Abstract
Objective
To estimate the absolute risks of adverse maternal and perinatal outcomes based on small differences in prepregnancy body mass (eg, 10% of body mass or 10-20 lbs).
Methods
This population-based cohort study (n=226,958) was drawn from all singleton pregnancies in British Columbia (Canada) from 2004-2012. The relationships between prepregnancy BMI (as a continuous, non-linear variable) and adverse pregnancy outcomes were examined using logistic regression models. Analyses were adjusted for maternal age, height, parity, and smoking in pregnancy. Adjusted absolute risks of each outcome are reported according to incremental differences in prepregnancy BMI and weight in pounds.
Results
A 10% difference in prepregnancy BMI was associated with at least a 10% lower risk of preeclampsia, gestational diabetes, indicated preterm delivery, macrosomia, and stillbirth. In contrast, larger differences in prepregnancy BMI (20-30% differences in BMI) were necessary to meaningfully reduce risks of cesarean delivery, shoulder dystocia, NICU stay ≥48 hours, and in-hospital newborn mortality. Prepregnancy BMI was not associated with risk of postpartum hemorrhage requiring intervention, severe maternal morbidity or maternal mortality, or spontaneous preterm delivery before 32 weeks of gestation.
Conclusion
These results can inform prepregnancy weight loss counseling by defining achievable weight loss goals for patients that may reduce their risk of poor perinatal outcomes.
Introduction
Despite the 2013 American College of Obstetricians and Gynecologists’ recommendation to provide preconception counseling for overweight (body mass index [BMI] 25 to <30) and obese (BMI ≥30) women,(1) there is insufficient data to inform such counseling. Previous studies have found that overweight and obese women and their fetuses are at increased risk of a number of important adverse outcomes compared to women at normal weights (BMI 18.5 to 25).(2-25) Few women, however, lose enough weight to shift entire BMI categories. In the non-pregnant obese population, a 10% reduction in body weight is recommended by the National Institutes of Health as an initial weight loss target to confer health benefits.(26) With regard to perinatal outcomes, the benefits of achievable magnitudes of weight loss have not been well-examined.
The ideal data to inform this counseling would come from randomized trials of preconceptional weight loss interventions. Such studies are difficult to conduct and would likely not have sufficient power to examine important rare outcomes, such as stillbirth.(27) Thus, population-based studies that compare the pregnancy outcomes of different women based on their prepregnancy BMIs are critical to provide estimates of the benefits of achievable weight loss prior to conception.
We conducted the current study to estimate the absolute risk of adverse maternal and newborn outcomes according to incremental differences in prepregnancy BMI. Our goal was to produce results that would be useful to clinicians providing preconception counseling about the reductions in risk associated with small, achievable amounts of weight loss.
Materials and Methods
In British Columbia, information from the antenatal, labor and delivery, and newborn records for all births >500 grams or ≥20 weeks of gestation is abstracted from the medical record into the British Columbia Perinatal Data Registry, a quality-controlled provincial database administered by Perinatal Services BC. Chart abstraction is standardized, and data quality is maintained by checks in the data entry software program, year-end checks and reports, and ongoing hospital and provincial-level quality checks . Abstraction is performed by health information management professionals, who complete a 2-year training program and pass a national certification examination. Validation studies have established the accuracy and completeness of this database.(28) The base population for this study was drawn from all singleton pregnancies in British Columbia from April 1, 2004 to March 31, 2012. Pregnancies complicated by preexisting diabetes or hypertension were excluded. Prepregnancy weight and height are based on maternal self-report or provider assessment, typically documented at the first antenatal visit. The study population was restricted to pregnancy records with available prepregnancy maternal weight and height. Ethics approval was obtained from the University of British Columbia/Children's and Women's Health Centre of British Columbia Research Ethics Board (#H13-01707).
The following adverse maternal and perinatal outcomes were examined: 1) preeclampsia, 2) gestational diabetes mellitus, 3) spontaneous preterm delivery before 32 weeks (spontaneous onset of labour or premature rupture of membranes (PROM), restricted to live births), 4) indicated preterm delivery <37 weeks (induction of labor or pre-labor cesarean delivery in the absence of PROM, restricted to live births), 5) macrosomia (birth weight ≥4500 grams), 6) shoulder dystocia, 7) birth injury secondary to shoulder dystocia (Erb's paralysis, Klumpke's paralysis, brachial plexus birth injuries, or fracture of the humerus or clavicle), 8) cesarean delivery, 9) postpartum hemorrhage requiring intervention to control bleeding (hysterectomy, blood transfusion, embolization/ligation of vessels, aspiration/dilation and curettage, uterine/vaginal packing, or suturing of uterus),10) anesthesia complications (aspiration pneumonitis or other pulmonary complications, cardiac arrest or failure, cerebral anoxia, or failed or difficult intubation during labor and delivery), 11) maternal mortality/severe morbidity (any of the following: venothromboembolic events, placental abruption, antepartum hemorrhage, puerperal sepsis, obstetric embolism (including amniotic fluid embolism and septic embolism), cardiomyopathy, renal failure, obstetric death, cardiac arrest, cerebrovascular hemorrhage or infarction, adult respiratory distress syndrome, or repair of bladder, urethra, or intestine), 12) stillbirth at ≥20 weeks, 13) neonatal intensive care unit (NICU) stay ≥48 hours, and 14) in-hospital newborn mortality. Outcomes were defined using either International Statistical Classification of Diseases and Related Health Problems 10th edition codes, Canadian Classification of Health Interventions codes, or British Columbia Perinatal Data Registry codes. A complete list of outcome codes used is presented in Appendix 1, available online at http://links.lww.com/xxx. Although congenital anomalies may be an outcome of interest, our database only includes anomalies detected at ≥20 weeks. Because this analysis would be vulnerable to selection bias, anomalies are only presented descriptively in Table 1.
Table 1.
Maternal and neonatal clinical characteristics according to prepregnancy BMI (N=226,958)
| Characteristic | All | <18.5 | 18.5-<25 | 25-<30 | 30-<35 | 35-<40 | ≥40 |
|---|---|---|---|---|---|---|---|
| 226,958 (100.0) | 8,854 (3.9) | 144,502 (63.7) | 46,317 (20.4) | 17,210 (7.6) | 6,695 (2.9) | 3,380 (1.5) | |
| Maternal age | 30.15.5 | 28.35.6 | 30.25.5 | 30.25.5 | 30.05.4 | 29.85.2 | 30.05.1 |
| Maternal height (inches) | 64.82.8 | 64.82.8 | 64.72.8 | 64.72.8 | 64.82.8 | 64.82.9 | 64.73.0 |
| Nulliparous | 111,463 (49.1) | 5,200 (58.7) | 74,427 (51.5) | 20,695 (44.7) | 7,160 (41.6) | 2,662 (39.8) | 1,319 (39.0) |
| Smoking in pregnancy | 20,775 (9.2) | 1,091 (12.3) | 11,425 (7.9) | 4,631 (10.0) | 2,207 (12.8) | 917 (13.7) | 504 (14.9) |
| Gestational weight gain (lbs)* | 32.913.1 | 34.912.3 | 34.211.8 | 32.513.8 | 28.115.0 | 23.716.2 | 19.317.2 |
| Preterm delivery (<37 weeks)** | 16,931 (7.5) | 816 (9.2) | 10,283 (7.1) | 3,458 (7.5) | 1,439 (8.4) | 586 (8.8) | 349 (10.3) |
| Birth weight† | 3427550 | 3187524 | 3391527 | 3505565 | 3548595 | 3572611 | 3619623 |
| Congenital anomalies at ≥20 weeks gestation | 10,782 (4.8) | 462 (5.2) | 6,841 (4.7) | 2,103 (4.5) | 834 (4.9) | 370 (5.5) | 172 (5.1) |
Data are mean SD or n (%).
37,645 (16.6) missing values
186 (0.1) missing values
56 (0.0) missing values
We examined the relationship between prepregnancy BMI and all outcomes in a crude analysis by BMI category according to the following categories: underweight (BMI<18.5), normal weight (BMI 18.5 to 25), overweight (BMI 25 to <30), class 1 obese (BMI 30 to <35), class 2 obese (BMI 35 to <40), and class 3 obese (BMI≥40). For each outcome, we performed a likelihood ratio test comparing a null logistic regression model to a model with only BMI. We limited additional analyses to outcomes for which there were at least 500 cases in order to maintain stable estimates along the prepregnancy BMI continuum. For those outcomes with ≥500 cases, we performed logistic regression analyses modeling prepregnancy BMI using restricted cubic splines. This flexible modeling method allows smooth, curvilinear shapes, and ensures the best possible transformation at each specific BMI value.(29) We used the default of five knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles.
Multivariable regression models were adjusted for maternal age, parity, smoking, and maternal height. To avoid adjusting away part of the association between increasing prepregnancy BMI and adverse pregnancy outcomes that is mediated by congenital anomalies, we did not adjust for congenital anomalies. Models for preeclampsia and cesarean delivery were restricted to nulliparas because the risk factors for these outcomes may differ by parity. Assumptions of linearity were assessed for continuous variables. After performing each logistic regression analysis, the predicted odds at each BMI value were estimated.(30) We then applied the expit function to obtain the predicted risks. Adjusted risks were presented at the population average values of all confounders, so that these results represent the average risk of each outcome at the population level. All analyses were performed using STATA 12.0.(31)
For those outcomes with a significant association with prepregnancy BMI (using α=0.01 due to the large sample size), we also presented the adjusted absolute risk (and 95% confidence interval) for each value of prepregnancy BMI from 17 to 50. To demonstrate how these results may inform prepregnancy counseling, we produced a table in which we transformed prepregnancy BMI to prepregnancy weight in pounds (ranging from 130 to 300 lbs.) further adjusted for height squared, and present the population-level absolute risks at the population average height (5’4.7”). Thisallows weight loss counseling for women of average height to refer to differences in prepregnancy weight, rather than differences in prepregnancy BMI, which may be more understandable to women.
Assuming that weight loss would reduce the risk of these adverse outcomes, the absolute risks presented in our tables can be used to estimate the number of women who would need to be at a specified lower target BMI value compared to a specified higher initial BMI value to prevent one adverse event. This parameter, which is the same as that used to determine the “number needed to treat”, is calculated as .
Results
Of the 334,861 pregnancies in the study base, 105,474 pregnancies (31.5%) with missing height or weight data were excluded. Pregnancies complicated by preexisting diabetes or hypertension [2,429 (0.7%)] were also excluded. Pregnancies with missing weight or height were similar to pregnancies with available data for macrosomia, cesarean delivery, maternal mortality/severe morbidity, and NICU stay ≥48 hours. However, pregnancies with missing height or weight data were less likely to be nulliparous, have gestational diabetes, or induced labor. Smoking in pregnancy, spontaneous preterm delivery <32 weeks, postpartum hemorrhage requiring intervention, maternal mortality or severe morbidity, stillbirth, and in-hospital newborn mortality were significantly more common among those with missing compared with available BMI data (Appendix 2, available online at http://links.lww.com/xxx).
After these exclusions, the final study population included 226,958 pregnancies. The majority of the pregnancies (63.7%) were among normal-weight women, 20.4% among overweight women, and 12.0% among obese women. Characteristics of the study population are presented by BMI category in Table 1. Maternal age, height, and congenital anomalies were similar across prepregnancy BMI categories. Smoking during pregnancy was least common among normal-weight women, and more common among underweight, overweight, and obese women.
In the crude analysis, increasing BMI category was associated with an increased proportion of pregnancies complicated by preeclampsia, gestational diabetes, indicated preterm delivery <37 weeks, shoulder dystocia, and cesarean delivery, with p-values <0.01(Table 2). Additionally, all fetal or newborn outcomes, including macrosomia, stillbirth, NICU stay ≥48 hours, and in-hospital newborn mortality, increased with increasing BMI category. Interestingly, spontaneous preterm delivery <32 weeks, postpartum hemorrhage requiring intervention, and maternal mortality/severe morbidity were not associated with increasing BMI category. Birth injuries secondary to shoulder dystocia increased with increasing BMI category, but were too rare to be examined using logistic regression, and are thus presented only in this crude analysis. With only 58 cases, anesthesia complications were too rare to see any association with prepregnancy BMI in this crude analysis or logistic regression models.
Table 2.
Absolute risks (%) of adverse maternal and perinatal outcomes according to prepregnancy BMI category (N=226,958)
| Outcome | All n=226,958 | <18.5 n=8,854 | 18.5-<25 n=144,502 | 25-<30 (n=46,317) | 30-<35 n=17,210 | 35-<40 n=6,695 | ≥40 n=3,380 | p-value* |
|---|---|---|---|---|---|---|---|---|
| Preeclampsia | 11,245 (5.0) | 211 (2.4) | 4,944 (3.4) | 2,952 (6.4) | 1,728 (10.0) | 858 (12.8) | 552 (16.3) | <0.001 |
| Gestational diabetes | 17,944 (7.9) | 479 (5.4) | 8,807 (6.1) | 4,496 (9.7) | 2,350 (13.7) | 1,110 (16.6) | 702 (20.8) | <0.001 |
| Spontaneous birth <32 weeks | 1,315 (0.6) | 73 (0.8) | 790 (0.6) | 287 (0.6) | 108 (0.6) | 4 (0.6) | 17 (0.5) | 0.025 |
| Indicated birth <37 weeks | 4,192 (1.9) | 183 (2.1) | 2,297 (1.6) | 924 (2.0) | 425 (2.5) | 225 (3.4) | 138 (4.1) | <0.001 |
| Macrosomia | 4,404 (1.9) | 39 (0.4) | 1,927 (1.4) | 1,280 (2.8) | 648 (3.8) | 304 (4.5) | 206 (6.1) | <0.001 |
| Shoulder dystocia | 8,231 (3.6) | 217 (2.5) | 5,008 (3.5) | 1,876 (4.1) | 698 (4.1) | 294 (4.4) | 138 (4.1) | <0.001 |
| Birth injury secondary to shoulder dystocia | 187 (0.1) | 3 (0.0) | 82 (0.1) | 60 (0.1) | 21 (0.1) | 11 (0.2) | 10 (0.3) | <0.001 |
| Cesarean delivery | 66,699 (29.4) | 1,927 (21.8) | 38,292 (26.5) | 15,337 (33.1) | 6,577 (38.2) | 2,887 (43.1) | 1,679(49.7) | <0.001 |
| Postpartum hemorrhage requiring intervention | 1,632 (0.7) | 76 (0.9) | 1,055 (0.7) | 310 (0.7) | 138 (0.8) | 44 (0.7) | 9 (0.3) | 0.010 |
| Anesthesia complications | 58 (0.0) | 4 (0.1) | 34 (0.0) | 11 (0.0) | 4 (0.0) | 5 (0.1) | 0 (0.1) | 0.864 |
| Maternal mortality/severe morbidity | 1,358 (0.6) | 67 (0.8) | 861 (0.6) | 280 (0.6) | 99 (0.6) | 31 (0.5) | 20 (0.6) | 0.526 |
| Stillbirth | 678 (0.3) | 23 (0.3) | 377 (0.3) | 154 (0.3) | 75 (0.4) | 28 (0.4) | 21 (0.6) | <0.001 |
| NICU stay ≥48 hours | 9,313 (4.1) | 463 (5.2) | 5,570 (3.9) | 1,959 (4.2) | 812 (4.7) | 327 (4.9) | 214 (6.0) | <0.001 |
| In-hospital newborn mortality | 933 (0.4) | 40 (0.5) | 524 (0.4) | 208 (0.5) | 95 (0.6) | 38 (0.6) | 28 (0.8) | <0.001 |
Data are n (%).
A likelihood ratio test was conducted to compare a null model to a model with the linear and cubic spline terms for BMI. Due to the large sample size, the threshold of significance was set at α=0.01
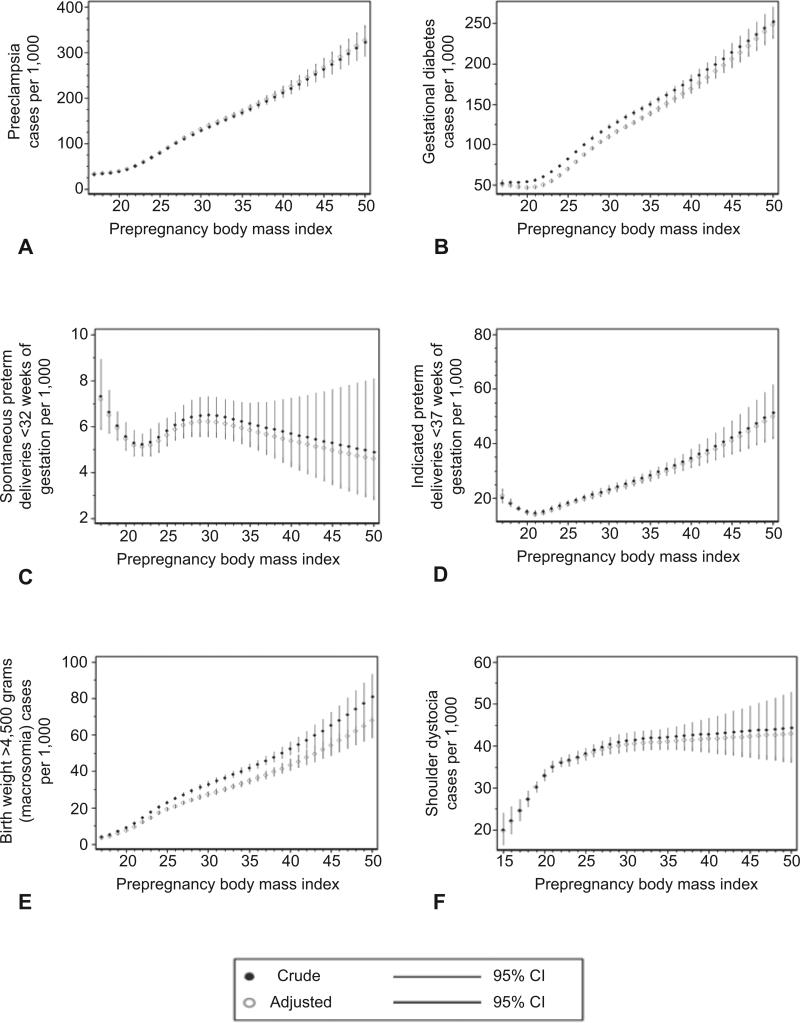
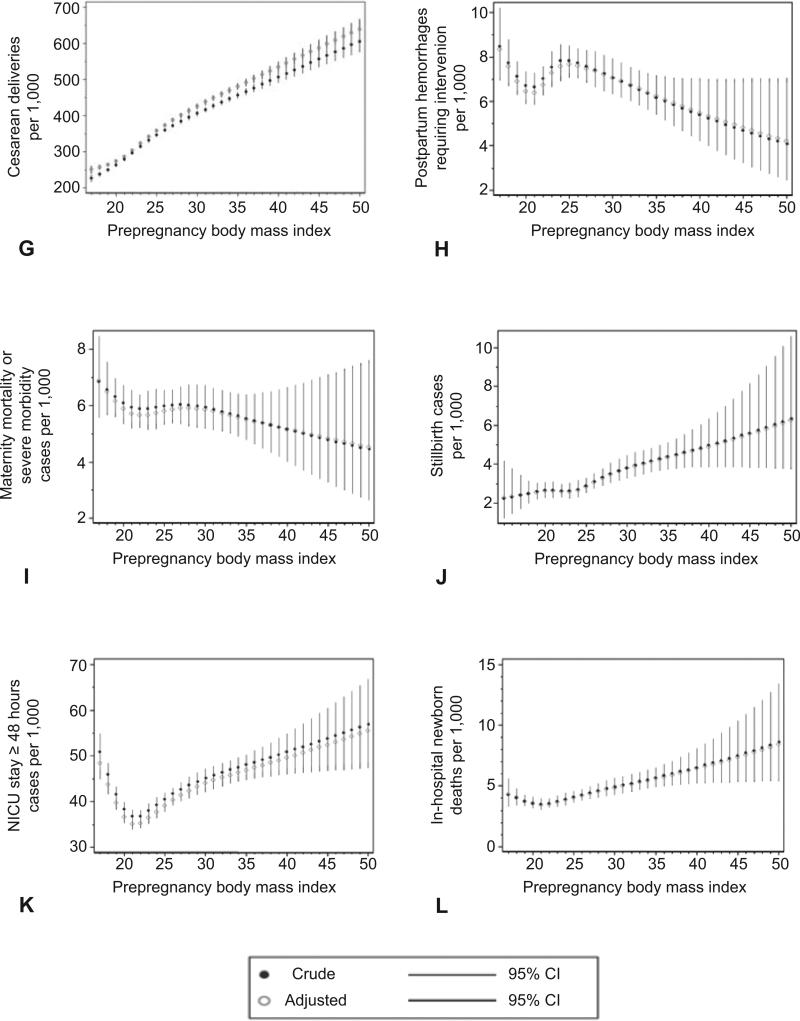
Figure 1 illustrates the estimated relationships between prepregnancy BMI and each adverse outcome by showing the crude and adjusted predicted risk at each BMI value. The risk of preeclampsia, gestational diabetes, cesarean delivery, macrosomia, and stillbirth showed an almost linear association with increasing BMI. In contrast, indicated preterm delivery <37 weeks and NICU stay ≥48 hours appeared to be more common among underweight women, reached a nadir at approximately a BMI of 20, and then increased consistently with BMI. Inhospital newborn mortality increased with increasing BMI, although the magnitude of the increase for this rare outcome was small, and the confidence intervals were wide. Finally, shoulder dystocia increased sharply until a BMI of 25, and then increased slowly until a BMI of 30, at which point it plateaued. Interestingly, for most of these outcomes, the risk increased with increasing BMI even among normal-weight and overweight women. No significant relationships were observed between prepregnancy BMI and spontaneous preterm delivery <32 weeks, postpartum hemorrhage requiring intervention, or maternal mortality or severe morbidity. The similarities between crude and adjusted estimates indicate that adjustment for confounders had little effect on the estimated risks.
Figure 1.
Crude and adjusted predicted risk per 1,000 of preeclampsia among nulliparas (A), gestational diabetes mellitus (B), spontaneous preterm delivery at less than 32 weeks of gestation (C), indicated preterm delivery at less than 37 weeks of gestation (D), macrosomia, birth weight greater than 4,500 grams (E), shoulder dystocia (F), cesarean delivery among nulliparas (G), postpartum hemorrhage requiring intervention to control bleeding (H), maternal mortality or severe morbidity (I), stillbirth (J), neonatal intensive care unit (NICU) stay of 48 hours or longer (K), in-hospital newborn mortality according to prepregnancy body mass index (L), with 95% confidence intervals (CI).
Table 3 quantifies the adjusted risk of each outcome according to prepregnancy BMI (with 95% confidence intervals) calculated at the population average values of all confounders. Similarly, Table 4 quantifies the risk of each outcome according to prepregnancy weight in pounds at the population average height (5 ft. 4.7 in.). These tables can inform prepregnancy weight loss counseling by comparing risks of each outcome among women with different prepregnancy body masses. For example, 21.4% of women with a prepregnancy BMI of 40 developed preeclampsia, 16.9% developed gestational diabetes, 53.5% had a cesarean delivery, 4.3% delivered a macrosomic baby, and 0.5% delivered a stillborn baby. These risks can be compared to risks among women with a BMI of 36, which represents a 10% reduction in body mass (for women of the same height). Among women with a prepregnancy BMI of 36, 18.0% developed preeclampsia, 14.5% developed gestational diabetes, 49.2% had a cesarean delivery, 3.6% had a macrosomic baby, and 0.4% delivered a stillborn baby. Similar comparisons can be made between any baseline BMI (using Table 3) or weight (using Table 4) and any target BMI or weight for each outcome examined.
Table 3.
Adjusted predicted absolute risks (%) of adverse maternal and perinatal outcomes according to maternal prepregnancy body mass index, with 95% confidence intervals (N=226,958)
| BMI | Preeclampsia | Gestational diabetes | Indicated birth <37 weeks | Macrosomia* | Shoulder dystocia | Cesarean delivery | Stillbirth | NICU stay ≥48 hours | In-hospital newborn mortality |
|---|---|---|---|---|---|---|---|---|---|
| 17 | 3.2 (2.8-3.6) | 5.1 (4.7-5.5) | 2.1 (1.8-2.3) | 0.3 (0.3-0.4) | 2.4 (2.2-2.7) | 25.0 (24.0-26.1) | 0.2 (0.2-0.3) | 4.8 (4.5-5.2) | 0.4 (0.3-0.6) |
| 18 | 3.4 (3.1-3.7) | 4.9 (4.7-5.1) | 1.8 (1.7-2.0) | 0.4 (0.4-0.5) | 2.7 (2.5-2.9) | 25.7 (25.0-26.4) | 0.2 (0.2-0.3) | 4.4 (4.2-4.6) | 0.4 (0.3-0.5) |
| 19 | 3.6 (3.4-3.8) | 4.7 (4.6-4.9) | 1.6 (1.5-1.7) | 0.6 (0.5-0.6) | 3.0 (2.9-3.2) | 26.4 (25.9-26.9) | 0.3 (0.2-0.3) | 4.0 (3.8-4.1) | 0.4 (0.3-0.4) |
| 20 | 3.9 (3.7-4.1) | 4.6 (4.5-4.8) | 1.5 (1.4-1.6) | 0.7 (0.7-0.8) | 3.3 (3.2-3.4) | 27.3 (26.9-27.8) | 0.3 (0.2-0.3) | 3.7 (3.5-3.8) | 0.4 (0.3-0.4) |
| 21 | 4.3 (4.1-4.5) | 4.7 (4.5-4.8) | 1.4 (1.3-1.5) | 0.9 (0.9-1.0) | 3.5 (3.4-3.7) | 28.6 (28.1-29.1) | 0.3 (0.2-0.3) | 3.5 (3.4-3.7) | 0.3 (0.3-0.4) |
| 22 | 5.0 (4.8-5.2) | 5.0 (4.8-5.1) | 1.5 (1.4-1.5) | 1.2 (1.1-1.3) | 3.6 (3.5-3.7) | 30.4 (30.0-30.8) | 0.3 (0.2-0.3) | 3.5 (3.4-3.6) | 0.4 (0.3-0.4) |
| 23 | 5.9 (5.7-6.1) | 5.4 (5.3-5.6) | 1.6 (1.5-1.7) | 1.5 (1.4-1.5) | 3.7 (3.5-3.8) | 32.3 (31.8-32.9) | 0.3 (0.2-0.3) | 3.6 (3.5-3.8) | 0.4 (0.3-0.4) |
| 24 | 6.9 (6.6-7.2) | 6.1 (5.9-6.3) | 1.7 (1.6-1.8) | 1.7 (1.6-1.8) | 3.7 (3.6-3.9) | 34.2 (33.6-34.7) | 0.3 (0.2-0.3) | 3.8 (3.6-3.9) | 0.4 (0.4-0.5) |
| 25 | 8.0 (7.7-8.3) | 6.9 (6.7-7.1) | 1.8 (1.7-1.9) | 1.9 (1.8-2.0) | 3.8 (3.6-3.9) | 35.8 (35.3-36.3) | 0.3 (0.3-0.3) | 3.9 (3.8-4.0) | 0.4 (0.4-0.5) |
| 26 | 9.1 (8.8-9.4) | 7.8 (7.6-8.0) | 1.9 (1.8-2.0) | 2.1 (2.0-2.2) | 3.8 (3.7-4.0) | 37.4 (36.9-37.8) | 0.3 (0.3-0.3) | 4.0 (3.9-4.2) | 0.4 (0.4-0.5) |
| 27 | 10.1 (9.8-10.5) | 8.7 (8.5-8.9) | 2.0 (1.9-2.1) | 2.2 (2.1-2.3) | 3.9 (3.8-4.1) | 38.8 (38.2-39.4) | 0.3 (0.3-0.4) | 4.1 (4.0-4.3) | 0.4 (0.4-0.5) |
| 28 | 11.2 (10.8-11.6) | 9.5 (9.2-9.7) | 2.1 (2.0-2.2) | 2.4 (2.3-2.5) | 4.0 (3.8-4.1) | 40.2 (39.5-40.9) | 0.3 (0.3-0.4) | 4.2 (4.0-4.4) | 0.5 (0.4-0.5) |
| 29 | 12.2 (11.7-12.7) | 10.2 (10.0-10.5) | 2.2 (2.0-2.3) | 2.6 (2.4-2.7) | 4.0 (3.8-4.2) | 41.4 (40.7-42.1) | 0.4 (0.3-0.4) | 4.3 (4.1-4.5) | 0.5 (0.4-0.5) |
| 30 | 13.1 (12.6-13.6) | 11.0 (10.7-11.3) | 2.3 (2.1-2.4) | 2.7 (2.6-2.9) | 4.0 (3.8-4.2) | 42.6 (41.9-43.4) | 0.4 (0.3-0.4) | 4.4 (4.2-4.6) | 0.5 (0.4-0.6) |
| 31 | 14.0 (13.5-14.5) | 11.6 (11.3-11.9) | 2.4 (2.2-2.5) | 2.9 (2.7-3.0) | 4.1 (3.9-4.2) | 43.8 (43.1-44.6) | 0.4 (0.3-0.5) | 4.5 (4.3-4.7) | 0.5 (0.4-0.6) |
| 32 | 14.8 (14.3-15.4) | 12.2 (11.9-12.6) | 2.5 (2.3-2.6) | 3.0 (2.9-3.2) | 4.1 (3.9-4.3) | 44.9 (44.2-45.7) | 0.4 (0.3-0.5) | 4.5 (4.3-4.7) | 0.5 (0.5-0.6) |
| 33 | 15.6 (15.0-16.2) | 12.8 (12.5-13.1) | 2.6 (2.4-2.7) | 3.2 (3.0-3.3) | 4.1 (3.9-4.3) | 46.0 (45.3-46.8) | 0.4 (0.4-0.5) | 4.6 (4.4-4.8) | 0.5 (0.5-0.6) |
| 34 | 16.4 (15.8-17.0) | 13.4 (13.0-13.7) | 2.7 (2.5-2.8) | 3.3 (3.1-3.5) | 4.1 (3.9-4.3) | 47.1 (46.3-47.9) | 0.4 (0.4-0.5) | 4.6 (4.4-4.9) | 0.5 (0.5-0.6) |
| 35 | 17.2 (16.5-17.8) | 13.9 (13.5-4.3) | 2.8 (2.6-2.9) | 3.5 (3.3-3.7) | 4.1 (3.9-4.3) | 48.2 (47.3-49.0) | 0.4 (0.4-0.5) | 4.7 (4.5-4.9) | 0.6 (0.5-0.6) |
| 36 | 18.0 (17.3-18.7) | 14.5 (14.1-14.9) | 2.9 (2.7-3.1) | 3.6 (3.4-3.8) | 4.1 (3.9-4.4) | 49.2 (48.3-50.2) | 0.4 (0.4-0.5) | 4.7 (4.5-5.0) | 0.6 (0.5-0.7) |
| 37 | 18.8 (18.0-19.6) | 15.1 (14.6-15.5) | 3.0 (2.8-3.2) | 3.8 (3.6-4.0) | 4.1 (3.9-4.4) | 50.3 (49.2-51.3) | 0.5 (0.4-0.5) | 4.8 (4.5-5.1) | 0.6 (0.5-0.7) |
| 38 | 19.6 (18.8-20.5) | 15.7 (15.2-16.2) | 3.1 (2.9-3.4) | 4.0 (3.7-4.2) | 4.1 (3.9-4.4) | 51.3 (50.2-52.5) | 0.5 (0.4-0.6) | 4.8 (4.6-5.2) | 0.6 (0.5-0.7) |
| 39 | 20.5 (19.5-21.5) | 16.3 (15.7-16.9) | 3.2 (3.0-3.5) | 4.1 (3.9-4.4) | 4.2 (3.8-4.5) | 52.4 (51.1-53.7) | 0.5 (0.4-0.6) | 4.9 (4.6-5.3) | 0.6 (0.5-0.8) |
| 40 | 21.4 (20.3-22.6) | 16.9 (16.3-17.6) | 3.4 (3.1-3.7) | 4.3 (4.0-4.7) | 4.2 (3.8-4.6) | 53.5 (52.0-54.9) | 0.5 (0.4-0.6) | 5.0 (4.6-5.3) | 0.6 (0.5-0.8) |
| 41 | 22.4 (21.1-23.7) | 17.6 (16.8-18.3) | 3.5 (3.2-3.8) | 4.5 (4.2-4.9) | 4.2 (3.8-4.6) | 54.5 (52.9-56.1) | 0.5 (0.4-0.7) | 5.0 (4.6-5.5) | 0.7 (0.5-0.8) |
| 42 | 23.4 (21.9-24.9) | 18.3 (17.4-19.1) | 3.7 (3.3-4.1) | 4.7 (4.3-5.2) | 4.2 (3.8-4.6) | 55.6 (53.8-57.3) | 0.5 (0.4-0.7) | 5.1 (4.6-5.6) | 0.7 (0.5-0.9) |
| 43 | 24.4 (22.8-26.0) | 19.0 (18.0-19.9) | 3.8 (3.4-4.3) | 5.0 (4.5-5.5) | 4.2 (3.8-4.7) | 56.6 (54.7-58.5) | 0.5 (0.4-0.7) | 5.1 (4.6-5.7) | 0.7 (0.5-0.9) |
| 44 | 25.4 (23.6-27.3) | 19.7 (18.7-20.8) | 4.0 (3.5-4.5) | 5.2 (4.7-5.8) | 4.2 (3.8-4.8) | 57.6 (55.6-59.7) | 0.5 (0.4-0.8) | 5.2 (4.6-5.8) | 0.7 (0.5-1.0) |
| 45 | 26.5 (24.5-28.5) | 20.4 (19.3-21.6) | 4.1 (3.6-4.7) | 5.4 (4.8-6.1) | 4.2 (3.7-4.8) | 58.7 (56.5-60.9) | 0.5 (0.4-0.8) | 5.2 (4.6-5.9) | 0.7 (0.5-1.0) |
| 46 | 27.6 (25.4-29.8) | 21.2 (19.9-22.5) | 4.3 (3.7-4.9) | 5.7 (5.0-6.4) | 4.2 (3.7-4.9) | 59.7 (57.3-62.0) | 0.6 (0.4-0.8) | 5.3 (4.7-6.0) | 0.8 (0.5-1.1) |
| 47 | 28.7 (26.3-31.2) | 22.0 (20.6-23.5) | 4.4 (3.8-5.2) | 5.9 (5.2-6.7) | 4.3 (3.7-4.9) | 60.7 (58.2-63.2) | 0.6 (0.4-0.9) | 5.3 (4.7-6.1) | 0.8 (0.5-1.1) |
| 48 | 29.8 (27.2-32.6) | 22.8 (21.3-24.4) | 4.6 (3.9-5.4) | 6.2 (5.4-7.1) | 4.3 (3.6-5.0) | 61.7 (59.1-64.3) | 0.6 (0.4-0.9) | 5.4 (4.7-6.2) | 0.8 (0.5-1.2) |
| 49 | 31.0 (28.2-34.0) | 23.6 (22.0-25.4) | 4.8 (4.0-5.7) | 6.5 (5.6-7.5) | 4.3 (3.6-5.1) | 62.7 (59.9-65.4) | 0.6 (0.4-1.0) | 5.5 (4.7-6.4) | 0.8 (0.5-1.2) |
| 50 | 32.2 (29.1-35.4) | 24.5 (22.7-26.4) | 5.0 (4.2-6.0) | 6.7 (5.8-7.9) | 4.3 (3.6-5.1) | 63.7 (60.8-66.5) | 0.6 (0.4-1.0) | 5.5 (4.7-6.5) | 0.8 (0.5-1.3) |
Data are n (%).
Macrosomia was defined as birth weight>4500 grams.
Analyses restricted to those without preexisting diabetes mellitus or chronic hypertension. Models for cesarean delivery and preeclampsia restricted to nulliparous women. Models adjusted for height, maternal age at delivery, parity, and maternal smoking in pregnancy. Risks are reported at the population average values of all covariates: height (1.64m, or 5.’4”), maternal age at delivery (30.1 years), nulliparity (50.9%), and smoking in pregnancy (9.15%).
Table 4.
Adjusted predicted absolute risks (%) of adverse maternal and perinatal outcomes according to maternal prepregnancy weight in pounds with 95% confidence intervals (N=226,958)
| lbs | Preeclampsia | Gestational diabetes | Indicated birth <37 weeks | Macrosomia* | Shoulder dystocia | Cesarean delivery | Stillbirth | NICU stay >48 hours | In-hospital newborn mortality |
|---|---|---|---|---|---|---|---|---|---|
| 100 | 2.9 (2.6-3.2) | 4.3 (4.1-4.5) | 1.5 (1.4-1.7) | 0.3 (0.3-0.4) | 2.8 (2.6-3.0) | 23.3 (22.5-24.1) | 0.3 (0.2-0.3) | 4.2 (3.9-4.5) | 0.4 (0.3-0.5) |
| 110 | 3.5 (3.3-3.7) | 4.5 (4.4-4.7) | 1.5 (1.4-1,6) | 0.5 (0.4-0.6) | 3.0 (2.9-3.2) | 25.6 (25.1-26.1) | 0.3 (0.2-0.3) | 3.9 (3.7-4.0) | 0.4 (0.3-0.4) |
| 120 | 4.1 (3.9-4.4) | 4.7 (4.6-4.9) | 1.5 (1.4-1,6) | 0.7 (0.6-0.8) | 3.3 (3.1-3.4) | 28.0 (27.5-28.6) | 0.3 (0.2-0.3) | 3.7 (3.5-3.8) | 0.4 (0.3-0.4) |
| 130 | 4.9 (4.7-5.1) | 5.0 (4.9-5.1) | 1.5 (1.4-1,6) | 1.0 (1.0-1.1) | 3.5 (3.4-3.6) | 30.5 (30.1-31.0) | 0.3 (0.2-0.3) | 3.6 (3.5-3.8) | 0.3 (0.3-0.4) |
| 140 | 6.1 (5.8-6.4) | 5.6 (5.4-5.7) | 1.6 (1.5-1,7) | 1.5 (1.4-1.6) | 3.7 (3.5-3.8) | 33.2 (32.6-33.7) | 0.2 (0.2-0.3) | 3.8 (3.6-3.9) | 0.3 (0.3-0.4) |
| 150 | 7.8 (7.5-8.1) | 6.7 (6.5-6.9) | 1.8 (1.7-1,8) | 1.9 (1.8-2.1) | 3.8 (3.7-4.0) | 35.9 (35.4-36.4) | 0.2 (0.2-0.3) | 3.9 (3.8-4.0) | 0.4 (0.3-0.4) |
| 160 | 9.9 (9.6-10.2) | 8.1 (7.0-8.3) | 2.0 (1.9-2.0) | 2.4 (2.3-2.5) | 3.9 (3.8-4.1) | 38.5 (37.9-39.0) | 0.3 (0.3-0.4) | 4.1 (3.9-4.2) | 0.4 (0.4-0.5) |
| 170 | 11.8 (11.4-12.3) | 9.5 (9.2-9.7) | 2.1 (2.0-2.3) | 2.7 (2.6-2.9) | 4.0 (3.8-4.2) | 40.9 (40.2-41.6) | 0.4 (0.3-0.4) | 4.2 (4.0-4.4) | 0.5 (0.4-0.5) |
| 180 | 13.6 (13.1-14.1) | 10.8 (10.5-11.1) | 2.3 (2.2-2.5) | 3.1 (2.9-3.2) | 4.1 (3.9-4.3) | 43.0 (42.3-43.8) | 0.4 (0.3-0.5) | 4.3 (4.1-4.5) | 0.5 (0.4-0.6) |
| 190 | 15.2 (14.6-15.8) | 12.0 (11.7-12.4) | 2.5 (2.3-2.6) | 3.3 (3.2-3.5) | 4.2 (4.0-4.4) | 45.0 (44.3-45.8) | 0.4 (0.4-0.5) | 4.4 (4.2-4.6) | 0.5 (0.5-0.6) |
| 200 | 16.6 (16.0-17.2) | 13.3 (12.9-13.6) | 2.6 (2.5-2.8) | 3.6 (3.4-3.8) | 4.2 (4.0-4.4) | 46.9 (46.1-47.7) | 0.4 (0.4-0.5) | 4.5 (4.3-4.8) | 0.6 (0.5-0.6) |
| 210 | 18.0 (17.3-18.7) | 14.5 (14.1-14.9) | 2.8 (2.6-3.0) | 3.8 (3.6-4.0) | 4.2 (4.0-4.5) | 48.7 (47.9-49.6) | 0.5 (0.4-0.6) | 4.6 (4.4-4.9) | 0.6 (0.5-0.7) |
| 220 | 19.4 (18.7-20.2) | 15.8 (15.4-16.3) | 2.9 (2.7-3.1) | 4.0 (3.8-4.3) | 4.3 (4.0-4.5) | 50.6 (49.6-51.5) | 0.5 (0.4-0.6) | 4.8 (4.5-5.0) | 0.6 (0.5-0.7) |
| 230 | 21.0 (20.0-21.9) | 17.2 (16.7-17.8) | 3.1 (2.9-3.3) | 4.3 (4.0-4.5) | 4.3 (4.0-4.6) | 52.4 (51.2-53.5) | 0.5 (0.4-0.6) | 4.9 (4.6-5.2) | 0.6 (0.5-0.7) |
| 240 | 22.6 (21.5-23.7) | 18.8 (18.1-19.5) | 3.3 (3.0-3.6) | 4.5 (4.2-4.8) | 4.3 (4.0-4.7) | 54.2 (52.8-55.5) | 0.5 (0.4-0.6) | 5.0 (4.6-5.4) | 0.6 (0.5-0.8) |
| 250 | 24.3 (23.0-25.7) | 20.4 (19.5-21.3) | 3.5 (3.1-3.8) | 4.8 (4.4-5.2) | 4.4 (4.0-4.8) | 56.0 (54.4-57.5) | 0.5 (0.4-0.7) | 5.1 (4.7-5.6) | 0.7 (0.5-0.9) |
| 260 | 26.1 (24.5-27.8) | 22.1 (21.1-23.2) | 3.6 (3.2-4.1) | 5.1 (4.6-5.5) | 4.4 (3.9-4.9) | 57.8 (56.0-59.5) | 0.5 (0.4-0.6) | 5.2 (4.7-5.8) | 0.7 (0.5-0.9) |
| 270 | 28.0 (26.1-29.9) | 23.9 (22.7-25.2) | 3.8 (3.4-4.4) | 5.4 (4.9-5.9) | 4.4 (3.9-5.0) | 59.5 (57.5-61.5) | 0.5 (0.4-0.6) | 5.3 (4.8-6.0) | 0.7 (0.5-1.0) |
| 280 | 29.9 (27.7-32.2) | 25.9 (24.4-27.4) | 4.1 (3.5-4.7) | 5.7 (5.1-6.4) | 4.5 (3.9-5.1) | 61.3 (59.1-63.5) | 0.5 (0.4-0.6) | 5.5 (4.8-6.2) | 0.8 (0.5-1.1) |
| 290 | 31.9 (29.4-34.6) | 27.9 (26.2-29.7) | 4.3 (3.6-5.1) | 6.0 (5.3-6.8) | 4.5 (3.9-5.2) | 63.0 (60.6-65.4) | 0.5 (0.4-0.6) | 5.6 (4.8-6.4) | 0.8 (0.5-1.1) |
| 300 | 34.0 (31.1-37.1) | 30.0 (28.0-32.1) | 4.5 (3.8-5.5) | 6.4 (5.6-7.3) | 4.6 (3.8-5.3) | 64.7 (62.1-67.2) | 0.5 (0.4-0.6) | 5.7 (4.9-6.7) | 0.8 (0.5-1.2) |
Data are n (%).
Macrosomia was defined as birth weight>4500 grams.
Analyses restricted to those without preexisting diabetes mellitus or chronic hypertension. Models for cesarean delivery and preeclampsia restricted to nulliparous women.
Models adjusted for height, maternal age at delivery, parity, and maternal smoking in pregnancy. Risks are reported at the population average values of all covariates: height (1.64m, or 5.’4”), maternal age at delivery (30.1 years), nulliparity (50.9%), and smoking in pregnancy (9.15%).
The number needed to “treat” (NNT) provides way to interpret our findings on a population level. Again, because the NIH recommends a 10% reduction in body weight to confer health benefits outside of pregnancy,(26) we defined “treatment” as a 10% difference in prepregnancy BMI. Compared to a baseline prepregnancy BMI of 40, 29.4 women would need to reduce their BMI to 36 to prevent 1 case of preeclampsia, 41.7 to prevent 1 case of gestational diabetes, and 23.3 to prevent 1 cesarean delivery. For less common outcomes, the NNT would be larger. For the same group, the NNT for macrosomia is 142.9 , and for stillbirth is 1000 . The NNT can be calculated from Tables 3 and 4 for any target body mass difference for all outcomes that we examined.
Discussion
This study found that prepregnancy BMI was associated with the absolute risk of many important obstetric outcomes, including preeclampsia, gestational diabetes, indicated preterm delivery, macrosomia, shoulder dystocia, cesarean delivery, stillbirth, NICU stay ≥48 hours, and in-hospital newborn mortality. These findings are consistent with previous investigations.(5-13,15,16,20) This paper adds to prior studies by examining BMI in smaller increments, which reflect achievable amounts of weight loss, and by estimating absolute risks, which are more relevant for clinical counseling than odds ratios. Clinicians can use Tables 3 and 4 to inform weight loss counseling and help patients set achievable weight loss goals to reduce their risk of poor pregnancy outcomes. The accuracy of the actual values decreases as women's characteristics deviate from the population averages. This is particularly pronounced when using the estimates based on weight for women far from the population average height, and less pronounced for estimates based on BMI, because height is an independent risk factor for some outcomes we examined.
Defining what magnitude of risk reduction is clinically meaningful is challenging. One approach is to define a threshold of change in risk as meaningful. If we define a 10% risk difference as meaningful, our study found that a 10% difference in prepregnancy BMI is associated with clinically meaningful risk differences for preeclampsia, gestational diabetes, indicated preterm delivery, macrosomia, and stillbirth. In contrast, larger differences in prepregnancy BMI (i.e., 20-30% differences, or shifting an entire BMI category or more) would be necessary to see meaningfully lower risks of cesarean delivery, shoulder dystocia, NICU stay ≥48 hours, and inhospital newborn mortality. We present the absolute risks so that clinicians and patients can determine what magnitude of expected reduction in risk is meaningful on an individual level. On a population level, interpreting our findings in terms of the number of women who would need to lose a specified amount of weight to prevent one adverse event illustrates the potential effectiveness of lowering prepregnancy BMI to reduce adverse pregnancy outcomes.
While this study confirmed the association between BMI and several perinatal outcomes, we found no relationship between BMI and risk of spontaneous preterm delivery <32 weeks, postpartum hemorrhage requiring intervention, or maternal mortality/severe morbidity. This was not entirely surprising, as the relationships between obesity and these outcomes are less certain in the literature.(3,5,8,17-19,21,23,24,32-34)
The results of our study must be interpreted in light of several limitations. Without longitudinal data, we cannot conclude that between-woman differences in prepregnancy BMI are equivalent to the same magnitudes of BMI loss for individual women. In the absence of data from randomized trials of weight loss interventions, studies that compare the outcomes of different women by prepregnancy BMIs provide the best available evidence to inform prepregnancy weight loss counseling.
Our database lacks information on race. The racial composition in British Columbia differs from that in the United States, with smaller black and Hispanic populations and larger East and Southeast Asian populations, although the percent of white women is comparable.(35,36) While universally available health care may mitigate some of the difference in risks of adverse perinatal outcomes by race in British Columbia, we cannot rule out bias due to confounding by race. We also do not know if the BMI distribution is different among those with missing vs. available BMI information in the database. It is challenging to speculate on the direction and magnitude of bias introduced by missing data for BMI or race,(37) however, and confirmation of our findings in a cohort with complete BMI and race data would be valuable.
Most reproductive-aged women report weight and height accurately, though some over- and underestimation of prepregnancy weight is expected.(38) Without specific information about the error in our database, the direction and magnitude of any mismeasurement cannot be determined. Finally, as there are fewer obese women in British Columbia than the United States,(39,40) we restricted the upper limit of BMI values examined to 50, and no estimates are provided for women with higher BMIs.
This population-based cohort study of the relationship between small differences in prepregnancy BMI and adverse pregnancy outcomes provides a unique contribution to the literature. By examining BMI as a continuous variable and presenting absolute risks for outcomes, our findings provide more clinically applicable data about the magnitude of the effect of different prepregnancy BMI values on the risk of adverse pregnancy outcomes. Our tables provide simple resources that clinicians can use for preconception weight loss counseling.
Supplementary Material
Précis.
Small differences in prepregnancy body mass index are associated with meaningfully lower preeclampsia, gestational diabetes, indicated preterm delivery, macrosomia, and stillbirth risks, while larger differences are necessary for other outcomes.
Acknowledgements
The authors thank Terri Pacheco, Perinatal Services British Columbia, for her assistance in compiling the study data.
Supported by the Child and Family Research Institute at the BC Children's and Women's Hospital, University of British Columbia. LS holds a Leadership Trainee Award from the Maternal-Child Health Bureau and was supported by Training Grant T32HD060454 in Reproductive, Perinatal and Pediatric Epidemiology from the National Institute of Child Health and Human Development, National Institutes of Health. JAH holds New Investigator awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. KPH is supported by National Institutes of Health Grants K12HD063087.
Footnotes
All inferences, opinions, and conclusions drawn in this publication are those of the authors, and do not reflect the opinions or policies of Perinatal Services BC.
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented as a poster at the annual Pregnancy meeting convened by the Society for Maternal-Fetal Medicine (SMFM) on February 3-8, 2014, and at the 2014 Society for Epidemiologic Research Annual Meeting on June 24-27, 2014.
References
- 1.American Congress of Obstetricians and Gynecologists (ACOG) Committee opinion: Obesity in pregnancy. American Congress of Obstetricians and Gynecologists (ACOG); Washington, DC: 2013. p. 549. Report No. [Google Scholar]
- 2.Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA. 2009;301(6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 3.Myles TD, Gooch J. Obesity as an indepdendent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol. 2002;100(5):959–964. doi: 10.1016/s0029-7844(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 4.Barnardo P, Jenkins J. Failed tracheal intubation in obstetrics: A 6-year review in a UK region. Anaesthesia. 2000;55(7):690–694. doi: 10.1046/j.1365-2044.2000.01536.x. [DOI] [PubMed] [Google Scholar]
- 5.Cnattingius S, Villamor E, Johansson S, Bonamy A, Persson M, Wikstrom A, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 6.Cnattingius S, Bergstrom R, Lipworth L, Kramer M. Prepregnancy weight and the risk of adverse pregnancy outcomes. New Engl J Med. 1998;338(3):147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 7.Hollowell J, Pillas D, Rowe R, Knight M, Brocklehurst P. The impact of maternal obesity on intrapartum outcomes in otherwise low risk women: Secondary analysis of the birthplace national prospective cohort study. Brit J Obstet Gynaec. 2013;121(3):343–355. doi: 10.1111/1471-0528.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty D, Magann E, Francis J, Morrison J, Newnham J. Pre-pregnancy body mass index and pregnancy outcomes. Int J Obstet Gynecol. 2006;95(3):242–247. doi: 10.1016/j.ijgo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Cedergreen MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 10.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate – A population-based screening study. Am J Obstet Gynecol. 2004;190(3):1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Pub Health. 2001;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson HE, O'Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106(6):1357–1364. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 13.Aviram A, Hod M, Yogev Y. Maternal obesity: Implications for pregnancy outcome and long-term risks – a link to maternal nutrition. Int J Obstet Gynecol. 2001;115(Suppl 1):S6–S10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18:234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing body mass index. Ann Epidemiol. 2005;15:475–482. doi: 10.1016/j.annepidem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Blomberg M. Maternal obesity, mode of delivery, and neonatal outcome. Obstetrics and Gynecology. 2013;122(1):50–55. doi: 10.1097/AOG.0b013e318295657f. [DOI] [PubMed] [Google Scholar]
- 17.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(3):561–568. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 18.McDonald SD. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: A systematic review. BMJ. 2010:341. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salihu H, Mbah AK, Alio AP, Kornosky JL, Whiteman VE, Belogolovkin V, et al. Nulliparity and preterm birth in the era of obesity epidemic. J Matern-Fetal Neo M. 2010;23(12):1444–1450. doi: 10.3109/14767051003678044. [DOI] [PubMed] [Google Scholar]
- 20.Gilboa SM, Correa A, Alverson C. Spline regression in an analysis of maternal prepregnancy body mass index and adverse birth outcomes: Does it tell us more than we already know? Ann Epidemiol. 2008;18:196–205. doi: 10.1016/j.annepidem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Halloran DR, Marshall NE, Kunovich RM, Caughey AB. Obesity trends and perinatal outcomes in black and white teenagers. Am J Obstet Gynecol. 2012;207(6):492.e1–492.e7. doi: 10.1016/j.ajog.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhyre JM. Anethetic management for the morbidly obese pregnant woman. Int Anesthesiol Clin. 2007;45(1):51–70. doi: 10.1097/AIA.0b013e31802b8a90. [DOI] [PubMed] [Google Scholar]
- 23.Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anaesthesia. 1993;79(6):1210–1218. doi: 10.1097/00000542-199312000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5):952–956. doi: 10.1016/s0029-7844(03)00861-5. [DOI] [PubMed] [Google Scholar]
- 25.Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61(1):26–48. doi: 10.1111/j.1365-2044.2005.04433.x. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health, National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. National Institutes of Health; Bethesda, MD: 1998. Report No.: NIH Publication No.98-4083. [PubMed] [Google Scholar]
- 27.Forsum E, Brantsaeter A, Olafsdottir A, Olsen S, Thorsdottir I. Weight loss before conception: A systematic literature review. Food Nutr Res. 2013;57:20522. doi: 10.3402/fnr.v57i0.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frosst GO, Joseph KS, Hutcheon JA, Kinniburgh BA, Lee L. Validity of data in a population-based perinatal database routinely used for surveillance and research.. Proceedings of the 27th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research; Seattle, WA.. 2014. [Google Scholar]
- 29.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. Springer; New York, NY: 2001. [Google Scholar]
- 30.Orsini N, Greenland S. A procedure to tabulate and plot results after flexbile modeling of a quantitative covariate. Stata J. 2011;11(1):1–29. [Google Scholar]
- 31.Stata Corp. Stata staistical software: Release 12. 2011.
- 32.Torloni MR, Betran AP, Daher S, Widmer M, Dolan SM, Menon R, et al. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. J Matern-Fetal Neo M. 2009;22(11):957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- 33.Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The preterm prediction study: Association between maternal body mass index and spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2005;192(3):882–886. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Grobman WA, Bailit JL, Murguia M, Wapner RJ, Reddy UM, Varner MW, et al. Frequency of factors associated with severe maternal morbidity. Obstet Gynecol. 2014;123(4):804–810. doi: 10.1097/AOG.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton BE, Martin JA, Osterman MJK, Curtin SC. Preliminary data for 2013. Nat Vit Stat Rep. 2014;63(2):e1–e19. [Google Scholar]
- 36.BC Stats . 2006 census fast facts: Ethnicity and visible minority characteristics of BC's population. BC Stats, a branch of Service BC, Ministry of Labour and Citizens' Services; Victoria, BC: 2008. pp. 2006–12. Report No. [Google Scholar]
- 37.Lash TL. Heuristic thinking and inference from observational epidemiology. Epidemiol. 2007;18(1):67–72. doi: 10.1097/01.ede.0000249522.75868.16. [DOI] [PubMed] [Google Scholar]
- 38.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11:137–144. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 39.British Columbia Perinatal Health Program . Perinatal health report 2008. British Columbia Perinatal Health Program; Vancouver, BC.: 2010. [Google Scholar]
- 40.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnancy women? prepregnancy obesity rends in 20 states, 2003-2009. Prev Med. 2013;56(6):372–378. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.