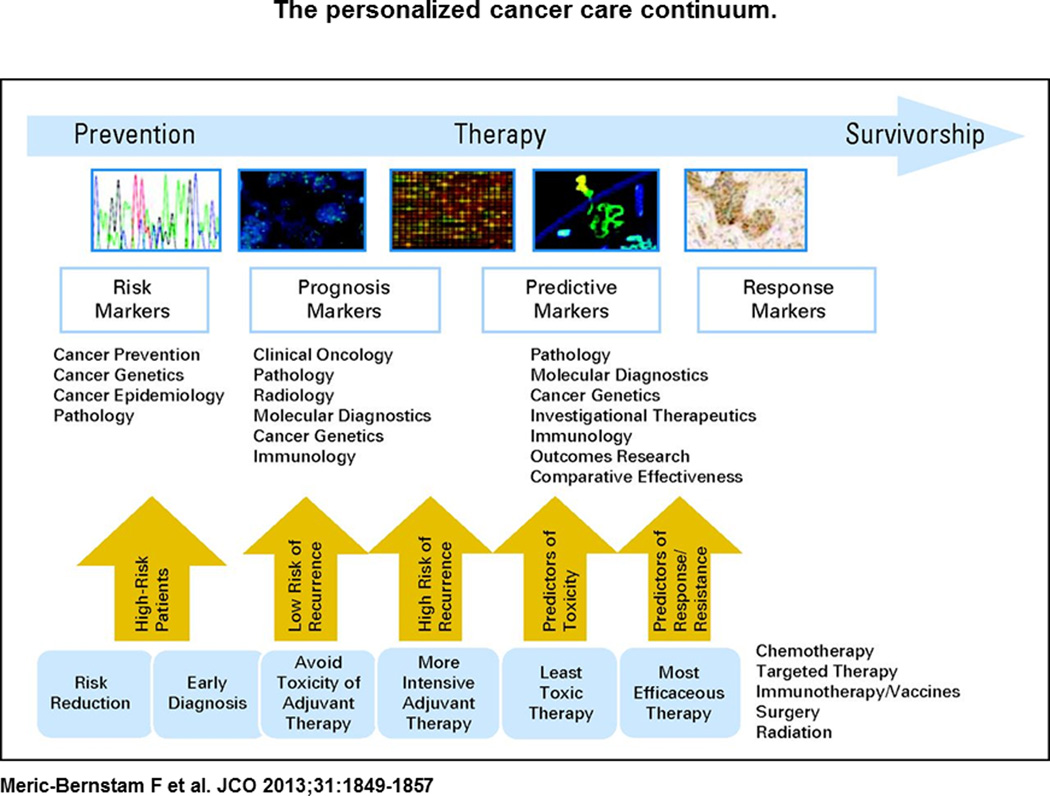
The field of oncology research spans a broad-range of topics including: (1) basic molecular biology of tumorigenesis (2) cancer prevention (3) early cancer detection and monitoring (4) risk stratification (5) locoregional cancer therapies and (6) systemic therapies for late stage disease. An understanding of oncogenesis based upon molecular changes facilitates the integration of these seemingly disparate fields into a unified whole based specifically upon tumor biology (see Figure 1). Given the availability and affordability of high-throughput sequencing modalities, we now have the tools to apply molecular knowledge to clinically relevant scenarios through the analysis of tumor specimens in individual patients in the context of their clinical course and as a function of time.
Figure 1.
The personalized cancer care continuum.
In conjunction with profiling efforts to feed back to patient care decisions, research endeavors carried out in both prospective and retrospective fashion should be undertaken to identify biomarkers that can be used to follow disease progression in real time. Investigative endeavors can be done in the non-Clinical Laboratory Improvements (CLIA) research environment, but may still require core facilities for analysis of tumor and serum specimens. Analysis can include hotspot mutation profiling, targeted gene approaches, whole exome sequencing (WES), whole genome sequencing (WGS), RNA-seq, mRNA and/or miRNA profiling, proteomics, epigenomics and metabolomics. In conjunction with tumor markers, serum-based assays looking at cell-free DNA (cfDNA), miRNA, and/or exosomes may be done is parallel to identify serum-based surveillance approaches that may be scalable across tumor types, particularly in disease where tumors are not amenable to serial biopsy.
2. What Type of Testing Is Available?
DNA, RNA, protein
The revolution in molecular profiling in cancer has been led by breakthroughs in sequencing technologies. We are now capable of analyzing tumors (or even cells) by multiple analytic platforms in parallel, allowing a comparison of genes, RNA and protein expression, and functional metabolism within the same sample allowing a deeper and more integrated evaluation of changes that occur during tumorigenesis. This multiplatform analysis offers the opportunity to identify and validate potential therapeutic targets for further intervention.
Whole genome sequencing (WGS) is an analytic process that evaluates the complete DNA sequence of a patient’s genome at a single time, including chromosomal, non-coding DNA, and mitochondrial DNA. Whole exome sequencing (WES) is an efficient process of selectively sequencing the exomes; exomes are the coding portions of the genome, which are translated into proteins. Therefore, this approach will offer a reflection of changes within the coding regions of genes offering insight into functionally relevant variations or mutations. It may, however, miss changes in regulatory or non-coding regions, which still may have some relevance for RNA/protein expression. Targeted exome sequencing refers to the use of targeted enrichment strategies for exome sequencing that the selective capture regions of interest from a DNA sample prior to sequencing. This can facilitate shorter analysis times and lower the cost of analysis given the restriction in data to be analyzed although it will only enrich for pre-selected exomes of interest. Epigenomics is the study of epigenetic changes made to genetic material, which leads to reversible alterations in DNA or histones that have a functional impact on gene expression. These alterations may occur in a relatively short timeframe and are also potential sites of therapeutic intervention.
Transcriptional profiling evaluates the expression level of RNA within a given sample. Techniques to do this include RNAseq, which allows the characterization of all RNA expression within a sample including non-coding RNA and miRNA if the capture specifics are optimized for this process. Using this technique, one can analyze coding SNPs, evaluate transcript isoforms, identify regulatory RNA, look at splice variants and compare the relative abundance of transcripts within a given sample. Single cell sequencing is now possible although it requires an amplification step for genomic or transcriptomic sequencing. In the case of single cell sequencing the effects of sample degradation, contamination, or the process of amplification itself can have a profound effect on coverage, background noise and possible inaccurate quantification. With stringent protocols and careful interpretation this technique can be used, but it is still limited in terms of widespread applicability for these reasons.
Proteomics is the study of protein structure and function and the proteome represents the entire complement of proteins expressed by a specific tissue or cell at a given time. Metabolomics is recognized to be a significant and relevant field in oncology focused on the study of metabolism and chemical reactions within a cell/tissue. This adds a functional perspective to tumor biology and is another site of potential therapeutic intervention. The use of circulating biomarkers is a field of great interest given the ease of phlebotomy in cancer patients as compared to direct tumor biopsies. Many groups are interested in evaluating the clinical utility of analyzing circulating free DNA (cfDNA), exosomes, or miRNA as potential tools for tumor profiling and for following patients for response and/or recurrence. Given the advances in technology that are capable of generating large volumes of data, analytic tools appropriate for these types of datasets have been created. The use of big data analytics will be a key foundation of any personalized medicine system and must be integrated into the system as it is being built.
DNA
Early approaches to DNA sequencing in cancer focused primarily on germline alterations that accompanied increased cancer risk syndromes. It has become clear that profiling of somatic, acquired mutations is also of great relevance to understanding tumor biology and absolutely critical when the goal is pairing appropriate patients to selected targeted therapies. Sanger sequencing was the original sequencing modality for sequencing the human genome and involves the detection of fluorescently labeled nucleotide sequences. Pyrosequencing is often used for DNA hot-spot sequencing of short-length DNA segments in commonly found exons with known mutations and is better suited for sequencing from formalin-fixed, paraffin embedded (FFPE) tissue than the Sanger technique [6]. Allele-specific polymerase chain reaction (PCR) utilizes primers spanning DNA sites of known interest and is often used for hotspot testing of known mutated genes in cancer. More recently next-generation sequencing tools (NGS) have been developed that array DNA molecules on a solid surface and can determine DNA sequence in situ, allowing millions of reactions to be monitored in parallel and large scale sequencing to occur. The development of NGS platforms has changed the approach to cancer genomics, allowing low-cost high-throughput analysis. However, the infrastructure must adapt to accommodate these changes in technology. Ultimately, an integrated approach to genomic and transcriptomic profiling (at the least) may be needed to identify driver mutations and regulated networks rather than the many associated passenger alterations with no functional relevance to cancer formation. Profiling candidates can be validated preclinically (e.g. cell lines and xenograft models) in which functional assays can be performed and rational drug combinations can be tested.
CLIA/non-CLIA
Molecular characterization in the Clinical Laboratory Improvements (CLIA) environment is a necessary for any test to be used in clinical decision-making (such as treatment selection).. However expanding CLIA-based testing is expensive and time consuming. Therefore a close bridge between hypothesis-generating research and clinically applied testing needs to exist. Dedicated processes need to exist for pre-CLIA testing and analysis. This would be a basic research and development approach to assay development, with validation in the CLIA-certified space once sufficient early data exists to support the necessary institutional commitment towards new tests and assays. Decisions of this sort require a multi-disciplinary committee dedicated to transitioning research findings into new clinically-applicable assays within a reasonable timeframe.
Many institutions have begun utilizing multiplex genomic tests for analysis of hotspot mutations in known oncogenes to identify actionable mutations in a cost-effective and timely manner. These approaches include mass spectrometric genotypic (e.g. Sequenome), SNaPshot (multiplex PCR), multiplex primer extension, and capillary electrophoresis as well as next-generation sequencing techniques (AmpliSeq, IonTorrent) [7]. Many are rapidly working towards targeted exome sequencing and even whole exome sequencing in the CLIA environment. Given the cost associated with CLIA NGS testing, thoughtful patient selection is needed for optimal resource utilization.
Validation steps in the non-CLIA environment
The coupling of basic research and clinical protocols is clearly needed for the ongoing identification of actionable targets and validation of therapies and combinatorial strategies. Given the significant investment in resources for the creation of a personalized medicine infrastructure, the tethering of these efforts to the existing research infrastructure is critical for the longevity and advancement of these projects. Efforts can be tied at various points during these processes. At the time of initial tumor/serum collection, samples can be evaluated in both the CLIA-environment with validated assays, as well as in the non-CLIA research environment where additional studies can be done to identify new therapeutic targets or study the effect of various agents upon these tumors.
Deeper NGS in the non-CLIA environment, as well as multiplatform analysis for parallel genomic, transcriptomic, proteomic, and epigenomic profiling are more cost-effective and readily available in the research environment. Tumor profiling for research purposes can discover potential targets for therapy, validate selected therapies, and evaluate tumoral susceptibility in order to inform the selection of assays being used in the CLIA-environment. In parallel, the establishment of in vitro cell lines and patient-derived xenografts are vital components of a co-clinical research environment in which patient samples can be assayed and evaluated functionally in the research environment. A multidisciplinary panel may assist in determining the most appropriate assays for standard-of-care as well as corollary testing for research intent, funded by grants or philanthropy.
Timeframe for processing and data analysis
Another challenge to the concept of real-time personalized medicine is the time currently required to obtain samples, analyze the tumors and offer meaningful interpretation of sequencing results. There are multiple sites at which this process can be streamlined. Delays in retrieving tissue samples for processing and analysis may be facilitated by coupling the research protocols with current standardized processes of tissue retrieval and analysis. Dedicated staff can be hired to track tissue banking and retrieval and monitor the progress of the processing in real-time. Tools for feeding back information to the involved clinician are also critical for creating an infrastructure that can be used for clinical decision-making [7].
3. Biospecimen Collection and Biospecimen Science
Protocols – static, dynamic?
The foundation for any personalized medicine platform is the acquisition of appropriate tissue for molecular assessment. Currently, circulating assays have not evolved enough to replace a directed tumor biopsy for these studies. The lesion targeted, as well as the timing of the biopsy are critical issues to consider when interpreting the data obtained. Various tumors may be more or less accessible to percutaneous biopsy and tissue collection protocols may need to be coupled with surgical specimen collection systems.
For initial assessment of tumor biopsy, profiling from surgical samples often offers an unparalleled opportunity for tissue acquisition. However, accessible tumors (primary tumors or metastases) may be biopsied serially or at various times during treatment with systemic therapy or while on targeted therapy, issues which must be considered in the data analysis. For hotspot mutational profiling of DNA, these differences may be subtle. With multiplatform analysis, including transcriptomics and proteomics, these changes with therapy or as a function of time and treatment may be very important. In theory, there may be alterations in the genomic signature between primary and metastatic lesions or in lesions during or after therapy. Paired samples from the same patient offer the best approach to making this comparison although there are logistical challenges to a longitudinal biopsy approach.
The ultimate use of the biopsy must also be considered when selecting timing and approach to biopsy. For treatment purposes a metastatic lesion may be the most appropriate sample to use in making decisions regarding targeted therapy, while for research purposes analysis of a primary tumor for studies of basic tumor biology and the process of tumorigenesis may be more informative. Ideally, paired primary tumor and metastases could be compared within the same patient, allowing assessment of tumoral changes as a function of time and treatment.
Tissue preservation and banking
The collection of tumors is only the beginning of the infrastructure that must be created for large volume molecular profiling. Standardized protocols for tissue preservation and banking need to be established prior to tumor collection with knowledge of the back end applications/techniques being utilized for sample analysis. Pre-analytic factors are clearly important for meaningful molecular diagnostic tests to be useful – specimen age, warm ischemia time, cold ischemia time, and characteristics of the fixation solution can all affect the results of any future analysis. Formalin-fixed paraffin embedded (FFPE) tissues are a convenient source of easily archived samples that are frequently used for pathologic preservation of human tissues. While useful for immunohistochemistry (IHC) and some DNA analysis, this technique has limitations for RNA and other proteomic profiling approaches given tissue degradation, protein dephosphorylation and alterations that occur ex vivo or during processing. The ideal approach for many protocols is to have samples fresh frozen in liquid nitrogen for future preservation. The choices of preservation type and analysis will be determined by the ultimate application and planned use of the tissues, but discussion of this topic should occur during the initial creation of any tissue collection protocol in order to be certain that the appropriate preservation technique is being utilized to allow the selected sequencing or analysis to occur on the back end.
Quality Control
Biopsy specimens are variable in size, location, and tissue type and need appropriate pathologic review and quality control prior to any analysis. Inclusion of surrounding non-tumor tissue may contaminate or dilute any profiling results, making meaningful conclusions and application of profiling data difficult. Collaborating pathologists are a necessary and integral component of a personalized medicine infrastructure. One approach is to create a dedicated tissue/DNA quality control team that evaluates each specimen for tissue type, cellularity, selection of tumor (versus stroma) for analysis, and/or general quality control of samples. The critical role of pathologists in this process cannot be over-emphasized.
4. Personalized Therapy Research: Surgical Opportunities
Cancer Detection and Early Detection of Recurrence
In general, surgeons see patients at a relatively early point in their cancer care. While many surgeons are involved in metastectomies, there is a large proportion of surgical oncology that focuses on the primary site of disease. This allows surgeons the unique opportunity to ask relevant clinical and research questions regarding cancer detection at an early stage of disease. Additionally, given the goal of an R0 resection in most cases, this offers a research opportunity to study cancer biomarkers at a time of high versus low volume disease (pre-/post-surgery).
Serum-based assays to identify circulating markers of disease are of great interest to clinicians of all types. There has been interest in the use of cell-free DNA (cfDNA), microRNA (miRNA), serum proteins, circulating tumor cells (CTCs), exosomes, and bone marrow micrometastases in order to identify and stratify cancer at an early stage. Additionally, in the post-resection setting, these same assays can offer insight into the presence of minimal residual disease or suggest disease recurrence prior to radiographic or clinical evidence of disease.
cfDNA
It is believed that the presence of circulating, cell-free DNA in the blood may be related to apoptosis or necrosis of cancer cells. Alternatively, other groups have proposed a more active process of DNA secretion. These fragments of circulating DNA can range from several hundred base pairs to large fragments of 20 kilobases. Additionally circulating tumor cells (CTCs) may also contribute to the load of cfDNA. Given the heterogeneity of clones within tumors as well as the potential for cfDNA shedding from normal cells, studies have been undertaken to examine the correlation between cfDNA and the profile of a known tumor. In multiple disease sites (e.g. bladder, breast cervical, colorectal, hepatocellular carcinoma, lung, lymphoma, melanoma, ovarian, pancreatic, prostate), detection of tumor-specific mutations and methylation changes have been seen in paired blood specimens in which cfDNA has been isolated [8]. Comparison of pre/post-surgery serum samples in breast cancer demonstrates corresponding changes in chromosomal number imbalance (CNI) between cfDNA and primary tumors. Additionally, post-resection there is a loss of cfDNA in the majority of patients [9]. This data suggests a need to investigate the potential role of cfDNA for assessment of minimal residual disease and for monitoring for recurrence.
miRNA
MicroRNA are small, non-coding RNAs of 20–22 nucleotides involved in many critical biologic processes and are known to be important in the formation of many tumor types. It is thought that miRNA can function as both oncogenes and tumor suppressors and the expression patterns of various miRNA can reflect the tumor environment in various tumor types, represent various differentiation states within a tumor, and function as a marker of response to therapy [10]. Given this, circulating miRNA have been examined as potential tumor markers in various cancer types [11].
Exosomes
Exosomes are small vesicles (50–150nm) released by many cell types, but also are found at increasing concentrations in cancer patients. There have been publications in various diseases describing a role for exosomes in modulating the tumor microenvironment as well as eliciting changes in the immune system [12–14]. Recently double-stranded genomic DNA has been isolated from exosomes of pancreatic cancer patients and has been seen to correlate with tumoral genomic DNA [15], suggesting that exosomal isolation and profiling may be another means of tumor detection, profiling, and surveillance.
Circulating tumor cells
Circulating tumor cells (CTCs) have been isolated from patients with various epithelial tumor types and are undergoing study as potential biomarkers of disease. They have been suggested as the origin of metastatic disease and levels of CTCs have been seen to correlate with survival outcomes [16]. In patients with operable disease, higher levels of CTCs appear to correlate with aggressive disease, increased metastases, and decreased time to relapse [17]. Beyond enumeration, CTCs are now being pursued as a circulating source of tumor biomarkers, for IHC, FISH, gene expression profiling and next generation sequencing.
5. Cancer Detection and Early Detection of Recurrence
The surgical oncologist’s role in high risk clinics and management of early as well as locally advanced disease creates unique research opportunities. The potential for early detection of disease or stratification of patients with early disease into lower and higher risk categories is an area of great potential for personalized medicine applications. Profiling of circulating factors and/or analysis of pre-malignant or atypical tissues at high risk for cancer may facilitate allocation of patients to different surveillance schedules (i.e. less vs more frequent based upon risk factors), selection of appropriate patients for chemoprevention, and may influence choice of treatment or timing of surgery in high risk patients. In head and neck cancers, for example, a recent study has demonstrated a correlation between mutant-tumor allele heterogeneity (MATH), which is calculated using a “MATH” score with overall survival outcomes, demonstrating that patients with more intra-tumoral heterogeneity have poorer treatment and survival outcomes [18]. These types of sequencing-based stratification criteria may assist in the allocation of patients to more or less aggressive treatment regimens given the variability in disease-specific risk.
Patients with a genetically-assessed higher risk may be better served in a high-risk clinic with shorter surveillance intervals and more aggressive surveillance strategies. There are known cancer-related syndromes that carry an increased risk for various types of cancer including BRCA in breast cancer, Li Fraumeni syndrome (TP53 mutation), Familial Adenomatous Polyposis (FAP) syndrome in colon cancer (APC mutation), Multiple Endocrine Neoplasia (MEN/RET mutation), and von Hippel Lindau (VHL mutation). Given the known risk of various neoplasms, patients from these families undergo early genetic testing, closer surveillance, and more aggressive surgical management of their disease. In breast cancer, more intensive screening with magnetic resonance imaging, chemoprevention and risk reducing surgery may be offered to women at high risk for breast cancer, as determined by family or personal history of breast cancer and genetic risk stratification. In colon cancer, patients with familial syndromes undergo more aggressive surveillance and are offered more aggressive surgery than patients with sporadic disease.
In colorectal cancers with microsatellite-instability (MSI), there are characteristic clinicopathological features with associated differences in survival, even when present in sporadic tumors. In patients with germline MSI (e.g. Hereditary Nonpolyposis Colon Cancer – HNPCC), they have been found to have longer survival times as compared to microsatellite-stable sporadic tumors. Additionally, there are known and emerging modifier genes that may modulate the risk of cancer development in patients with deleterious germline mutations. The surgical cancer prevention field is optimally suited to incorporate new technologies to refine timing and modalities for cancer screening and to optimize patient selection for risk-reducing surgery.
As molecular tumoral profiling on a large scale through projects such as The Cancer Genome Atlas Project (TCGA) begins to clarify early changes in cancer development, efforts to extrapolate this information to screening and staging efforts may be useful. Currently, histopathological criteria are used for staging purposes in most diseases. In the era of molecular profiling, we may begin to identify very early changes that may allow better risk stratification of early, precancerous lesions. These efforts can be coupled with studies on cancer biomarkers and with approaches to chemoprevention with the goal of early detection and/or prevention.
Local-Regional Treatment
As we utilize more molecular candidates as markers of early or high risk disease, we can begin to apply that information for surgical decision-making. Endocrine surgeons are currently utilizing profiling techniques to examine BRAF, RAS, RET/PTC and PAX8/PPARgamma in order to increase the detection of a follicular neoplasm [19]. Based upon the risk for malignancy as established through profiling of fine-needle aspiration (FNA) specimens, patients are now being allocated to more or less aggressive surgical strategies given their risk of malignancy [20]. In the pancreas, the presence of a cyst often is a situation in which the malignant potential needs to be evaluated and will inform the need for surgical intervention. Molecular analysis of cystic fluid has been used to differentiate neoplasms from non-neoplastic lesions. Currently the presence of K-RAS in cyst fluid places the patient at a higher risk for mucinous cystic neoplasms. Somatic mutations in GNAS also seem to be specific for intraductal papillary mucinous neoplasms (IPMNs) [21]. While these studies are still preliminary, they demonstrate the potential of these types of screening approaches in minimizing surgical morbidity when unnecessary and offering early intervention for patients at the highest risk of malignancy.
Surgical Technique
From a surgical standpoint, the use of novel imaging techniques for intraoperative assessment of tumoral spread would be ideal. There has been much interest in the field of intraoperative imaging, and efforts by many groups are underway to improve intraoperative assessment of cancer spread. Various research groups have studied techniques for intraoperative assessment of tumoral spread/extent, including commonly used approaches such as frozen sections of margins or specimen radiographs in breast cancer [22] and also including novel techniques such as radiofrequency spectroscopy [23,24], optical coherence tomography [23,24], and molecular margin analysis in head and neck cancer [25].
Several practice-changing techniques are now utilized for assessment of high-risk regional nodal basins, such as sentinel lymph node sampling in melanoma and breast cancer. These techniques are applied to patients deemed sufficiently high risk for lymph node metastases and offer a minimally invasive technique for lymph node sampling, minimizing the risk of the morbidity of complete lymphadenectomy unless clinically indicated [26]. The use of minimally invasive techniques such as laparoscopy have also been applied to gastric, pancreatic, and peritoneal carcinomas with the goal of minimizing morbidity in patients who have disseminated disease. In the future the combination of visual assessment, cytology, and pathologic review may be coupled with additional levels of assessment, including molecular profiling to detect markers of high risk cancer or disease dissemination.
Risk of Recurrence
The interest in the use of molecular indicators of patients at greater risk for locoregional recurrence has been best examined in breast cancer using techniques such as the Oncotype DX, which can offer a predictor of breast cancer recurrence for early-stage disease based upon a 21 gene panel [27]. This same technique has been applied to stage II and III colon cancer patients [28] and early-stage prostate cancer to identify patients at higher risk of recurrence and to inform treatment decisions. This level of characterization also allows the potential targeting of pathways associated with an increase in locoregional recurrence within the identified high-risk subset of patients. Currently, clinicopathological criteria are utilized in various diseases to inform decisions regarding neoadjuvant or adjuvant therapies, such as chemotherapy and/or radiation. Looking forward, molecular profiling data may also be included to allow personalization of the extent of surgery needed or the need for adjuvant chemotherapy or radiation therapy.
The surgical approach to patients with isolated locoregional disease includes techniques such as regional perfusion strategies. Historically, melphalan has been the cytotoxic drug of choice although combinations using tumor necrosis factor and/or interferon have been investigated. In the era of selectively targeted therapies and immune modulators such as ipilimumab (anti-CTLA4), the rational combination of immunotherapies with regional approaches to cytotoxic therapy may be of great utility to patients. In patients with localized hepatic disease, hepatic arterial infusion of chemotherapy or radioactive beads can be utilized for regional targeting of agents to the specific site of disease. And hyperthermic, intraperitoneal chemotherapy (HIPEC) also offers opportunities for more specific targeting of perfusion to the area of disease. All of these techniques offer opportunities for the application of novel targeted therapies or new technologies for more specific targeting to the disease of interest while minimizing associated morbidity.
Neoadjuvant therapy as a Discovery Platform
The use of multimodality care is a common component of oncology, and discussions regarding the timing and sequencing of treatments should arise from a multidisciplinary team perspective. Neoadjuvant versus adjuvant timing of systemic therapy is one commonly discussed theme. There are practical benefits to neoadjuvant therapy such as tumor downstaging and influences on potential resectability of the tumor by up-front systemic therapy. In addition, the neoadjuvant approach allows assessment of tumoral response to systemic or regional therapies, offering an in vivo assessment of treatment efficacy. The observation of a given treatment within a specific patient offers an opportunity for optimal treatment selection, as well as an opportunity to test therapeutic regimens. Post-operative therapies can be selected or modified based upon pre-surgical patient responses. As previously described, the timing of preoperative therapy followed by surgery also allows for identification of biomarkers of responsiveness or non-responsiveness to therapies, as well as offering a testing ground for the pharmacodynamics of the response within an individual patient. After surgery, the tumor profiling may allow for further stratification into various therapeutic regimens based upon the residual burden of disease, molecular characteristics, and clinical behavior while on therapy.
Window of Opportunity Trials
As surgeons, we also have the opportunity to combine investigative research studies with routine clinical care. Window of opportunity trials allow the evaluation of short-term (1–4 week) treatment with new or established therapeutics prior to the surgical treatment. As routine surgical planning and scheduling often has 1–4 weeks of delays to surgery, this novel research approach capitalizes on this “window of opportunity”. The endpoints are generally histologic or molecular in nature, such as changes in cellular proliferation, cell death/apoptosis, or modulation of specific target signaling pathways. Evaluation of pre- and post-treatment tumor samples will also inform studies of adaptive tumoral responses to various therapies. The short window functions to offset any delay in therapy that might alter surgical resectability, but still allows in vivo functional assessment of a given therapy within a specific patient [29].
Clinical Decision-making
Ultimately, combining molecular profiling and basic research endeavors with day-to-day clinical care will allow the application of personalized therapy programs to develop. As mentioned, the volume of data and complexity of target choice will require decision support programs to assist practicing clinicians in selecting the most relevant data to inform their clinical decision-making.
An issue faced by many practicing clinicians is for whom to offer molecular profiling and in what clinical context is this most beneficial and/or cost-effective? An important focus of this discussion should be the overall utility of this information as it pertains to direct patient care decisions (i.e. how will this information help the patient?). At this time, it is unlikely that early stage cancer patients who are curable with current therapies will benefit from genomic characterization. However, there may be utility in patients at a high risk for recurrence, and clearly there may be benefit to patients with metastatic or inoperable tumors where investigational cancer therapeutics are being considered. Currently, candidate gene testing is standard of care for only a handful of cancer types, and mainly for systemic therapy selection: for patients with advanced metastatic colon cancer (RAS), advanced lung cancer (EGFR, ALK), advanced melanoma (RAF), and invasive breast cancer (Her2). In all of these scenarios testing is performed for systemic therapy selection. Further research is needed to determine the utility of molecular characterization for local-regional therapy selection and determination of optimal timing of local-regional treatment and systemic treatment.
6. Complementary Research
Pairing basic research endeavors with clinical studies will allow the translation of data bi-directionally, allowing clinical observations to inform basic research and hopefully allowing basic research findings to be applied in the patient-care setting. Genetically modified mouse models (GEMMs) have historically been utilized as a model of disease in which novel hypothesis may be tested. The use of these GEMMs in parallel with patient care is often referred to as “co-clinical trials.” However, many of these preclinical models do not accurately predict the clinical responsiveness of various agents in actual cancer patients. Therefore, patient-derived xenografts have been utilized as an alternative method of basic science hypothesis-testing prior to clinical applications. Since actual patient tumors are engrafted into immune-compromised mice, these models allow testing of an actual patient’s tumor in real time. The limitations are clearly the absence of a host immune response. However, these models can be invaluable when testing new therapies and assessing individual patient responses to new agents [30].
7. Multidisciplinary Approach
The Strength of Surgeons
Creating a functional personalized medicine program ultimately requires the work of many teams and various skill sets to optimally leverage “big data”, large datasets of clinically annotated molecular data, for discovery and implementation of personalized therapy.
This infrastructure will be reliant upon pathologists, medical oncologists, data analysts and informaticians, to name just a few. Surgeons play a critical role in the system, functioning as a hub of connectivity between these various groups. Historically, surgeons and pathologists have worked closely together given the analysis of surgical specimens and the importance of pathologic technique and analysis in the surgical setting. Additionally, given the window in which most surgical oncologists interface with patients, we have the opportunity to assess changes in tumors prior to various therapies, as well as after neoadjuvant therapies and have the opportunity to combine surgical resection or biopsy with clinical and research studies.
This is a very exciting time in oncology. To capitalize on the rapid advances in science and technology, training in molecular oncology, principles of biomarker analysis and experimental therapeutics are essential for future cancer surgeons. We need to be prepared not only to be team-players but also leaders in personalized cancer care.
Acknowledgements
This research was supported in part by the National Cancer Institute T32 CA009599 (GMB, FMB), the Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy (FMB), the Cancer Center Support Grant, CCSG P30 CA016672 (FMB), the National Center for Research Resources Grants 3UL1RR024148 (FMB).
Footnotes
Disclosures: No relevant conflicts of interest.
References
- 1.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou AM, Ringborg U, Schilsky RL. Strategies to overcome clinical, regulatory, and financial challenges in the implementation of personalized medicine. Am Soc Clin Oncol Educ Book. 2013;33:118–125. doi: 10.14694/EdBook_AM.2013.33.118. [DOI] [PubMed] [Google Scholar]
- 6.Cronin M, Ross JS. Comprehensive next-generation cancer genome sequencing in the era of targeted therapy and personalized oncology. Biomark Med. 2011;5:293–305. doi: 10.2217/bmm.11.37. [DOI] [PubMed] [Google Scholar]
- 7.Meric-Bernstam F, Farhangfar C, Mendelsohn J, Mills GB. Building a personalized medicine infrastructure at a major cancer center. J Clin Oncol. 2013;31:1849–1857. doi: 10.1200/JCO.2012.45.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 9.Umovitz HBBJ, Schutz E, Singh G, Mitchell WM, Black DM, Babiera G, Bedrosian I, Kuerer HM, Mills GB, Meric-Bernstam F. Modulation of breast cancer cell-free DNA with surgical resection. J Clin Oncol. 2013;31 Abstr 11060. [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borges FT, Melo SA, Ozdemir BC, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaeffer D, Reis FP, Johnson SJ, et al. The CR3 motif of Rrp44p is important for interaction with the core exosome and exosome function. Nucleic Acids Res. 2012;40:9298–9307. doi: 10.1093/nar/gks693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahlert C, Melo SA, Protopopov A, et al. Identification of Double Stranded Genomic DNA Spanning all Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J Biol Chem. 2014 doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 17.Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–695. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 18.Mroz EA, Tward AD, Pickering CR, et al. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer. 2013;119:3034–3042. doi: 10.1002/cncr.28150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19:1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14:1458–1471. doi: 10.1245/s10434-006-9236-0. [DOI] [PubMed] [Google Scholar]
- 23.Haka AS, Volynskaya Z, Gardecki JA, et al. In vivo margin assessment during partial mastectomy breast surgery using raman spectroscopy. Cancer Res. 2006;66:3317–3322. doi: 10.1158/0008-5472.CAN-05-2815. [DOI] [PubMed] [Google Scholar]
- 24.Karni T, Pappo I, Sandbank J, et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007;194:467–473. doi: 10.1016/j.amjsurg.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg D, Harden S, Masayesva BG, et al. Intraoperative molecular margin analysis in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:39–44. doi: 10.1001/archotol.130.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Boland GM, Gershenwald JE. Sentinel lymph node biopsy in melanoma. Cancer J. 2012;18:185–191. doi: 10.1097/PPO.0b013e31825046c7. [DOI] [PubMed] [Google Scholar]
- 27.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webber EM, Lin JS, Evelyn PW. Oncotype DX tumor gene expression profiling in stage II colon cancer. Application: prognostic, risk prediction. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glimelius B, Lahn M. Window-of-opportunity trials to evaluate clinical activity of new molecular entities in oncology. Ann Oncol. 2011;22:1717–1725. doi: 10.1093/annonc/mdq622. [DOI] [PubMed] [Google Scholar]
- 30.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]