Abstract
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central auditory system. Altered GABAergic neurotransmission has been found in both the inferior colliculus and the auditory cortex in animal models of presbycusis. Edited magnetic resonance spectroscopy (MRS), using the MEGA-PRESS sequence, is the most widely used technique for detecting GABA in the human brain. However, to date there has been a paucity of studies exploring changes to the GABA concentrations in the auditory region of patients with presbycusis. In this study, sixteen patients with presbycusis (5 males/11 females, mean age 63.1 ± 2.6 years) and twenty healthy controls (6 males/14 females, mean age 62.5 ± 2.3 years) underwent audiological and MRS examinations. Pure tone audiometry from 0.125 to 8 KHz and tympanometry were used to assess the hearing abilities of all subjects. The pure tone average (PTA; the average of hearing thresholds at 0.5, 1, 2, and 4 kHz) was calculated. The MEGA-PRESS sequence was used to measure GABA+ concentrations in 4 × 3 × 3 cm3 volumes centered on the left and right Heschl’s gyri.
GABA+ concentrations were significantly lower in the presbycusis group compared to the control group (left auditory regions: p = 0.002, right auditory regions: p = 0.008). Significant negative correlations were observed between PTA and GABA+ concentrations in the presbycusis group (r = −0.57, p = 0.02), while a similar trend was found in the control group (r = −0.40, p = 0.08). These results are consistent with a hypothesis of dysfunctional GABAergic neurotransmission in the central auditory system in presbycusis, and suggest a potential treatment target for presbycusis.
Introduction
Presbycusis or age-related hearing loss is the most common sensory deficit in the elderly, most often characterized by a decline in frequency audibility towards high frequencies, which are particularly important for speech recognition. Poor speech discrimination, especially in a noisy environment, reflects impaired temporal processing within the central auditory system (Mazelova et al., 2003; Snell and Frisina, 2000).
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central auditory system (Caspary et al., 1995). Decreased concentrations of GABA have been found in both the inferior colliculus (Caspary et al., 1995; Caspary et al., 1990; Syka, 2010) and the auditory cortex (Syka, 2010) in animal models of presbycusis. Measurement of GABA in the human brain using magnetic resonance spectroscopy (MRS) is hindered because resonances of GABA overlap with other metabolites such as glutamate (Glu), N-acetylaspartate (NAA), total creatine (Cr) and macromolecules (MM), and the concentration of GABA is relatively low (0.5–1.9 mmol/dm3) (Govindaraju et al., 2000).
Recently, measurements of GABA, largely free from overlap with more abundant molecules, have been established using spectral editing techniques (Mescher et al., 1998; Rothman et al., 1993), and this has been applied successfully to measure GABA in a number of neurologic, psychiatric and developmental disorders (Edden et al., 2012a; Foerster et al., 2013; Rowland et al., 2013) and in healthy control subjects (Boy et al., 2011; Edden et al., 2009; Gao et al., 2013; Puts and Edden, 2012; Puts et al., 2011). However, to date there has been a paucity of studies exploring changes in GABA concentration in auditory regions of patients with presbycusis. In this study, therefore, J-difference edited MRS was used to investigate GABA concentrations in the auditory region of patients with presbycusis and healthy controls and their relationship to audiological outcomes.
Material and methods
Subjects
The study was approved by the Shandong University institutional review board and each participant provided informed consent.
Sixteen patients with presbycusis (presbycusis group, 5 males/11 females, mean age 63.1 ± 2.6 years) were recruited in this study. Hearing loss was defined as a speech-frequency pure tone average (PTA) of thresholds at 0.5, 1, 2, and 4 kHz (air conduction) in the better hearing ear as per the definition of hearing loss adjudicated by the World Health Organization. The PTA value of 25 decibel hearing level (dB HL) was accepted as the limit for normal hearing threshold (Lin et al., 2011). Inclusion criteria were: 1) 60 years of age and older, 2) PTA > 25 dB HL in the better hearing ear. Exclusion criteria were: 1) previous history of otological surgery, ototoxic drug therapy, noise exposure or hearing aid use, 2) ear diseases that affect hearing thresholds and sensorineural hearing losses other than presbycusis, 3) head trauma, lesions of the facial nerve, disorders of the cervical spine, neurological or psychiatric disease, 4) conductive hearing loss (the mean air-bone differences at 0.5, 1, 2 and 4 KHz) above 10 dB in one or both ears, 5) asymmetrical hearing loss with a difference in air-conduction thresholds exceeding 20 dB in at least 2 frequencies between 0.5, 1, 2 and 4 KHz. The complete list of exclusion criteria has been previously described (Van Eyken et al., 2006).
Twenty age- and gender-matched healthy controls (control group, 6 males/14 females, mean age 62.5 ± 2.3 years, PTA ≤ 25 dB HL in the better hearing ear) were recruited for this study. All controls were in good health, and had no history of neurological or psychiatric disease.
All subjects were right-handed. None of the subjects were musical professionals. No subject had a history of long-term alcohol consumption. Smoking, drinking alcohol and taking caffeine were prohibited for 12 hours prior to MR measurement.
Assessment of auditory function
Otoscopic examination was performed in all subjects to remove the cerumen and confirm an intact tympanic membrane. Pure tone audiometry and tympanometry were used to assess the hearing abilities of all subjects.
Tympanometry was performed with a Madsen Electronics Zodiac 901 Middle Ear Analyser to confirm optimal middle ear conditions. Pure tone audiometry was performed with a Madsen Electronics Midimate 622 Clinical/Diagnostic audiometer, coupled with TDH-39P Telephonics headphones. Air conduction was measured at 0.125, 0.25, 0.5, 1, 2, 4, and 8 KHz, and bone conduction at 0.25, 0.5, 1, 2, and 4 KHz. Hearing thresholds were detected with a resolution of 5 dB steps. The PTA of all subjects’ ears were calculated.
MRI and voxel localization
All subjects were scanned on a 3T scanner (Philips ‘Achieva’ TX, Best, The Netherlands) using an eight-channel phased-array head coil for receive. T1-weighted three-dimensional TFE images were used as a localizer, acquired with the following parameters: TR = 8.2 ms; TE = 3.7 ms; slice thickness = 1 mm; matrix 256 × 256; field of view = 24 × 24 cm2; and flip angle = 8°. Images were reconstructed with 1 × 1 × 1 mm3 isotropic voxels. The volumes of interest (VOIs) with a size of 4 × 3 × 3 cm3 were centered on the Heschl’s gyrus in both auditory regions (as shown in Figure 1). Criteria for determination of Heschl’s gyrus have been described previously (Rojas et al., 1997). The VOIs also included portions of insula, parietal and frontal operculum. All of the VOIs were placed in a manner that enabled avoidance of the lateral ventricle and skull.
Figure 1.
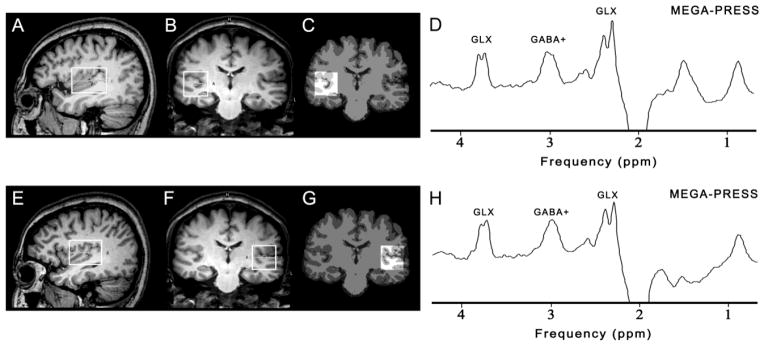
The position of volumes of interest (4 × 3 × 3 cm3) in the right (above) and left auditory region (below) on sagittal (A, E) and coronal (B, F) T1-weighted images. The corresponding results of brain segmentation are shown for the right (C) and left auditory region (G). Representative GABA+-edited MEGA-PRESS spectra from the right auditory region (D) and the left auditory region (H) are shown.
1H-MRS spectroscopy, editing, and VOI segmentation
The 3 ppm resonance of GABA was measured using a MEGA-PRESS sequence (Mescher et al., 1998), using the following parameters: TR 2000 ms; TE 68 ms; 320 averages; acquisition bandwidth 1000 Hz; and total acquisition time 11 minutes. During odd-numbered acquisitions, a frequency-selective, Gaussian inversion pulse was applied to the 3CH2 resonance of GABA at 1.9 ppm, affecting the weakly J-coupled triplet peak at 3.02 ppm (EDIT-ON). During even-numbered acquisitions, the same pulse was applied symmetrically to the other side of the water peak, at 7.5 ppm (EDIT-OFF), in order to minimize the residual water signal upon subtraction. The resulting edited spectrum is derived from the difference between the ON and OFF spectra. Chemical Shift Selective Suppression (CHESS) was used for water suppression. FASTMAP shimming of the VOI was performed automatically prior to each acquisition. For quantification, a shorter measurement (8 averages) of the unsuppressed water signal was obtained.
Because the signal detected at 3.02 ppm using these experimental parameters is also expected to contain contributions from both macromolecules (MM) and homocarnosine (Rothman et al., 1997), in the rest of this manuscript this signal is labeled GABA+ rather than GABA, to indicate the potential presence of these other compounds. The MEGA-PRESS data were analyzed using ‘Gannet’ (GABA-MRS Analysis Tool) in Matlab 2010b (Mathworks) with Gaussian curve fitting to the GABA+ peaks (Edden et al., 2013). 3 Hz exponential line broadening was applied.
The ratios of the integrals of the GABA+ and water signals, making corrections for T1 and T2 relaxation times and partial volume effects, were used to calculate water-scaled GABA+ concentration in mmol/L (mM) using the formula (Gasparovic et al., 2006; Mullins et al., 2014):
Where IG and IW are the fitting integrals of GABA+ (G) and water (W) as determined by ‘Gannet’, [H2O] is the pure water concentration (55,550mmol/L), VISw is a factor accounting for MR water visibility and tissue proton density (0.65), and fGM, fWM, fCSF are the fractions of water attributable to GM, WM, and CSF respectively (Gasparovic et al., 2006). The relaxation attenuation factors are given by the equation RW_y = exp[− TE/T2W_y](1 − exp[−TR/T1W_y]), where T1W_y and T2W_y are the T1 and T2 relaxation times of water in compartment y. Similarly, RG is the relaxation attenuation factor for GABA. The relaxation times used were as follows: GM water: T1 = 1331ms, T2 = 110ms; WM water: T1 = 832ms, T2 = 79.6ms; CSF: T1 = 3817ms, T2 = 503ms (Lu et al., 2005; Piechnik et al., 2009; Wansapura et al., 1999); GABA: T1 = 1310ms, T2 = 88ms (Edden et al., 2012b; Puts et al., 2013). MMcor is a macromolecular correction factor (0.45) given by the fraction of the GABA+ peak that is thought to be GABA (Mullins et al., 2014).
Gannet provides normalized residual fitting errors of GABA+, which can be interpreted quantitatively to assess measurement quality. Only spectra with a fitting error of GABA+ below 10% were included in the final analysis.
Each pixel in the 3D T1-weighted brain images was segmented as gray matter (GM), white matter (WM), or cerebrospinal fluid (CSF) using an automatic brain segmentation program, FAST (FMRIB’s automated segmentation tool) in the FSL package (Oxford University, Oxford, UK) (Zhang et al., 2001). VOIs were co-registered to the anatomical images using the “Re-creation of VOI” Matlab tool (Montelius et al., 2008) (as shown in Figure 1). Tissue GM fractions were obtained by calculating the ratio of GM volume to the GM+WM volumes in the VOIs. The concentrations of GABA in CSF were considered to be negligible.
Statistical analysis
Results are presented as mean ± standard deviation (SD). A two-tailed t-test was performed to test for differences of GABA+ concentration between patients with presbycusis and healthy controls. Linear correlation was used to examine the association between GABA+ concentration and PTA in the presbycusis and control group. The threshold of significance was set at a p-value of 0.05. Statistical analyses were carried out using the statistical package SPSS 13.0.
Results
Hearing function results
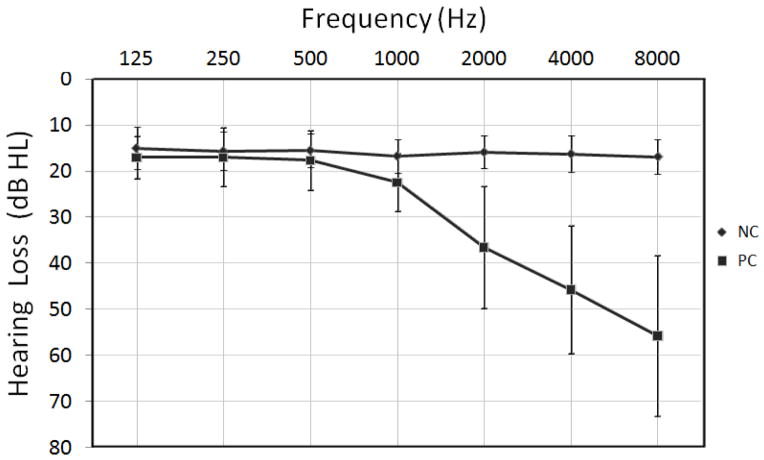
No significant difference in PTA between the left and right ears was found (presbycusis group, p = 0.44; control group, p = 0.79), therefore the hearing thresholds from both ears were averaged. The control group displayed mean hearing thresholds that were less than 20 dB HL above all the frequencies (as shown in Figure 2). The presbycusis group showed mean hearing thresholds that were less than 20 dB HL at frequencies up to 0.5 KHz. There was a small but steady increase of the mean hearing thresholds at higher frequencies, with a 45.8 dB hearing loss at 4 kHz and a 55.8 dB hearing loss at 8 kHz in the presbycusis group (as shown in Figure 2). The PTA of the presbycusis group was significantly higher than those of the control group (p < 0.001). Tympanometric examination showed a type A curve in all subjects. The type A curve shows that there is normal pressure in the middle ear with normal mobility of the eardrum and the conduction bones.
Figure 2.
Hearing thresholds of the presbycusis (PC) and normal control (NC) group as assessed using pure tone audiometry. Hearing thresholds from both ears are averaged. Data are shown as means ± standard deviation (SD) from 125 Hz to 8000 Hz (air conduction).
Comparisons of GABA+ concentration between groups
Edited spectra were successfully collected from all 36 subjects, and the fitting error of GABA+ in all spectra was below 10%. Representative GABA-edited spectra are shown in Figure 1. The GABA+ concentrations in left and right auditory regions among all subjects are summarized in Table 1. GABA+ concentrations were significantly lower in the presbycusis group compared to the control group (left auditory regions: p = 0.002, right auditory regions: p = 0.008).
Table 1.
Demographic, MRS, and segmentation data of the presbycusis and normal control group
| PC | NC | p-value | |
|---|---|---|---|
| Number | 16 | 20 | — |
| Age (years) | 63.1 ± 2.6 | 62.5 ± 2.3 | 0.45 |
| Female, Number (%) | 11 (68.8) | 14 (70.0) | 0.94 |
| Left auditory region: | |||
| GABA+ level (mM) | 1.25 ± 0.40 | 1.65 ± 0.29 | 0.002 |
| GABA+ fitting errors (%) | 5.23 ± 1.56 | 5.80 ± 1.83 | 0.33 |
| GM/(G M+WM) (%) | 51.10 ± 2.44 | 52.02 ± 2.33 | 0.26 |
| Right auditory region: | |||
| GABA+ level (mM) | 1.01 ± 0.26 | 1.28 ± 0.31 | 0.008 |
| GABA+ fitting errors (%) | 6.22 ± 1.58 | 5.76 ± 2.04 | 0.47 |
| GM/(G M+WM) (%) | 50.90 ± 1.84 | 51.39 ± 1.58 | 0.40 |
Data are given as mean ± standard deviation.
PC = presbycusis, NC = normal control, GM = gray matter, WM = white matter. mM = mmol/L, GABA = gamma-aminobutyric acid, GABA+ = GABA plus co-edited macromolecules.
Correlation of GABA+ concentration with hearing function
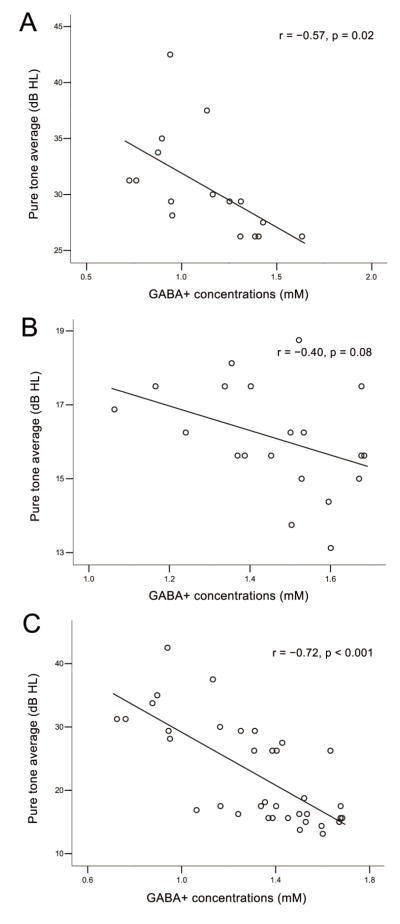
The GABA+ concentrations were averaged from the left and right auditory regions, and the PTA from both ears of all subjects were also averaged. Significant negative correlations were observed between PTA and GABA+ concentrations in the presbycusis group (r = −0.57, p = 0.02) (as seen in Figure 3). A trend toward a similar correlation was found in the control group (r = −0.40, p = 0.08) (as seen in Figure 3). Significant negative correlations were also observed between PTA and GABA+ concentrations in all subjects (r = −0.72, p < 0.001) (as seen in Fig. 3).
Figure 3.
Correlations between pure tone average (PTA) and GABA+ concentrations in the presbycusis group (A), normal control group (B) and all subjects (C). A significant negative correlation was observed between PTA and GABA+ concentrations in the presbycusis group (r = −0.57, p = 0.02). A trend toward correlation was seen in the normal control group (r = −0.40, p = 0.08). A significant negative correlation was also observed between PTA and GABA+ concentrations in all subjects (r = −0.72, p < 0.001)
Tissue segmentation and GABA+ fitting error
The mean GM tissue fraction GM/(GM + WM) was 51.10% and 50.90% in the left and right auditory regions, respectively, in the presbycusis group, and 52.02% and 51.39%, respectively, in the control group (Table 1). These were not significantly different (left auditory region, p = 0.26; right auditory region, p = 0.40).
The mean GABA+ fitting error was 5.23% and 6.22% in the left and right auditory regions, respectively, in the presbycusis group, and 5.80% and 5.76%, respectively, in the control group (Table 1). These were also not significantly different (left auditory region, p = 0.33; right auditory region, p = 0.47).
Discussion
Our study demonstrates that GABA+ concentrations were reduced in auditory regions of patients with presbycusis, as compared to age- and gender-matched healthy controls. Significant negative correlations between GABA+ concentrations and PTA were also observed in patients with presbycusis. To the best of our knowledge, this is the first in vivo demonstration of reduced GABA+ concentrations in patients with presbycusis, and the first in vivo human demonstration of a relationship between GABA and auditory function.
Previous animal studies have demonstrated changes in GABAergic neurotransmission in the inferior colliculus (Caspary et al., 1999; Caspary et al., 1990; Ouda and Syka, 2012), cochlear nuclei (Tang et al., 2014) and auditory cortex (Caspary et al., 2013; Ling et al., 2005; Ouda et al., 2008) of the Fischer 344 rat (a model of presbycusis), including decreased concentrations of GABA, decreased GABA release, decreased GABAB receptor binding and decreased numbers of presynaptic terminals. Therefore, reduced GABA+ concentrations in patients with presbycusis might reflect dysfunctional GABAergic neurotransmission in the central auditory system.
The observed reduction in GABA+ concentrations could be indicative of GABAergic neuronal loss in presbycusis. This is consistent with a number of previous animal studies: decreased numbers of GABA immunoreactive neurons were found in the inferior colliculus (Caspary et al., 1995; Caspary et al., 1990; Syka, 2010), cochlear nuclei (Syka, 2010) and auditory cortex (Syka, 2010) of the Fischer 344 rat using immunocytochemical and neurochemical methods. One study has shown a relationship between MRS GABA concentrations and genes coding for glutamic acid decarboxylase (GAD), a decarboxylase involved in the production of GABA (Marenco et al., 2010). Cytoplasmic GABA is primarily produced from glutamate via the tonically active 67 kilodalton form of GAD (GAD67), and vesicular GABA is controlled in the main via the phasically active 65 kilodalton form of GAD (GAD65) (Stagg et al., 2011). It is therefore also possible that the current finding of reduced GABA+ concentrations in patients with presbycusis reflects a decline in the efficacy of production in GABA. Consistent with this, several studies of the Fischer 344 rat found reduced levels of GAD65 and GAD67 in the inferior colliculus (Burianova et al., 2009; Ouda and Syka, 2012; Raza et al., 1994) and auditory cortex (Burianova et al., 2009; Ling et al., 2005).
To our knowledge, no previous study has demonstrated altered GABA concentrations in patients with presbycusis. One MRS study indicated that there was no significant differences in GABA concentrations of the auditory cortex between patients with presbycusis and healthy controls (Profant et al., 2013). However, the study has analyzed non-edited short-TE spectra at field strengths of 3.0T, which have limited ability to resolve GABA from glutamate and glutamine (Puts and Edden, 2012).
PTA is often used to calculate the degree of hearing loss in speech frequency. In this study, significant negative correlations between GABA+ concentrations and PTA were found in the presbycusis group. Findings reported in animal studies may be related: lower levels of GABA transporter were observed in the ventral cochlear nucleus of the wistar rat (a model of presbycusis), which exhibited higher auditory brainstem response (ABR) thresholds (Alvarado et al., 2014). Moreover, significant negative correlations were also observed between PTA and GABA+ concentrations in all subjects. The results might demonstrate a mechanistic connection between the GABAergic neurotransmission and hearing function. GABA is the main inhibitory neurotransmitter in the central auditory system, and there is ample evidence that GABA-mediated inhibition plays a critical role in processing acoustic information (Caspary et al., 1995). Previous animal studies have suggested that decreased inhibition may lead to increased, and less well-controlled, excitatory activity with reduced tuning of the neuronal receptive fields (Palombi and Caspary, 1996; Walton et al., 2002). Discrimination of the temporal parameters of sounds is significantly reduced when the inhibitory fine-tuning of receptive fields is decreased (Narayan et al., 2005). A reduction in temporal acuity limits gap detection thresholds and gap duration difference limens, which play a vital role in processing of complex sounds, including language (Gordon-Salant and Fitzgibbons, 1993; Gordon-Salant et al., 2007). In addition, decreased inhibition in the auditory neuraxis of animals results in impaired localization of a sound source in the environment (Litovsky and Delgutte, 2002; Pecka et al., 2007). Therefore, reduced GABA+ concentrations in auditory regions of patients with presbycusis could contribute significantly to the deterioration of hearing function. Viewed in the light of the prior literature, our results suggest that the GABAergic neurotransmission may be a potential treatment target for presbycusis.
The majority of GABA is located in two pools within neurons - the cytoplasm and the presynaptic vesicles (Kwon et al., 2014). However, MRS is only capable of detecting total GABA within the prescribed localized region, and it cannot distinguish between these separate functional pools of GABA (Stagg et al., 2011). Therefore, the scope of our study to introduce GABAergic neurotransmission as a treatment target for presbycusis may be limited. There are other factors associated with hearing function. For instance, significantly decreased metabolic rates of oxygen and glucose were found in the auditory cortex of totally deaf patients using positron emission tomography (PET) (Ito et al., 1993). With the cochlear implant device, the metabolic activity returned to near normal levels (Ito et al., 1990; Ito et al., 1993). However, there is no clear correspondence between metabolic rates as measured by PET and the concentrations of metabolites measured by MRS. It is difficult in a cross-sectional study to separate the causes and effects of deafness.
The data acquisition strategy for this study has several limitations. First, the relatively low amplitude of the GABA signal of this method required the use of relatively large VOIs (4 × 3 × 3 cm3), a volume that contains, but is by no means restricted to, primary auditory cortex. The VOIs also included portions of insula, parietal and frontal operculum, so it is not solely involved in auditory function. An approach enabling the use of smaller VOIs may prove more efficient for region-specific analyses of brain GABA changes. Secondly, the edited GABA signal that was detected contains a significant contribution (~50% of the signal) from co-edited macromolecules, and homocarnosine. New methods for macromolecule suppression are being developed which can detect ‘pure GABA’ (Edden et al., 2012c). However, the two major methods available have substantial practical limitations: acquiring MM-only spectra is time-consuming and reduces the GABA-to-noise ratio; acquiring MM-suppressed edited experiments make the acquisition substantially more sensitive to field changes (whether due to heating or movement). Our current belief is that the GABA+ measurement is the most robust one available, although it does suffer from contaminations. The in vivo concentration of homocarnosine is much lower than that of GABA (Govindaraju et al., 2000), so isolated changes in homocarnosine are less likely to be driving the observed changes.
Conclusion
In conclusion, reduced GABA+ concentrations were found in the patients with presbycusis, and significant negative correlations were observed between PTA and GABA+ concentrations. These results are consistent with a hypothesis of dysfunctional GABAergic neurotransmission in the central auditory system in presbycusis, and suggest a potential treatment target for presbycusis.
Highlights.
Reduced GABA levels were found in auditory regions of patients with presbycusis.
Significant negative relations between GABA level and auditory function were found.
These results suggest a potential treatment target for presbycusis.
Acknowledgments
This study applies application and processing tools developed under NIH grants P41 EB015909 and R01 EB016089; RAEE also receives support from these grants. Project also supported by the National Natural Science Foundation of China (Grant No. 81171380/H1807).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarado JC, Fuentes-Santamaria V, Gabaldon-Ull MC, Blanco JL, Juiz JM. Wistar rats: a forgotten model of age-related hearing loss. Front Aging Neurosci. 2014;6:29. doi: 10.3389/fnagi.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Experimental Gerontology. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL. Age-related GABAA receptor changes in rat auditory cortex. Neurobiology of Aging. 2013;34:1486–1496. doi: 10.1016/j.neurobiolaging.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert D, Mostofsky S. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012a;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: application to GABA at 3 Tesla. J Magn Reson Imaging. 2012b;35:229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med. 2012c;68:657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging. 2013 doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RAE, Mohamed MA, Welsh RC, Carlos RC, Barker PB, Feldman EL. An Imbalance Between Excitatory and Inhibitory Neurotransmitters in Amyotrophic Lateral Sclerosis Revealed by Use of 3-T Proton Magnetic Resonance Spectroscopy. JAMA Neurology. 2013;70:1009. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA. Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. J Speech Lang Hear Res. 2007;50:1181–1193. doi: 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ito J, Sakakibara J, Honjo I, Iwasaki Y, Yonekura Y. Positron emission tomographic study of auditory sensation in a patient with a cochlear implant. Arch Otolaryngol Head Neck Surg. 1990;116:1437–1439. doi: 10.1001/archotol.1990.01870120083015. [DOI] [PubMed] [Google Scholar]
- Ito J, Sakakibara J, Iwasaki Y, Yonekura Y. Positron emission tomography of auditory sensation in deaf patients and patients with cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:797–801. doi: 10.1177/000348949310201011. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Benjamin J, Myers EH, Qiu M, Schneider KC, Rothman DL, Constable RT, Ment LR. GABA, Resting-State Connectivity and the Developing Brain. Neonatology. 2014;106:149–155. doi: 10.1159/000362433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Delgutte B. Neural correlates of the precedence effect in the inferior colliculus: effect of localization cues. J Neurophysiol. 2002;87:976–994. doi: 10.1152/jn.00568.2001. [DOI] [PubMed] [Google Scholar]
- Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC. Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging. 2005;22:13–22. doi: 10.1002/jmri.20356. [DOI] [PubMed] [Google Scholar]
- Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS, Kolachana B, Radulescu E, Zhang F, Callicott JH, Straub RE, Shen J, Weinberger DR. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35:1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Popelar J, Syka J. Auditory function in presbycusis: peripheral vs. central changes. Exp Gerontol. 2003;38:87–94. doi: 10.1016/s0531-5565(02)00155-9. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Montelius M, et al. ESMRMB. 2008. Oct 1–3, MATLAB tool for segmentation and re-creation of 1H-MRS volumes of interest in MRI image stacks. Antalya/TR. [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan R, Ergun A, Sen K. Delayed inhibition in cortical receptive fields and the discrimination of complex stimuli. J Neurophysiol. 2005;94:2970–2975. doi: 10.1152/jn.00144.2005. [DOI] [PubMed] [Google Scholar]
- Ouda L, Druga R, Syka J. Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Experimental Gerontology. 2008;43:782–789. doi: 10.1016/j.exger.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Ouda L, Syka J. Immunocytochemical profiles of inferior colliculus neurons in the rat and their changes with aging. Front Neural Circuits. 2012;6:68. doi: 10.3389/fncir.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. Physiology of the aged Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. J Neurophysiol. 1996;76:3114–3125. doi: 10.1152/jn.1996.76.5.3114. [DOI] [PubMed] [Google Scholar]
- Pecka M, Zahn TP, Saunier-Rebori B, Siveke I, Felmy F, Wiegrebe L, Klug A, Pollak GD, Grothe B. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci. 2007;27:1782–1790. doi: 10.1523/JNEUROSCI.5335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P. Functional changes in CSF volume estimated using measurement of water T2 relaxation. Magn Reson Med. 2009;61:579–586. doi: 10.1002/mrm.21897. [DOI] [PubMed] [Google Scholar]
- Profant O, Balogova Z, Dezortova M, Wagnerova D, Hajek M, Syka J. Metabolic changes in the auditory cortex in presbycusis demonstrated by MR spectroscopy. Exp Gerontol. 2013;48:795–800. doi: 10.1016/j.exger.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Puts NA, Barker PB, Edden RA. Measuring the longitudinal relaxation time of GABA in vivo at 3 Tesla. J Magn Reson Imaging. 2013;37:999–1003. doi: 10.1002/jmri.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31:16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res. 1994;77:221–230. doi: 10.1016/0378-5955(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale P, Sheeder J, Simon J, Reite M. Sex-specific expression of Heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry. 1997;154:1655–1662. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Prichard JW, Petroff OA. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magn Reson Med. 1997;38:924–929. doi: 10.1002/mrm.1910380611. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Edden RA, Kontson K, Zhu H, Barker PB, Hong LE. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25:83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol. 2011;4:573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J. The Fischer 344 rat as a model of presbycusis. Hear Res. 2010;264:70–78. doi: 10.1016/j.heares.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhu X, Ding B, Walton JP, Frisina RD, Su J. Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience. 2014;259:184–193. doi: 10.1016/j.neuroscience.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken E, Van Laer L, Fransen E, Topsakal V, Lemkens N, Laureys W, Nelissen N, Vandevelde A, Wienker T, Van De Heyning P, Van Camp G. KCNQ4: a gene for age-related hearing impairment? Hum Mutat. 2006;27:1007–1016. doi: 10.1002/humu.20375. [DOI] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]