Abstract
Aim
To compare and evaluate the treatment outcomes of a nurse-led rheumatology clinic and a rheumatologist-led clinic in patients with low disease activity or in remission who are undergoing biological therapy.
Background
Patients with chronic inflammatory arthritis treated with biological therapy are usually monitored by rheumatologists. Nurse-led rheumatology clinics have been proposed in patients with low disease activity or in remission.
Design
Randomized controlled trial.
Methods
A 12-month follow-up trial was conducted between October 2009 and August 2011, where 107 patients were randomized into two groups with a 6-month follow-up to a nurse-led rheumatology clinic based on person-centred care (intervention group; n = 53) or to a rheumatologist-led clinic (control group; n = 54). The hypothesis was that the nurse-led clinic outcomes would not be inferior to those obtained from a rheumatologist-led clinic at the 12-month follow-up. The primary outcome was disease activity measured by Disease Activity Score 28.
Results
A total of 47 patients in the intervention group and 50 in the control group completed the 12-month trial. The trial revealed no statistically significant differences between groups in mean change of Disease Activity Score 28, Visual Analogue Scales for pain, the Health Assessment Questionnaire, satisfaction with or confidence in obtaining rheumatology care.
Conclusion
Patients with stable chronic inflammatory arthritis undergoing biological therapy could be monitored by a nurse-led rheumatology clinic without difference in outcome as measured by the Disease Activity Score 28.
Keywords: biological therapy, intervention, nurse-led rheumatology clinic, person-centred care, randomized controlled trial
Introduction
The primary goal of treating patients with chronic inflammatory arthritis (CIA) is remission or low disease activity achieved by controlling the symptoms and inflammation (Smolen et al. 2010, Braun et al. 2011, Gossec et al. 2012). The term ‘CIA’ refers to rheumatoid arthritis (RA) and spondyloarthritis (van Eijk-Hustings et al. 2012). Cut-off points have been developed to determine whether a patient is in clinical remission or a state of low disease activity. No single instrument can adequately describe the disease process for every patient. Disease activity is, therefore, evaluated by composite measures comprising patient- and practitioner-reported outcomes. One frequently used composite measure is the Disease Activity Score 28 (DAS28) (Fransen et al. 2004). Previous research has led to the development of biological therapy for patients with an inadequate response to traditional disease-modifying anti-rheumatic drug (DMARD) therapies. Biological DMARD therapies have transformed the rheumatology landscape and are rapidly becoming more common as new therapies are developed for a greater number of indications (Furst et al. 2013). Previous research has shown that biological therapy leads to improvements in disease activity, health status, physical function and quality of life (Nam et al. 2010). Disease activity and inflammation in patients with CIA have declined over the past decade since the introduction of biological therapy (Simard et al. 2011).
In Sweden, patients with CIA undergoing biological therapy are usually monitored by a rheumatologist every 6 months to evaluate the effect of the medication and the disease activity measured by the DAS28. Data are stored in the Swedish Rheumatology Quality (SRQ) register (van Vollenhoven & Askling 2005, Ovretveit et al. 2013). Biological therapies are either intravenous infusions administered by a nurse or self-administered subcutaneous injections. Patients perceive the regular contact with a nurse in conjunction with the infusions as secure, invigorating and leading to involvement (Larsson et al. 2009). When treated with subcutaneous injections, a nurse teaches the patients how to administer the injection, but some patients report missing regular contact with a nurse (Larsson et al. 2010). The nurse's role has developed into that of an expert with in-depth nursing knowledge and competence of how to provide evidence-based care and support patients to become co-actors in the care (Arvidsson et al. 2003, Palmer & El Miedany 2010, Oliver 2011). Collaboration between patient and nurse is a prerequisite for participation and making patients co-actors (Sahlsten et al. 2008). In qualitative studies, patients have described how nurses provide added value to patient care by their holistic approach. The nurse creates familiarity and patients dare to open up to a nurse who listens attentively to their problems (Arvidsson et al. 2006, Ryan et al. 2006, Larsson et al. 2012).
Background
Nurse-led clinics are established as a complement to physician-led clinics in the management of, for example, cardiovascular and pulmonary diseases as well as diabetes and cancer (Stromberg et al. 2001, Kirby 2005, Cooper et al. 2010, Chin et al. 2011). Patients experienced greater well-being and satisfaction with the care in nurse-led clinics as well as improved quality of care (Wong et al. 2005, Lewis et al. 2009, Clark et al. 2010, Chin et al. 2011, Schadewaldt & Schultz 2011). To enhance the quality of care by means of a more holistic approach, nurse-led rheumatology clinics have been proposed for patients with low disease activity or in remission who are undergoing biological therapy (Palmer & El Miedany 2010, Oliver 2011). Nurse-led clinics will enable the rheumatologist to prioritize and allocate more time to patients with early RA or high disease activity who require more frequent monitoring or change in medication, leading to the desired treatment outcome (Grigor et al. 2004, Schipper et al. 2012).
A systematic review (Ndosi et al. 2011) only identified four small UK and Dutch randomized controlled trials (RCTs) focusing on nurse-led rheumatology clinics that employ traditional therapies among patients with RA. Patients who visit a nurse-led rheumatology clinic every or every second month report a high level of satisfaction. They also have greater knowledge of the disease and treatment in addition to positive results in terms of disease activity, functioning and health as well as less pain. A more extensive RCT (n = 287) from Denmark demonstrated that nurse-led consultations increased self-efficacy beliefs in patients with established RA who received traditional therapies. Disease activity, physical disability, pain and fatigue did not differ over 1 year between nurse-led follow-up, traditional monitoring by a rheumatologist and no planned consultation (Primdahl et al. 2012). When reviewing the literature, no trial was found within rheumatological care that focused on comparing treatment outcomes from a nurse-led rheumatology clinic, where every second visit to the rheumatologist was replaced by one to a rheumatology nurse in patients undergoing biological therapy who had low disease activity or were in remission. Accordingly, the hypothesis of this RCT was that the treatment outcomes measured by the DAS28 in patients with low disease activity or in remission, undergoing biological therapy at a nurse-led clinic, would not be inferior to those of a rheumatologist-led clinic at the 12-month follow-up.
The study
Aim
The aim of this trial was to compare and evaluate treatment outcomes of a nurse-led rheumatology clinic and a rheumatologist-led clinic in patients with low disease activity or in remission undergoing biological therapy.
Methods
Design
An RCT was designed and the intention was to replace one of the two annual rheumatologist monitoring visits by a nurse-led rheumatology monitoring visit in patients undergoing biological therapy. Patients with CIA undergoing biological therapy with low disease activity or in remission completed a pre-test before inclusion, a test at the 6-month and the 12-month follow-up visit. The trial was conducted at a rheumatology clinic in Sweden. The hospital had 30 inpatient beds for patients with rheumatic diseases and 5500 outpatient visits annually for 3500 patients, of whom 600 were undergoing biological therapy.
Participants
Inclusion criteria were patients with CIA, undergoing biological therapy and DAS28 ≤3·2. In this trial, patients with RA, undifferentiated arthritis, undifferentiated spondyloarthritis (USpA) and psoriatic arthritis (PsA) were included if they had peripheral arthritis. Patients with recurrent infections or adverse events due to the biological therapy were excluded. A total of 270 patients were assessed by a rheumatologist, of whom 125 met the inclusion criteria and were invited to participate. The participants who agreed to take part in the trial (n = 107) were randomly assigned to either the nurse-led rheumatology clinic (intervention group; n = 53) or the rheumatologist-led clinic (control group; n = 54). Randomization took the form of sealed envelopes containing assignment to one of the two groups. The envelopes were mixed and when a patient met the inclusion criteria, an envelope was randomly picked. At inclusion, 18 patients decided not to participate, nine men and nine women (Figure 1), mean age 46·4 years (sd 17·1, 25–72 years) and a mean disease duration of 12·8 years (sd 8·9, 1–35 years). Those who agreed to participate were significantly older (P < 0·01), but there were no other significant differences in demographic and clinical characteristics between the participants and non-participants.
Figure 1.
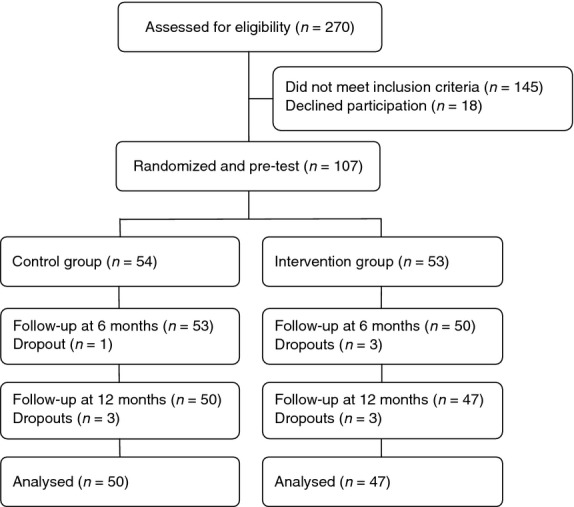
Flow chart of the participants in the study.
A pre-trial power analysis was based on the primary outcome DAS28 score. A mean difference of 0·6 was considered a moderate improvement, while a difference of 1·2 was deemed clinically significant or good (van Riel et al. 1996). Based on a change of 0·6 in the DAS28 score and a sd of 1·0 (Rezaei et al. 2012), the power analysis demonstrated that 95 patients would be a sufficient number to detect a clinically moderate difference between groups at a 5% significance level with at least 90% power. It was decided to include 107 patients to allow for the predicted 10% dropout. The primary outcome measure was change in the DAS28 over a 12-month period.
Intervention
Rheumatologist-led clinic
The usual care for patients with CIA undergoing biological therapy in Sweden is monitoring by a rheumatologist every 6 months for 30 minutes to evaluate the effect of the medication and the disease activity measured by the DAS28 (van Vollenhoven & Askling 2005). The rheumatologist assessed disease activity by examining tender and swollen joints based on a 28-joint count in addition to evaluating the results of laboratory tests. The patients were able to contact the rheumatology clinic between the scheduled follow-up visits.
Nurse-led rheumatology clinic
A nurse-led rheumatology clinic based on person-centred care focusing on patient needs was designed. The purpose of person-centred care is to give patients the opportunity to talk about themselves as a person and to allow their illness narrative to constitute a starting point for building collaboration, which encourages and empowers them to play an active role in their biological therapy and find solutions to their problems (Ekman et al. 2011). Patients were monitored for 30 minutes by a rheumatology nurse after 6 months, followed by 30 minutes monitoring by a rheumatologist after 12 months. The patients had the opportunity to contact the nurse during the 12-month trial period. The nurse assessed the patients' disease activity by examining tender and swollen joints based on the 28-joint count in addition to evaluating the results of laboratory tests in the same way as a rheumatologist. Drug treatment was discussed in terms of administration, adherence, side effects and laboratory tests as well as patients' global health. Patients' narratives constituted the starting point of a dialogue to meet individual needs and included lifestyle and psychosocial aspects. Such narratives created a common understanding of the illness experience, which, together with the symptoms of disease, provided the nurse with a good foundation for discussing and planning care and treatment with the patients (Ekman et al. 2011). Five Registered Nurses with 22–39 years' professional experience and 9–20 years' experience of managing rheumatic diseases in both inpatient and outpatient rheumatology care participated in the trial. They had undergone special training from a rheumatologist and RA instructors to assess swollen and tender joints based on the 28-joint count to make an evidence-based assessment of disease activity. RA instructors are specially trained patients who instruct healthcare staff how to examine the joints of the hands, wrists, feet and ankles, and provide information about living with the disease. If necessary, the nurse could contact the rheumatologist for advice or to obtain a prescription.
Data collection
Data collection took place between October 2009–August 2011. Data were collected at baseline, 6 and 12 months and entered into the SRQ register. The monitoring by the rheumatology nurse (intervention group) and the rheumatologist (control group) included an assessment of the number of swollen and tender joints based on the DAS28. The participants indicated their perceived global health the previous week (0–100, best to worst) on a 100-mm Visual Analogue Scale (VAS). The Health Assessment Questionnaire (HAQ), VAS for pain and the Numerical Rating Scale (NRS) for assessment of satisfaction with and confidence in obtaining rheumatology care were used. An assessment of disease activity, medication record, employment status and any adverse events were also documented. The primary outcome was disease activity measured by the DAS28. All patients were monitored by the rheumatologist at baseline and after 12 months.
Instruments
The DAS28 is a validated index of RA disease activity (Prevoo et al. 1995) and a composite measurement comprising patient-reported (number of tender joints based on the 28-joint count and global assessment, VAS for global health) and practitioner-reported [number of swollen joints based on the 28-joint count and erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP)] scores. The outcome of the DAS28 is a number on a scale from 0 to 10, where the values >5·1, <3·2 and <2·6 indicate high disease activity, low disease activity and remission respectively (Fransen et al. 2004). The DAS28 is also used to measure disease activity in other inflammatory joint diseases, such as peripheral PsA and USpA (Fransen et al. 2006, Saber et al. 2010, Glintborg et al. 2011), and constitutes a variable for evaluating disease activity in patients treated by means of biological therapy (Vander Cruyssen et al. 2005). In this trial, the abbreviation DAS28 was used when the calculation included the ESR, while DAS28-CRP was employed when the calculation included CRP, and the correlation between them was found to be good (Wells et al. 2009).
The HAQ is a self-administered, disease-specific questionnaire for assessing activity limitation in patients with arthritis. It measures the ability to perform 20 items and assesses the degree of difficulty involved in performing activities of daily living during the previous week. The activities are grouped into eight categories of functioning: dressing, rising, eating, walking, hygiene, reach, grip and usual activities. The total score ranges from 0–3 and a higher score indicates a greater degree of disability (Ekdahl et al. 1988).
The VAS was used to assess pain during the previous week (VAS 0–100 mm). The anchor points of the scale are 0 (no pain) and 100 (worst possible pain) (Joos et al. 1991).
The level of satisfaction with and confidence in the rheumatology care was assessed by the NRS scored from 0 to 10. The questions were, ‘How satisfied are you with the rheumatology care?’ The anchor points of the scale are 0 (not at all satisfied) and 10 (completely satisfied). ‘How confident are you of obtaining help from your rheumatology clinic when you have joint problems?’ The anchor points of the scale are 0 (no confidence and 10 (complete confidence) (Moe et al. 2010, Eriksen et al. 2011).
Validity and reliability
Validated instruments with good psychometric properties were used to enhance the validity and reliability of the data. The instruments in the SRQ register intended to provide a standardized evaluation of the clinical and research aspects involved in monitoring the biological therapy (van Vollenhoven & Askling 2005). The DAS28, HAQ and VAS pain are well-validated (Ekdahl et al. 1988, Joos et al. 1991, Prevoo et al. 1995) and have been used in routine disease assessments for measuring the response level of biological therapy in many clinical settings (e.g. Europe) (Furst et al. 2013).
Ethical considerations
The regional Ethics Committee at Lund University, Sweden, approved the trial (No. 2009/245). The research conforms to the ethical principles for medical research on human beings set out in the declaration of Helsinki (WMA 2008) and the national guidelines on ethical principles (Swedish Research Council 2011). According to the Northern Nurses' Federation (2003), this trial fulfils the four requirements on research: information, consent, confidentially and safety of the participant (Northern Nurses' Federation 2003). Written informed consent was obtained from patients at their first visit before inclusion in the trial. They were advised about their right to withdraw at any time. This trial was registered at http://clinicaltrials.gov under the identification code NCT01071447.
Data analysis
Statistical analyses were performed using SPSS version 19.0 for Windows. Differences in disease activity, activity limitation, pain, satisfaction with and confidence in obtaining rheumatology care between groups were analysed by means of an independent sample t-test and within the groups by a paired t-test. Due to the properties of the scales, VAS Pain, VAS Global health, HAQ, NRS Satisfaction and NRS Confidence were analysed by both parametric (t-test) and non-parametric (Mann–Whitney U test and Wilcoxon signed ranks test) methods. Values of P < 0·05 were considered statistically significant.
Results
Participant characteristics
In all, 97 participants completed the trial. The baseline characteristics of the intervention and control groups as well as the dropouts are presented in Table 1 in terms of sociodemographic, clinical and outcome variables (Table 1). There were similar numbers of men and women in the intervention group (45% vs. 55%) and the control group (44% vs. 56%), but a greater difference within the dropout group (30% vs. 70%). The mean age was 55·0 (sd 12·3, range 34–81 years) in the intervention group and 55·8 years (sd 13·2, range 21–77 years) in the control group. The dropouts were slightly younger with a mean age of 51·1 years (16·0, range 24–70 years). After 6 months, 50 patients (94%) in the intervention group were monitored by a rheumatology nurse at the follow-up visit, while 53 patients (98%) in the control group were monitored by the rheumatologist. In total, 47 patients (89%) in the intervention group and 50 patients (93%) in the control group completed the 12-month trial. The reasons for dropping out were medical (changed therapy or other diseases), moving to another area and death. The flow chart depicted in Figure 1 visualizes the flow during the trial.
Table 1.
Baseline sociodemographic and clinical characteristics of the intervention and control groups including dropouts
| Characteristics | Intervention group | Control group | Dropouts |
|---|---|---|---|
| (n = 47) | (n = 50) | (n = 10) | |
| Sex | |||
| Male | 21 (45%) | 22 (44%) | 3 (30%) |
| Female | 26 (55%) | 28 (56%) | 7 (70%) |
| Age (years) | |||
| Mean age (sd) | 55·0 (12·3) | 55·8 (13·2) | 51·1 (16·0) |
| Range | 34–81 | 21–77 | 24–70 |
| Civil status | |||
| Living alone | 12 (26%) | 11 (22%) | 4 (40%) |
| Co-habiting | 35 (74%) | 39 (78%) | 6 (60%) |
| Education | |||
| Primary school | 15 (32%) | 14 (28%) | 5 (50%) |
| Secondary school | 15 (32%) | 15 (30%) | 3 (30%) |
| Third level education | 17 (36%) | 21 (42%) | 2 (20%) |
| Rheumatic disease | |||
| Rheumatoid arthritis (RA) | 25 (53%) | 35 (70%) | 8 (80%) |
| Undifferentiated arthritis (UA) | 1 (2%) | 3 (6%) | 0 |
| Undifferentiated spondyloarthritis (USpA) | 10 (21%) | 6 (12%) | 1 (10%) |
| Peripheral psoriatic arthritis (PsA) | 11 (23%) | 6 (12%) | 1 (10%) |
| Disease duration (years) | |||
| Mean disease duration (sd) | 17·3 (10·9) | 16·2 (12·1) | 14·0 (6·9) |
| Range | 1–44 | 1–52 | 1–21 |
| Disease activity DAS28 | |||
| Mean (sd) | 1·97 (0·67) | 2·14 (0·71) | 2·39 (0·60) |
| Range | 0·61–3·20 | 0·53–3·06 | 1·47–3·13 |
| Disease activity DAS28-CRP | |||
| Mean (sd) | 2·43 (0·58) | 2·53 (0·64) | 2·65 (0·49) |
| Range | 1·74–4·10 | 1·71–4·12 | 2·02–3·23 |
| Health Assessment Questionnaire (HAQ) | |||
| Mean (sd) | 0·45 (0·42) | 0·63 (0·55) | 0·43 (0·59) |
| Range | 0–2·13 | 0–2·50 | 0–1·88 |
| VAS Pain (mm) | |||
| Mean (sd) | 17·8 (11·5) | 24·2 (22·2) | 17·2 (16·9) |
| Range | 0–48 | 0–79 | 0–59 |
| Satisfaction (0–10) | |||
| Mean (sd) | 9·6 (0·80) | 9·4 (1·28) | 9·8 (0·42) |
| Range | 7–10 | 3–10 | 9–10 |
| Confidence (0–10) | |||
| Mean (sd) | 9·7 (0·66) | 9·3 (1·45) | 9·8 (0·63) |
| Range | 7–10 | 2–10 | 8–10 |
Primary outcome
Disease activity was assessed by the DAS28 and DAS28-CRP total score. There were no statistically significant differences in changes in the DAS28 (P = 0·66) or DAS28-CRP (P = 0·70) between the intervention and control group. The DAS28 is calculated using the results of the number of swollen and tender joints based on the 28-joint count, the VAS for global health and the ESR or CRP. There was a statistically significant, but not clinically relevant, difference in mean change of the individual CRP measurement (P = 0·03) between the intervention and the control group. In each of the other individual measurements, there were no significant mean changes between the groups.
Within both groups, there were small, but not clinically relevant, deteriorations in the DAS28 score (intervention group 0·14; P = 0·19; control group 0·20; P = 0·048) between baseline and the 12-month follow-up. Within the control group, there were small, but not clinically relevant, deteriorations in CRP (1·20; P = 0·01) between baseline and the 12-month follow-up. In each of the other individual measurements (number of swollen and tender joints based on the 28-joint count, VAS for global health and ESR), there were no significant mean changes within the groups (Table 2).
Table 2.
Comparison of mean change after 12 months between and within the intervention group (Nurse-led rheumatology clinic) (n = 47) and control group (Rheumatologist-led clinic) (n = 50)
| Intervention group – Control group | Intervention group | Control group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference of change | (95% CI) | P | Mean change | (95% CI) | P | Mean change | (95% CI) | P | |
| DAS28 | −0·06 | −0·34, 0·22 | 0·66 | 0·14 | −0·07, 0·34 | 0·19 | 0·20 | 0·00, 0·39 | 0·05 |
| DAS28-CRP | 0·05 | −0·28, 0·19 | 0·70 | 0·14 | −0·03, 0·31 | 0·10 | 0·10 | −0·07, 0·26 | 0·24 |
| ESR (mm/h) | −1·05 | −3·97, 1·86 | 0·47 | 1·09 | −0·40, 2·57 | 0·15 | 2·14 | −0·36, 4·64 | 0·09 |
| CRP (mg/L) | −1·07 | −2·02, −0·12 | 0·03 | 0·13 | −0·10, 0·35 | 0·26 | 1·20 | 0·29, 2·10 | 0·01 |
| Swollen joints (28) | 0·13 | −2·18, 0·61 | 0·60 | 0·13 | −0·27, 0·53 | 0·52 | 0·00 | −0·29, 0·29 | 1·00 |
| Tender joints (28) | 0·33 | −0·47, 1·13 | 0·42 | 0·47 | −0·05, 0·99 | 0·08 | 0·14 | −0·47, 0·75 | 0·65 |
| VAS Global health (mm)* | 4·29 | −2·58, 11·16 | 0·22 | 2·49 | −2·59, 7·56 | 0·33 | −1·80 | −6·57, 2·97 | 0·45 |
| VAS Pain (mm)* | −0·24 | −7·89, 7·40 | 0·95 | 0·98 | −4·74, 6·69 | 0·73 | 1·22 | −4·02, 6·47 | 0·64 |
| HAQ* | 0·02 | −0·10, 0·13 | 0·79 | 0·04 | −0·04, 0·12 | 0·34 | 0·02 | −0·05, 0·10 | 0·51 |
| NRS Satisfaction* | 0·25 | −0·37, 0·88 | 0·43 | −0·19 | −0·57, 0·18 | 0·31 | −0·44 | −0·94, 0·06 | 0·09 |
| NRS Confidence* | 0·20 | −0·29, 0·69 | 0·42 | 0·00 | −0·22, 0·22 | 1·00 | −0·20 | −0·63, 0·23 | 0·36 |
Independent sample t-test for comparison between and Paired t-test for comparison within the groups.
Also analysed by Mann–Whitney U test and Wilcoxon signed ranks test with non-significant (P > 0·05) results.
DAS, Diseases Activity Score (scale 0–10); ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; Swollen and Tender joint (28 count); VAS, Visual Analogue Scales, VAS Global health (scale 0–100 best to worse), VAS Pain (scale 0–100 best to worse); HAQ, Health Assessment Questionnaire (0–3 best to worse); NRS, Numerical Rating Scale, NRS Satisfaction and NRS Confidence (0–10 worse to best).
Secondary outcomes
Physical difficulties in performing activities of daily living were measured by the HAQ. There were no statistically significant (P = 0·79) differences in mean change after 12 months in the HAQ or in pain assessed by VAS (P = 0·95) between the intervention and the control group. Nor were there any statistically significant differences between the groups after 12 months in terms of satisfaction with (P = 0·43) and confidence (P = 0·42) in obtaining rheumatology care. Within the intervention group and the control group, there were no statistically significant mean changes in HAQ, VAS pain, satisfaction with or confidence in obtaining rheumatology care (Table 2).
Discussion
The hypothesis of this RCT was that the treatment outcomes measured by the DAS28 at a nurse-led clinic would not be inferior to those from a rheumatologist-led clinic at the 12-month follow-up. This hypothesis was supported by the trial. There were no differences in the treatment outcomes measured by DAS28 at a nurse-led clinic compared with a rheumatologist-led clinic at the 12-month follow-up. Monitoring of the patients' biological therapy in the nurse-led rheumatology clinic resulted in no deterioration compared with monitoring in the rheumatologist-led clinic. When comparing disease activity between the two groups, the primary outcome revealed no significant difference. The main finding of this trial was that follow-up care by a rheumatology nurse for patients with stable CIA can be delivered with comparable safety and effectiveness to that provided by a rheumatologist. Similar results were found in other RCTs of nurse-led clinics and in different patient groups where the visit to a physician was replaced by one to a nurse (Nathan et al. 2006, Schadewaldt & Schultz 2011). A nurse-led rheumatology clinic based on person-centred care is one way to implement the European League Against Rheumatism (EULAR) recommendations pertaining to the role of nurses in the management of CIA, which state that, ‘Nurses should participate in comprehensive disease management to control disease activity, to reduce symptoms and to improve patient-preferred outcomes’ (van Eijk-Hustings et al. 2012, p. 15). A nurse-led clinic can meet the EULAR recommendations, as it provides patients with improved knowledge of CIA and its management as well as enhancing communication, continuity and satisfaction with care (van Eijk-Hustings et al. 2012). Other nurse-led clinics have reported similar clinical outcomes with high levels of satisfaction with consultations undertaken by a nurse compared to a physician (Stables et al. 2004, Laurant et al. 2005). The result of this trial did not reveal any differences in satisfaction with or confidence in obtaining care between the two groups. This may be due to the fact that patients in the intervention group were monitored just once by the nurse during the 12 months. Nevertheless, the hypothesis was supported by the trial and the nurse-led clinic intervention based on person-centred care with focus on the whole person proved safe and purposeful. The satisfactory results of the trial could be due to the nurse assessing the disease activity from a person-centred perspective and basing the care on patients' view of their life situation. Medical assessment is important and should form the basis of care, but the patients' own experiences of their condition must also be taken into consideration. The nurse's role is to provide care based on the patient's needs and to support her/him on the healthcare journey (Oliver 2011).
This trial has demonstrated that there is no clinically relevant difference in outcomes between a nurse-led rheumatology clinic and a rheumatologist-led clinic in patients with low disease activity or in remission, undergoing biological therapy. Previous research has revealed that care provided by a nurse-led clinic has a similar long-term clinical outcome to inpatient and day patient team care in patients with RA and is thus an effective innovation (Tijhuis et al. 2003). Data indicate that a holistic approach to patient care is an important element of good nursing in nurse-led clinics. Nurses acknowledge each patient as a unique person and try to develop a holistic understanding of the patients' life context (Shiu et al. 2012). Person-centred care includes a holistic approach and as an RCT does not capture the qualitative aspects, this RCT was complemented by a qualitative study. Larsson et al. (2012) interviewed patients about their experiences of the encounter with the nurse and the patients reported that such encounters led to a sense of security, familiarity and participation. A nurse-led rheumatology clinic for biological therapy contributed added value for patients within rheumatology care, which became more complete by replacing every second visit to a rheumatologist with one to a nurse. A nurse and a rheumatologist complement each other, as they encounter the patients from different perspectives (Larsson et al. 2012). This is consistent with experiences of patients from nurse-led clinics in diabetes care (Edwall et al. 2008).
Today, the role of the nurse involves comprehensive duties, but ultimately enhanced care is achieved by a more holistic approach (Palmer & El Miedany 2010) and person-centred care that supports patients in making an informed decision to improve their well-being. In essence, the expertise of the nurse specialist is multifaceted, involving a number of important components such as regular assessment of disease activity, support, information sharing, coordination and continuity of care (Oliver 2011).
Limitations
Due to the growing demand for evidence-based practice, RCTs are considered the best method for testing the effect of nursing interventions, as they yield strong evidence. Through randomization and the use of a comparison group, i.e. standard care, the trial was as close as possible to clinical practice. RCTs are sometimes criticized for their artificiality (Polit & Beck 2012), but the present intervention can be directly integrated into the clinical routine. To strengthen internal validity, it is important to ensure that the intervention nurses cannot influence aspects of the process such as recruitment, randomization or data analysis (Lindsay 2004). In this trial, the nurses did not participate in any aspect of the research process other than the intervention. The researchers were not involved in the intervention or assessment of the patients. Furthermore, data were registered in the SRQ using validated instruments. Reliability refers to the extent to which results are consistent over time and whether the result of a trial can be reproduced with a similar methodology (Polit & Beck 2012). The structure of the nurse-led clinic was designed for the trial, and both the nurse and the rheumatologist followed national and international management and treatment guidelines (van Vollenhoven & Askling 2005); thus, this trial can be reproduced with a similar methodology. A limitation is the short time perspective (12 months), as a 2-year follow-up would better identify the long-term effects. A threat to reliability is loss of data due to dropout (Polit & Beck 2012). In this trial, 107 patients were enrolled from 125 potential participants (86%). The retention rate was high with 90% of the patients completing the 12-month follow-up. The dropout rate of 11% in the intervention group and 7% in the control group is acceptable and understandable.
Research indicates that a DAS28 measurement by a nurse is as effective as standard care provided by a rheumatologist (van Hulst et al. 2010). In this trial, the rheumatology nurses had between 9–20 years' experience of managing rheumatology diseases in both inpatient and outpatient settings and had received education from a rheumatologist and RA instructors. Although nurses' examination of patients' joints is fairly new in Sweden, this is not the case in other countries where nurses have assessed the joints of patients for decades (Hill 1997, Temmink et al. 2001). External validity concerns the extent to which evidence from RCT settings can be generalized to real-world clinical practice (Polit & Beck 2012). Previous research on traditional therapies indicates that consultation with an expert rheumatology nurse in a drug monitoring clinic may add value in terms of improving patients' perceived ability to cope with arthritis (Ryan et al. 2006). Implementation of nursing consultations as part of follow-up care in patients with stable RA is recommended (Primdahl et al. 2012). This trial comprised patients with CIA and low disease activity or in remission, thus a broader patient population than in previous research, which in most cases only included patients with RA.
What is already known about this topic
Rheumatology nurses are able to assess the patients' disease activity by examining tender and swollen joints when monitoring traditional therapies.
Regular contact with a nurse every 1–3 months at a nurse-led rheumatology clinic adds value to care for patients with rheumatoid arthritis treated by means of traditional therapies.
What this paper adds
Patients with stable chronic inflammatory arthritis undergoing biological therapy could be monitored by a nurse-led rheumatology clinic without any difference in outcome as measured by the Disease Activity Score 28.
Replacing one of the two annual rheumatologist visits by a nurse-led visit is safe and effective when monitoring biological therapy.
This randomized controlled trial demonstrated that a nurse-led rheumatology clinic, based on person-centred care, provides favourable follow-up care in patients with stable chronic inflammatory arthritis undergoing biological therapy.
Implications for practice
The follow-up care of patients undergoing biological therapy could be developed by a nurse-led rheumatology clinic based on person-centred care as a complement to a rheumatologist-led clinic.
A nurse-led rheumatology clinic based on person-centred care is one way of implementing the European League Against Rheumatism recommendations regarding the nurse's role in the management of chronic inflammatory arthritis.
This study indicates that a nurse-led rheumatology clinic should be made available to a broader patient population and not only to patients with rheumatoid arthritis in rheumatology units.
Conclusions
The nurse-led rheumatology clinic intervention based on person-centred care focusing on the whole person proved safe and purposeful. In monitoring of biological therapy treatment, outcomes of patients at a nurse-led rheumatology clinic were not inferior to those from a rheumatologist-led clinic at the 12-month follow-up. Patients with CIA undergoing biological therapy, with low disease activity or in remission, could be monitored by a nurse-led rheumatology clinic without any difference in outcome as measured by DAS28.
The clinical importance of this trial is the finding that biological therapy follow-up care can be effectively performed by a nurse-led clinic based on person-centred care. Thus, replacing every second visit to a rheumatologist by one to a rheumatology nurse facilitates the implementation of such clinics in everyday practice. If rheumatology nurses assume more comprehensive roles after specialized training, this trial would be applicable to the monitoring of therapies other than biological therapy and thus to a broader population. Further research is needed to evaluate the long-term effect and cost-effectiveness of a nurse-led rheumatology clinic for monitoring various therapies for patients with CIA.
Acknowledgments
The authors would like to thank the patients, nurses and rheumatologists who participated in this trial.
Funding
The authors thank the Swedish Rheumatism Association, the Norrbacka-Eugenia Foundation, the Rheumatism District of Gothenburg, the South Regional Health Care Committee, the Swedish Association of Health Professionals, the Association of Rheumatology Nurses in Sweden and the Inger Bendix Foundation for Medical Research for financial support.
Conflict of interest
No conflict of interest has been declared by the authors.
Author contributions
All authors have agreed on the final version and meet at least one of the following criteria (recommended by the ICMJE: http://www.icmje.org/ethical_1author.html):
substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
drafting the article or revising it critically for important intellectual content.
References
- Arvidsson B, Jacobsson L. Petersson IF. Rheumatology care in Sweden-the role of the nurse. Musculoskeletal Care. 2003;1(2):81–83. doi: 10.1002/msc.43. [DOI] [PubMed] [Google Scholar]
- Arvidsson SB, Petersson A, Nilsson I, Andersson B, Arvidsson BI, Petersson IF. Fridlund B. A nurse-led rheumatology clinic's impact on empowering patients with rheumatoid arthritis: a qualitative study. Nursing and Health Sciences. 2006;8(3):133–139. doi: 10.1111/j.1442-2018.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H, Dijkmans B, Dougados M, Emery P, Geher P, Hammoudeh M, Inman RD, Jongkees M, Khan MA, Kiltz U, Kvien T, Leirisalo-Repo M, Maksymowych WP, Olivieri I, Pavelka K, Sieper J, Stanislawska-Biernat E, Wendling D, Ozgocmen S, van Drogen C, van Royen B. van der Heijde D. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Annals of the Rheumatic Diseases. 2011;70(6):896–904. doi: 10.1136/ard.2011.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin WY, Lam CL. Lo SV. Quality of care of nurse-led and allied health personnel-led primary care clinics. Hong Kong Medical Journal. 2011;17(3):217–230. [PubMed] [Google Scholar]
- Clark CE, Smith LF, Taylor RS. Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM, Loeb SJ. Smith CA. The primary care nurse practitioner and cancer survivorship care. Journal of the American Academy of Nurse Practitioners. 2010;22(8):394–402. doi: 10.1111/j.1745-7599.2010.00528.x. [DOI] [PubMed] [Google Scholar]
- Edwall LL, Hellstrom AL, Ohrn I. Danielson E. The lived experience of the diabetes nurse specialist regular check-ups, as narrated by patients with type 2 diabetes. Journal of Clinical Nursing. 2008;17(6):772–781. doi: 10.1111/j.1365-2702.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- van Eijk-Hustings Y, van Tubergen A, Bostrom C, Braychenko E, Buss B, Felix J, Firth J, Hammond A, Harston B, Hernandez C, Huzjak M, Korandova J, Kukkurainen ML, Landewe R, Mezieres M, Milincovic M, Moretti A, Oliver S, Primdahl J, Scholte-Voshaar M, de la Torre-Aboki J, Waite-Jones J, Westhovens R, Zangi HA, Heiberg T. Hill J. EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Annals of the Rheumatic Diseases. 2012;71(1):13–19. doi: 10.1136/annrheumdis-2011-200185. [DOI] [PubMed] [Google Scholar]
- Ekdahl C, Eberhardt K, Andersson SI. Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scandinavian Journal of Rheumatology. 1988;17(4):263–271. doi: 10.3109/03009748809098795. [DOI] [PubMed] [Google Scholar]
- Ekman I, Swedberg K, Taft C, Lindseth A, Norberg A, Brink E, Carlsson J, Dahlin-Ivanoff S, Johansson IL, Kjellgren K, Liden E, Ohlen J, Olsson LE, Rosen H, Rydmark M. Sunnerhagen KS. Person-centered care–ready for prime time. European Journal of Cardiovascular Nursing. 2011;10(4):248–251. doi: 10.1016/j.ejcnurse.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Eriksen K, Rochester RP. Hurwitz EL. Symptomatic reactions, clinical outcomes and patient satisfaction associated with upper cervical chiropractic care: a prospective, multicenter, cohort study. BMC Musculoskeletal Disorders. 2011;12:219. doi: 10.1186/1471-2474-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen J, Creemers MC. Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43(10):1252–1255. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- Fransen J, Antoni C, Mease PJ, Uter W, Kavanaugh A, Kalden JR. Van Riel PL. Performance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitors. Annals of the Rheumatic Diseases. 2006;65(10):1373–1378. doi: 10.1136/ard.2006.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE, Keystone EC, So A, Braun J, Breedveld FC, Burmester GR, De Benedetti F, Dorner T, Emery P, Fleischmann R, Gibofsky A, Kalden JR, Kavanaugh A, Kirkham B, Mease P, Rubbet-Roth A, Sieper J, Singer NG, Smolen JS, Van Riel PL, Weisman MH. Winthrop K. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Annals of the Rheumatic Diseases. 2013;72(Suppl II):i2–34. doi: 10.1136/annrheumdis-2011-201036. [DOI] [PubMed] [Google Scholar]
- Glintborg B, Ostergaard M, Dreyer L, Krogh NS, Tarp U, Hansen MS, Rifbjerg-Madsen S, Lorenzen T. Hetland ML. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registry. Arthritis & Rheumatism. 2011;63(2):382–390. doi: 10.1002/art.30117. [DOI] [PubMed] [Google Scholar]
- Gossec L, Smolen JS, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, van der Heijde D, FitzGerald O, Aletaha D, Balint P, Boumpas D, Braun J, Breedveld FC, Burmester G, Canete JD, de Wit M, Dagfinrud H, de Vlam K, Dougados M, Helliwell P, Kavanaugh A, Kvien TK, Landewe R, Luger T, Maccarone M, McGonagle D, McHugh N, McInnes IB, Ritchlin C, Sieper J, Tak PP, Valesini G, Vencovsky J, Winthrop KL, Zink A. Emery P. European League against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Annals of the Rheumatic Diseases. 2012;71(1):4–12. doi: 10.1136/annrheumdis-2011-200350. [DOI] [PubMed] [Google Scholar]
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, Kincaid W. Porter D. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–269. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- Hill J. The expanding role of the nurse in rheumatology. British Journal of Rheumatology. 1997;36(4):410–412. doi: 10.1093/rheumatology/36.4.410. [DOI] [PubMed] [Google Scholar]
- van Hulst LT, Creemers MC, Fransen J, Li LC, Grol R, Hulscher ME. van Riel PL. How to improve DAS28 use in daily clinical practice? – a pilot study of a nurse-led intervention. Rheumatology (Oxford) 2010;49(4):741–748. doi: 10.1093/rheumatology/kep407. [DOI] [PubMed] [Google Scholar]
- Joos E, Peretz A, Beguin S. Famaey JP. Reliability and reproducibility of visual analogue scale and numeric rating scale for therapeutic evaluation of pain in rheumatic patients. The Journal of Rheumatology. 1991;18(8):1269–1270. [PubMed] [Google Scholar]
- Kirby M. Extending nursing roles in diabetes to achieve clinical targets. Journal of Diabetes Nursing. 2005;9(6):231–235. [Google Scholar]
- Larsson I, Bergman S, Fridlund B. Arvidsson B. Patients' dependence on a nurse for the administration of their intravenous anti-TNF therapy: a phenomenographic study. Musculoskeletal Care. 2009;7(2):93–105. doi: 10.1002/msc.140. [DOI] [PubMed] [Google Scholar]
- Larsson I, Bergman S, Fridlund B. Arvidsson B. Patients' independence of a nurse for the administration of subcutaneous anti-TNF therapy: a phenomenographic study. International Journal of Qualitative Studies on Health and Well-being. 2010;5(2):5146. doi: 10.3402/qhw.v5i2.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson I, Bergman S, Fridlund B. Arvidsson B. Patients' experiences of a nurse-led rheumatology clinic in Sweden – a qualitative study. Nursing and Health Sciences. 2012;14(4):501–507. doi: 10.1111/j.1442-2018.2012.00723.x. [DOI] [PubMed] [Google Scholar]
- Laurant M, Reeves D, Hermens R, Braspenning J, Grol R. Sibbald B. Substitution of doctors by nurses in primary care. Cochrane Database Systematic Review. 2005;2:CD001271. doi: 10.1002/14651858.CD001271.pub2. [DOI] [PubMed] [Google Scholar]
- Lewis R, Neal RD, Williams NH, France B, Wilkinson C, Hendry M, Russell D, Russell I, Hughes DA, Stuart NS. Weller D. Nurse-led vs. conventional physician-led follow-up for patients with cancer: systematic review. Journal of Advanced Nursing. 2009;65(4):706–723. doi: 10.1111/j.1365-2648.2008.04927.x. [DOI] [PubMed] [Google Scholar]
- Lindsay B. Randomized controlled trials of socially complex nursing interventions: creating bias and unreliability? Journal of Advanced Nursing. 2004;45(1):84–94. doi: 10.1046/j.1365-2648.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Moe RH, Uhlig T, Kjeken I, Hagen KB, Kvien TK. Grotle M. Multidisciplinary and multifaceted outpatient management of patients with osteoarthritis: protocol for a randomised, controlled trial. BMC Musculoskeletal Disorders. 2010;11:253. doi: 10.1186/1471-2474-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, Worthy G, Landewe R, Smolen JS, Emery P. Buch MH. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Annals of the Rheumatic Diseases. 2010;69(6):976–986. doi: 10.1136/ard.2009.126573. [DOI] [PubMed] [Google Scholar]
- Nathan JA, Pearce L, Field C, Dotesio-Eyres N, Sharples LD, Cafferty F. Laroche CM. A randomized controlled trial of follow-up of patients discharged from the hospital following acute asthma: best performed by specialist nurse or doctor? Chest. 2006;130(1):51–57. doi: 10.1378/chest.130.1.51. [DOI] [PubMed] [Google Scholar]
- Ndosi M, Vinall K, Hale C, Bird H. Hill J. The effectiveness of nurse-led care in people with rheumatoid arthritis: a systematic review. International Journal of Nursing Studies. 2011;48(5):642–654. doi: 10.1016/j.ijnurstu.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Northern Nurses' Federation. Ethical guidelines for nursing research in the Nordic countries. Vård i Norden. 2003;23(4):1–19. [Google Scholar]
- Oliver SM. The role of the clinical nurse specialist in the assessment and management of biologic therapies. Musculoskeletal Care. 2011;9(1):54–62. doi: 10.1002/msc.190. [DOI] [PubMed] [Google Scholar]
- Ovretveit J, Keller C, Forsberg HH, Essen A, Lindblad S. Brommels M. Continuous innovation: developing and using a clinical database with new technology for patient-centred care – the case of the Swedish quality register for arthritis. International Journal for Quality in Health Care. 2013;25(2):118–124. doi: 10.1093/intqhc/mzt002. [DOI] [PubMed] [Google Scholar]
- Palmer D. El Miedany Y. Biological nurse specialist: goodwill to good practice. British Journal of Nursing. 2010;19(8):477–480. doi: 10.12968/bjon.2010.19.8.47632. [DOI] [PubMed] [Google Scholar]
- Polit DF. Beck CT. Nursing Research. Generating and Assessing Evidence for Nursing Practice. 9. Philadephia, PA: Wolters Kluwer Health/Lippincott Williams and Wilkins; 2012. [Google Scholar]
- Prevoo ML, Van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB. van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Primdahl J, Wagner L, Holst R. Horslev-Petersen K. The impact on self-efficacy of different types of follow-up care and disease status in patients with rheumatoid arthritis – a randomized trial. Patient Education and Counseling. 2012;88(1):121–128. doi: 10.1016/j.pec.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Rezaei H, Saevarsdottir S, Forslind K, Albertsson K, Wallin H, Bratt J, Ernestam S, Geborek P, Pettersson IF. van Vollenhoven RF. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2-year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Annals of the Rheumatic Diseases. 2012;71(2):186–191. doi: 10.1136/annrheumdis-2011-200038. [DOI] [PubMed] [Google Scholar]
- van Riel PL, van Gestel AM. van de Putte LB. Development and validation of response criteria in rheumatoid arthritis: steps towards an international consensus on prognostic markers. British Journal of Rheumatology. 1996;35(Suppl 2):4–7. doi: 10.1093/rheumatology/35.suppl_2.4. [DOI] [PubMed] [Google Scholar]
- Ryan S, Hassell AB, Lewis M. Farrell A. Impact of a rheumatology expert nurse on the wellbeing of patients attending a drug monitoring clinic. Journal of Advanced Nursing. 2006;53(3):277–286. doi: 10.1111/j.1365-2648.2006.03725.x. [DOI] [PubMed] [Google Scholar]
- Saber TP, Ng CT, Renard G, Lynch BM, Pontifex E, Walsh CA, Grier A, Molloy M, Bresnihan B, Fitzgerald O, Fearon U. Veale DJ. Remission in psoriatic arthritis: is it possible and how can it be predicted? Arthritis Research & Therapy. 2010;12(3):R94. doi: 10.1186/ar3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlsten MJ, Larsson IE, Sjostrom B. Plos KA. An analysis of the concept of patient participation. Nursing Forum. 2008;43(1):2–11. doi: 10.1111/j.1744-6198.2008.00090.x. [DOI] [PubMed] [Google Scholar]
- Schadewaldt V. Schultz T. Nurse-led clinics as an effective service for cardiac patients: results from a systematic review. International Journal of Evidence-Based Healthcare. 2011;9(3):199–214. doi: 10.1111/j.1744-1609.2011.00217.x. [DOI] [PubMed] [Google Scholar]
- Schipper LG, Vermeer M, Kuper HH, Hoekstra MO, Haagsma CJ, Broeder AA, Riel PV, Fransen J. van de Laar MA. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Annals of the Rheumatic Diseases. 2012;71(6):845–850. doi: 10.1136/annrheumdis-2011-200274. [DOI] [PubMed] [Google Scholar]
- Shiu AT, Lee DT. Chau JP. Exploring the scope of expanding advanced nursing practice in nurse-led clinics: a multiple-case study. Journal of Advanced Nursing. 2012;68(8):1780–1792. doi: 10.1111/j.1365-2648.2011.05868.x. [DOI] [PubMed] [Google Scholar]
- Simard JF, Arkema EV, Sundstrom A, Geborek P, Saxne T, Baecklund E, Coster L, Dackhammar C, Jacobsson L, Feltelius N, Lindblad S, Rantapaa-Dahlqvist S, Klareskog L, van Vollenhoven RF, Neovius M. Askling J. Ten years with biologics: to whom do data on effectiveness and safety apply? Rheumatology (Oxford) 2011;50(1):204–213. doi: 10.1093/rheumatology/keq326. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M, Aletaha D, Buch M, Gossec L, Huizinga T, Bijlsma JW, Burmester G, Combe B, Cutolo M, Gabay C, Gomez-Reino J, Kouloumas M, Kvien TK, Martin-Mola E, McInnes I, Pavelka K, van Riel P, Scholte M, Scott DL, Sokka T, Valesini G, van Vollenhoven R, Winthrop KL, Wong J, Zink A. van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Annals of the Rheumatic Diseases. 2010;69(6):964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables RH, Booth J, Welstand J, Wright A, Ormerod OJ. Hodgson WR. A randomised controlled trial to compare a nurse practitioner to medical staff in the preparation of patients for diagnostic cardiac catheterisation: the study of nursing intervention in practice (SNIP) European Journal of Cardiovascular Nursing. 2004;3(1):53–59. doi: 10.1016/j.ejcnurse.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Stromberg A, Martensson J, Fridlund B. Dahlstrom U. Nurse-led heart failure clinics in Sweden. European Journal of Heart Failure. 2001;3(1):139–144. doi: 10.1016/s1388-9842(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Swedish Research Council. Good Research Practice, Sweden Research Council. Report series 3:2011 Bromma; Sweden. 2011. Retrieved from http://www.vr.se/download/18.3a36c20d133af0c1295800030/Good+Research+Practice+3.2011_webb.pdf. [Google Scholar]
- Temmink D, Hutten JB, Francke AL, Rasker JJ, Abu-Saad HH. van der Zee J. Rheumatology outpatient nurse clinics: a valuable addition? Arthritis & Rheumatism. 2001;45(3):280–286. doi: 10.1002/1529-0131(200106)45:3<280::AID-ART261>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tijhuis GJ, Zwinderman AH, Hazes JM, Breedveld FC. Vlieland PM. Two-year follow-up of a randomized controlled trial of a clinical nurse specialist intervention, inpatient, and day patient team care in rheumatoid arthritis. Journal of Advanced Nursing. 2003;41(1):34–43. doi: 10.1046/j.1365-2648.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- Vander Cruyssen B, Van Looy S, Wyns B, Westhovens R, Durez P, Van den Bosch F, Veys EM, Mielants H, De Clerck L, Peretz A, Malaise M, Verbruggen L, Vastesaeger N, Geldhof A, Boullart L. De Keyser F. DAS28 best reflects the physician's clinical judgment of response to infliximab therapy in rheumatoid arthritis patients: validation of the DAS28 score in patients under infliximab treatment. Arthritis Research & Therapy. 2005;7(5):R1063–R1071. doi: 10.1186/ar1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vollenhoven RF. Askling J. Rheumatoid arthritis registries in Sweden. Clinical and Experimental Rheumatology. 2005;23(5 Suppl 39):S195–S200. [PubMed] [Google Scholar]
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D. van Riel PL. Validation of the 28-joint Disease Activity Score (DAS28) and European League against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Annals of the Rheumatic Diseases. 2009;68(6):954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WMA. World Medical Association declaration of Helsinki. Ethical principles for medical research involving human subjects. 2008. Retrieved from http://www.wma.net/en/30publications/10policies/b3/17c.pdf.
- Wong FK, Mok MP, Chan T. Tsang MW. Nurse follow-up of patients with diabetes: randomized controlled trial. Journal of Advanced Nursing. 2005;50(4):391–402. doi: 10.1111/j.1365-2648.2005.03404.x. [DOI] [PubMed] [Google Scholar]


