Abstract
BACKGROUND
We test the hypothesis that cardiac magnetic resonance (CMR) imaging-based indices of four-dimensional (4D) (three dimensions (3D) + time) right ventricle (RV) function have predictive values in ascertaining invasive pulmonary arterial systolic pressure (PASP) measurements from right heart catheterization (RHC) in patients with pulmonary arterial hypertension (PAH).
METHODS
We studied five patients with idiopathic PAH and two age and sex-matched controls for RV function using a novel contractility index (CI) for amplitude and phase to peak contraction established from analysis of regional shape variation in the RV endocardium over 20 cardiac phases, segmented from CMR images in multiple orientations.
RESULTS
The amplitude of RV contractility correlated inversely with RV ejection fraction (RVEF; R2 = 0.64, P = 0.03) and PASP (R2 = 0.71, P = 0.02). Phase of peak RV contractility also correlated inversely to RVEF (R2 = 0.499, P = 0.12) and PASP (R2 = 0.66, P = 0.04).
CONCLUSIONS
RV contractility analyzed from CMR offers promising non-invasive metrics for classification of PAH, which are congruent with invasive pressure measurements.
Keywords: right ventricular function, ventricular sphericity, contractility index, classification, pulmonary arterial hypertension (PAH)
Introduction
Idiopathic pulmonary arterial hypertension (PAH) is a progressive and debilitating disease characterized by pathological lesions of the pulmonary arteries leading to an increase in pulmonary artery pressure that may exceed systemic levels. The degree of rise in pulmonary artery pressure has modest prognostic significance in these patients as opposed to the ability of the right ventricle (RV) to cope with the progressive increase in pulmonary arterial pressure, which determines the patients’ functional capacity and survival.1–4 Severe PAH is associated with high morbidity and mortality. Hemodynamics and pulmonary artery pressures are important prognostic indicators of pulmonary hypertension (PH), which have been shown to correlate well with disease progression. However, confirmation of high baseline RV pressures requires right heart catheterization (RHC) – an invasive diagnostic measure. The average time from onset of symptoms until clinical diagnosis of PAH may be significantly reduced using an early non-invasive confirmation of high baseline pressures long before a catheterization examination is warranted.
The morphology and function of the RV in PAH assume prognostic importance, and cardiac magnetic resonance (CMR) imaging is the gold standard for evaluation of RV structure and function. Measurements of RV volumes, RV ejection fraction (RVEF), and RV fractional area change are established parameters for the assessment of RV function.5 However, there is paucity of a sensitive index to quantify RV function in the progression of PAH. Further, there is no proven and accurate means to directly measure right heart pressures analogous to the invasive RHC using non-invasive imaging. Therefore, in this study, we propose the quantification of a novel index of RV contractility, which may help in longitudinal assessment of RV function in PAH, by using standard CMR images. More specifically, we evaluate RV morphology and function using time-resolved cine CMR imaging, in three dimensions (3D), with the goal of investigating the correlation between RV contractility and RHC-based pulmonary arterial systolic pressures (PASPs) in patients with idiopathic PAH.
Methods
The methods followed in this study have been organized into sections, which begin with a description of the imaging data and our study cohort, followed by the definition and the methodology for quantification of our novel index of RV contractility and, finally, the metrics for statistical investigation against the ground-truth RHC pressure measurements.
Study cohort
A total of five patients with idiopathic PAH as defined by World Health Organization (WHO) criteria6 and two normal controls were included in this pilot study to define a novel metric of RV function. Standard metrics of global RV function, including end systolic and end diastolic volumes (ie, ESV and EDV), as well as ejection fraction (RVEF), were recorded. All patients had blinded RHC and clinical-indicated CMR imaging studies (two weeks ± five days apart). The study was approved by the Allegheny-Singer Research Institute - West Penn Allegheny Health System Institutional Review Board, and informed patient consent was obtained for CMR imaging and analysis. The research was conducted in accordance with the principles of the Declaration of Helsinki.
RV reconstruction
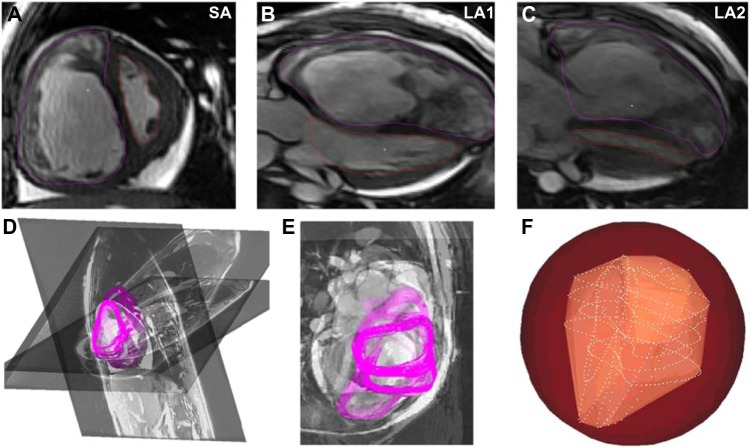
The RV endocardium was segmented on a slice-by-slice basis (ie, two dimensions (2D)) from time-resolved cine steady-state free precession (SSFP) CMR image sequences, over 20 cardiac phases, using semi-automated contour tracing routines written in MATLAB (The MathWorks, Inc.). These reconstructions were prepared by first segmenting 2D contours of the RV from long- and short-axis time-series images, and then fusing these contours into 3D models at each cardiac phase based on the relative orientation information computed from the DICOM header information of the individual image sequences. The accurately oriented and co-registered 2D contours were stitched into surface models using 3D Delaunay triangulation followed by loop subdivision- based smoothing using ParaView filters (Kitware, Inc.). A minimum of at least two long-axis cardiac image stacks through the RV and one short-axis image stack composed of slices normal to the ventricular axis were chosen for this reconstruction process, which is illustrated in Figure 1. The RV is contoured in purple and the left ventricle is contoured in red (ie, Fig. 1A, B, and C) in three slice orientations through the heart, viz., one short-axis stack and two long-axis stacks (ie, SA, and LA1 and LA2, respectively, in Fig. 1). Figure 1D and E shows the 3D reconstruction of these slices presented in their native orientation along with the segmented contours from each cardiac phase shown superimposed.
Figure 1.
Illustration of the methodology followed for reconstruction of the right ventricular endocardium at each cardiac phase, for a representative PAH patient dataset with elevated RV pressure.
Contractility index (CI)
A novel CI for the RV function was quantified based on the extraction of complete four-dimensional (4D; ie, 3D + time) signatures of RV shape variation over the cardiac cycle. These 4D signatures were established by first computing the regional shape difference of the reconstructed RV shapes at each cardiac phase with a virtual sphere fitting around the RV at end-diastole and encapsulating the RV throughout the cardiac cycle. Figure 1F illustrates the Delaunay-triangulated surface reconstruction of the RV at a single cardiac phase (in yellow) along with the constituent point cloud (ie, white points) from the oriented 2D segmentation contours, which were used to create it. The red enclosing sphere shown in Figure 1F is the aforementioned virtual sphere fitting around the RV, which enveloped the RV throughout the cardiac cycle and formed a constant reference for examining temporally evolving RV morphology through the cardiac cycle.
The CI was defined by both the amplitude of regional shape difference and the phase to peak contraction evidenced at the instant of maximum shape difference with this surrounding sphere. Cumulative wall displacement of the RV endocardial wall was quantified indirectly by means of a regional Hausdorff distance (HD) metric7,8 computed using an in-house plug-in for ParaView (Kitware, Inc.) at each of the vertices of the endocardial segmentation,9,10 establishing point-correspondences with the fixed enclosing sphere surrounding the RV. A vector was assigned to each of vertex of the endocardial surface at each cardiac phase based on the regional distance between corresponding points identified using the HD metric between the endocardium and the enclosing sphere.
Next, endocardium-averaged HD characteristic curves were prepared for each phase of the cardiac cycle. The fundamental frequency component of time-varying RV endocardial HD from the enclosing sphere was computed using a method of Fourier decomposition for this HD characteristic curve over time for each patient, given knowledge of the time-period of the cardiac cycle (viz., 20 phases), the Fourier decomposition of endocardial displacement.11 The amplitude and phase of the fitted fundamental frequency Fourier curve, fit to the data, were recorded to quantify the cardiac phase of peak contraction, and these two parameters (viz., CI magnitude and CI phase) constituted our CI for a patient-specific RV. The cardiac phase for peak RV mechanical contraction was reported in a numeric scale of 0°–360° (ie, degrees), where 360° represents one complete cardiac cycle.
Statistical analysis
The continuous variables analyzed in this study were expressed as mean ± standard deviation, and categorical variables were expressed as a percentage. Logistic regression analysis was conducted to correlate the amplitude and phase of the proposed CI with the degree of PAH as determined from the PASP data obtained from RHC, as well as with RVEF. The threshold for normal PASP was considered as 25 mmHg. Receiver-operating characteristics were studied to determine the sensitivity and specificity of the CI in predicting PAH automatically based on each amplitude and phase of peak RV contraction. Additionally, the accuracy in determining absolute RHC-based PASP values using a linear combination of this biomarker along with RVEF was also investigated by performing bivariate least-squares fitting with optimal linear coefficients.
A P-value of ≤0.05 was considered significant. Statistical analyses were conducted using SPSS v13 (SPSS Inc.).
Results
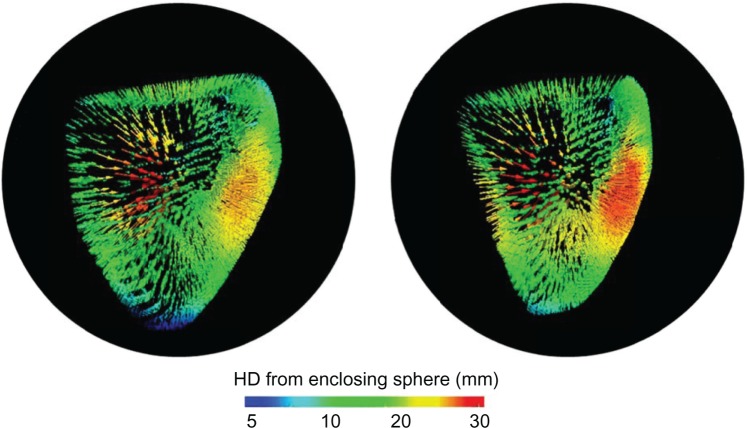
The five patients with PAH considered for this study had a mean age of 52 ± 19.5 years, and there were also two males. The mean PASP was 86.5 ± 21.7 mmHg. The mean RVEF was 38.5 ± 13.3%. The mean RV EDV was 229.5 ± 104.2 mL, and the mean RV ESV was 149.6 ± 95.7 mL. RV contraction was visualized over time as a colormap of the computed HD (see Fig. 2) with vectors indicating the regional distance between corresponding vertices on the fixed enclosing sphere and RV endocardium, at each cardiac phase. Time-series animations of the HD colormaps revealed visual evidence of subtle mechanical dyssynchrony across the RV endocardium, over the cardiac cycle.
Figure 2.
Vectors colored by the HD from the enclosing sphere (black) to the RV endocardium at the end-diastole (left) and end-systole (right) for an illustrative PAH patient.
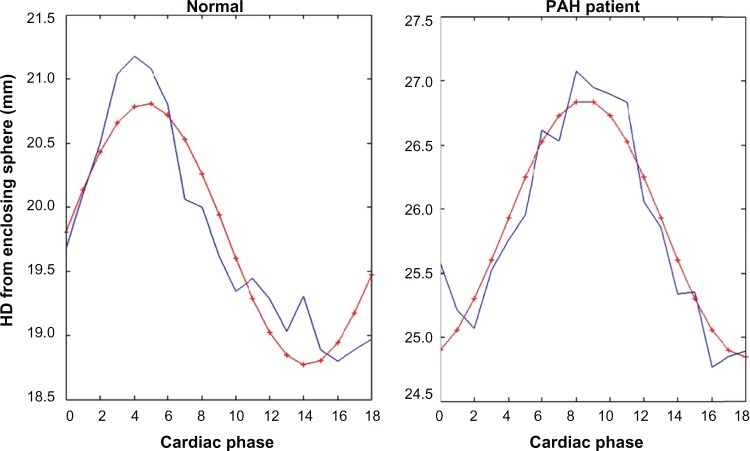
The time-varying HDs between the RV endocardium and its enclosing sphere for an illustrative normal patient and PA patient are shown in Figure 3, along with the superimposed first-order Fourier curve fits. The CI index-based amplitude of mean RV contraction (averaged across the RV endocardium) for the patient cohort was 1.59 ± 0.78 mm, and the first-order Fourier fit-based phase for peak RV contraction was 140° ± 16.9°. The normal controls had a similar amplitude of mean endocardial contraction, but the normal phase of peak RV contraction was 97.5° ± 12.0°, which was significantly earlier than the PAH patients (P = 0.03). Therefore, the CI phase was effective at clustering and distinguishing patients and normal controls.
Figure 3.
Illustrations of the smooth first-order Fourier curve fits (red) used to the CI parameters of amplitude and phase of peak contraction from the time-varying RV endocardium-averaged HD (blue) computed from the enclosing reference sphere, for a normal control (left) and a PAH patient (right). Note: the first cardiac phase is shown as the zeroth phase, and 19 phases are plotted.
The amplitude of contraction evidenced by our CI metric was inversely proportional to both RVEF (R2 = 0.64, P = 0.03) and PASP obtained from RHC (R2 = 0.71, P = 0.02) in the PAH patient cohort. The phase of peak RV contractility as per the CI metric was also inversely proportional to both RVEF (R2 = 0.499, P = 0.12) and PASP (R2 = 0.66, P = 0.04) in the PAH cohort.
A study of the receiver-operating characteristics revealed 100% sensitivity and specificity in accurately classifying PAH patients using a CI phase threshold of 111.5°, given a normal PASP threshold of 25 mmHg. The RVEF was also found to be an effective means to classify PAH patients with 100% accuracy using a normal RVEF threshold of >57.2%.
Finally, we examined the accuracy of determining absolute PASP values using a linear combination of our new CI phase biomarker with RVEF, with the goal of investigating the potential value of using a non-invasive imaging-based RV function metric as a predictive tool for catheterization pressures. Bivariate least-squares fitting for an optimal linear combination of CI phase and RVEF yielded a predicted PASP, which matched the true RHC-based PASP with a root mean square error margin of 17.54 mmHg (R2 = 0.60) when an optimal linear combination of CI phase and RVEF was employed.
Discussion
Right ventricular morphology
The geometry of the normal RV is complex. It consists of an inflow (sinus) and an outflow (conus) portion separated by the crista supraventricularis. It is crescent shaped in cross-section, formed by the concave RV free wall opposite to the convex interventricular septum. Functionally, the RV pumps the same stroke volume as the left ventricle (LV), but using 25% of the stroke work because of the low resistance of the pulmonary vasculature, making the RV wall thinner and more compliant than the LV wall. Normal RV contraction acts in a sequential peristaltic wave directed from inflow to infundibulum. Right ventricular free wall and interventricular septum contribute equally to RV performance. Spatially, RV wall deformation consists of three components (inward, longitudinal, and circumferential traction because of LV contraction), of which longitudinal shortening is the major contributor of overall RV performance.12 These constituent components of RV function were clearly discernible based on our novel multislice CMR segmentation and RV endocardium reconstruction approach demonstrated in this manuscript.
RV morphological changes in PAH
In PAH, the first adaptation to increase RV afterload is RV hypertrophy followed by progressive contractile impairment leading to overt RV failure. RV dilatation occurs in order to compensate for a reduced fractional shortening by an increase in preload, so that stroke volume is maintained. As contractile dysfunction progresses, RV failure occurs, and it is characterized by high RV filling pressures, diastolic dysfunction,13 and reduced cardiac output. With hypertrophy and dilatation, the RV progressively increases in its sphericity, its cross-sectional area enlarges, and the interventricular septum flattens, causing also LV diastolic dysfunction.14 The novel CI presented in this manuscript reports magnitude of RV contraction through a distance metric (viz., HD) comparing the RV endocardial surface with a surrounding sphere, and, therefore, is analogous to a report on RV sphericity at end-diastole and end-systole. Hypertensive RVs were observed to be distinctly more spherical in shape as per this definition than the normal RVs, when they were compared for morphology at end-diastole. In addition, our CI computation revealed insights into RV function as well since the RV function as well since the amplitude of the CI metric was inversely proportional to RVEF in the PAH patients in this study (R2 = 0.64, P = 0.03), which indicates that an increasing CI amplitude was congruent with diminishing contractile performance of the RV.
Assessment of RV function
Measurements of RV volumes, RVEFs and RV fractional area changes are established parameters for the assessment of RV function. Further, the tricuspid annular plane systolic excursion (TAPSE) assessment on 2D echocardiography has been validated as a sensitive and specific index of RV function,15 and tissue Doppler imaging and strain rate imaging have been used to quantify RV mechanical dyssynchrony.16,17 These parameters have established utility in RV failure, but each has their own limitations. The fractional area change of RV in assessing RV function is limited by its being highly afterload dependent, especially in PAH – an effect that might be compounded by tricuspid regurgitation, which by reducing systolic afterload augments RV systolic function. TAPSE, on the other hand, is measured on the M-mode 2D echocardiography and is often subject to intercept angle bias and also carries significant interobserver and intraobserver variabilities.18,19 In addition, the myocardial Tei index has an established role in the assessment of RV function, which is irrespective of the heart rate, but potential limitations exist, namely, that the index is not entirely load independent.12 Therefore, although established as accurate quantifications of RV function, the abovementioned indices are load dependent and observer angle dependent. The studied index of RV contractility in this study, on the other hand, viz., CI phase and magnitude, was found to be both sensitive and specific for the assessment of progressive RV function in PAH, and is based on a gold standard for structural imaging of the heart – CMR. The RVEF – an established index of RV function – tended to also show a gradual decline from normal RV function with normal PAP in controls to RV functional decline in patients with severe PAH in this study cohort; however, our computed phase of RV contractility demonstrated improved separation between patients with severe PASP and controls with normal PASP.
There are other CMR-based indices of RV morphology and hemodynamics that have been shown to have significant correlations with pulmonary arterial flow parameters reported in the literature, including left ventricular septal-to-free wall curvature ratio,20 which correlates with RV systolic pressures, as well as pulmonary arterial average velocity and minimum area,21 which both correlate with PASP. However, our metrics of RV contractility – CI phase and amplitude – provide biomarkers of RV function based on a full 4D (ie, 3D + time) picture of RV function for the first time in the literature. Further, our method of reconstructing the RV endocardium at each cardiac phase based on co-registration of standard cine CMR acquisitions in multiple orientations is also novel. This not only guarantees optimal utilization of images available from clinically indicated CMR studies but also provides an accurate rendition of patient-specific RV function that factors in out-of-plane motion of the heart, which are otherwise lost using reconstructions, form image stacks in any one orientation alone.
Limitations
We do not have long-term follow-up data of our patients. Therefore, we could not establish the utility of the CI as an index of longitudinal assessment of PAH. We have also not been able to establish its role in the assessment of response to therapy in these patients. As the relationship of CI with PAH was consistent and statistically demonstrated to be significant across a range of PASPs, we could extrapolate that this index may have potential for longitudinal assessment and prognostication in patients with PAH.
Technically, with regard to the method for CI computation, our approach of employing first-order Fourier curve fits for extracting the magnitude and phase of peak RV contraction was highly accurate in terms of identifying the instant of peak contraction but was limited in terms of its accuracy in assessing the magnitude of RV contraction because it was imperfect in curve-fitting the peaks of the raw data, as shown in Figure 3, where this is made clear by the discrepancy between the red and blue HD curves. However, the smoothness imparted by the first-order fit was desirable in the interest compensating for the likely effects of image-segmentation inaccuracies affecting the temporal variation of RV endocardium- averaged HD metric.
Conclusions
In this pilot study, we test the hypothesis that imaging-based indices of 4D (3D + time) RV function may have value in classification of patients with PH and establish relationships between non-invasive imaging-based metrics and pulmonary arterial pressures measured from RHC. The RV CI – our novel index composed of a metric for both magnitude and phase of peak RV contraction – was found to be correlated inversely with increasing PASP and decreasing RVEF in our study cohort, therefore, establishing it as a promising index for non-invasively classifying PAH based on CMR image data. The CI phase and amplitude metrics may further reveal quantitative signs of RV dysfunction in severe PAH. Further study with a larger patient cohort is required in order to investigate the potential value of extrapolating these metrics for clinical prognostication in the longitudinal assessment of patients with PAH.
Footnotes
Author Contributions
Conceived and designed the experiments: PGM, RBW. Analyzed the data: PGM, SMA, RBW, MD, RWWB. Wrote the first draft of the manuscript: PGM, SMA. Contributed to the writing of the manuscript: PGM, SMA, RBW, MD, RWWB. Agree with manuscript results and conclusions: PGM, SMA, RBW, MD, RWWB. Jointly developed the structure and arguments for the paper: PGM, SMA. Made critical revisions and approved final version: PGM. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–8. doi: 10.1097/00019501-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–9. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval J, Bauerle O, Palomar A, et al. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89:1733–44. doi: 10.1161/01.cir.89.4.1733. [DOI] [PubMed] [Google Scholar]
- 5.Boxt LM, Katz J, Kolb T, Czegledy FP, Barst RJ. Direct quantitation of right and left ventricular volumes with nuclear magnetic resonance imaging in patients with primary pulmonary hypertension. J Am Coll Cardiol. 1992;19:1508–15. doi: 10.1016/0735-1097(92)90611-p. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff F. Dimension und äußeres maß. Mathematische Annalen. 1918;79(1):157–79. [Google Scholar]
- 8.Commandeur F, Velut J, Acosta O. A VTK algorithm for the computation of the Hausdorff distance. VTK J. 2011. Available from: http://www.vtkjournal.org/browse/publication/839.
- 9.Menon PG, Morris L, Staines M, Lima J, Lee DC, Gopalakrishnan V. SPIE medical imaging. International Society for optics and photonics; San Diego, CA: 2014. Novel MRI-derived quantitative biomarker for cardiac function applied to classifying ischemic cardiomyopathy within a Bayesian rule learning framework; pp. 90341L–90341L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhyapak SM, Menon PG, Mehra A, Tully S, Rao Parachuri V. Rapid quantification of mean myocardial wall velocity in ischemic cardiomyopathy by cardiac magnetic resonance: an index of cardiac functional abnormalities during the cardiac cycle. J Clin Exp Cardiol. 2014;5(288):2. [Google Scholar]
- 11.Menon PG, Adhyapak SM, Parachuri VR. Analysis of cardiac MRI based regional timing of left ventricular mechanical contraction as a biomarker for electrical dyssynchrony in heart-failure patients. In: Zhang YJ, Tavares YMRS, editors. Computational Modeling of Objects Presented in Images. Fundamentals, Methods, and Applications. Pittsburgh, PA: Springer International Publishing; 2014. pp. 48–56. [Google Scholar]
- 12.Kukulski T, Hubbert L, Arnold M, Wranne B, Hatle L, Sutherland GR. Normal regional right ventricular function and its change with age: a Doppler myocardial imaging study. J Am Soc Echocardiogr. 2000;13:194–204. doi: 10.1067/mje.2000.103106. [DOI] [PubMed] [Google Scholar]
- 13.Chen EP, Craig DM, Bittner HB, Davis RD, Van Trigt P. Pharmacological strategies for improving diastolic dysfunction in the setting of chronic pulmonary hypertension. Circulation. 1998;97:1606–12. doi: 10.1161/01.cir.97.16.1606. [DOI] [PubMed] [Google Scholar]
- 14.Louie EK, Lin SS, Reynertson SI, Brundage BH, Levitsky S, Stuart S. Pressure and volume loading of the right ventricle have opposite effects on left ventricular ejection fraction. Circulation. 1995;92:819–24. doi: 10.1161/01.cir.92.4.819. [DOI] [PubMed] [Google Scholar]
- 15.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 16.López-Candales A, Dohi K, Bazaz R, Edelman K. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue Doppler imaging. Am J Cardiol. 2005;96:602–6. doi: 10.1016/j.amjcard.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 17.López-Candales A, Dohi K, Rajagopalan N, et al. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound. 2005;3:23. doi: 10.1186/1476-7120-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueti OM, Camargo EE, Ueti Ade A, de Lima-Filho EC, Nogueira EA. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88:244–8. doi: 10.1136/heart.88.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Candales A, Rajagopalan N, Saxena N, Gulyasy B, Edelman K, Bazar R. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006;98:973–7. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Dellegrottaglie S, Sanz J, Poon M, et al. Pulmonary hypertension: accuracy of detection with left ventricular septal-to-free wall curvature ratio measured at cardiac MR. Radiology. 2007;243(1):63–9. doi: 10.1148/radiol.2431060067. [DOI] [PubMed] [Google Scholar]
- 21.Sanz J, Kuschnir P, Rius T, et al. Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology. 2007;243(1):70–9. doi: 10.1148/radiol.2431060477. [DOI] [PubMed] [Google Scholar]