Introduction
The availability of combination antiretroviral therapy (cART) has led to substantial reduction in morbidity and mortality in HIV-infected patients; however, life expectancy remains reduced especially in HIV-infected patients who initiate cART with CD4 T-cell counts less than 200 cells/μl [1]. Increased immune activation in patients on long-term suppressive cART [2–4] has been associated with increased mortality [5,6] and both AIDS and non-AIDS-defining illnesses [7–10], suggesting that chronic immune activation may have a potential role in driving increased morbidity and mortality.
Causes of HIV-associated immune activation
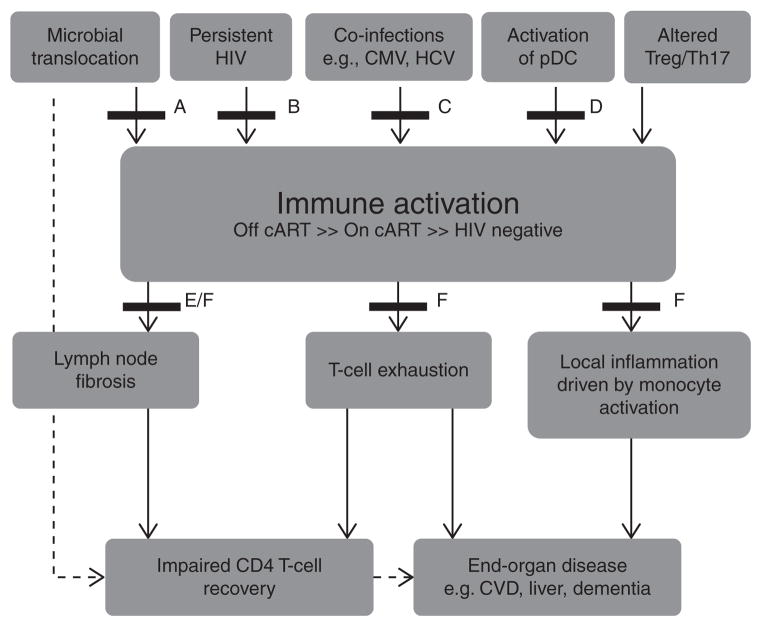
The mechanisms driving systemic immune activation in chronic HIV infection are multifactorial (reviewed in [11], Fig. 1) [12] and include the translocation of microbial products from the gastrointestinal tract [13,14], low-level HIV viremia [15,16], and coinfections with other persistent viral pathogens including cytomegalo-virus (CMV) and hepatitis C virus (HCV) [17]. A recent study in simian immunodeficiency virus-infected macaques demonstrated a significant increase in immune activation and coagulation markers, including D-dimers, following exogenous administration of lipopolysaccharide (LPS) [18]. The excessive production of interferon alpha (IFN-α) [19–22] and pro-inflammatory cytokines leading to upregulation of pro-apoptotic molecules [23–26], lymph node fibrosis [27], dysfunction of CD4 T-regulatory cells (T-regs) [28,29], and depletion of CD161++/(mucosal-associated invariant T cells, MAIT) [30,31] are likely to also contribute.
Fig. 1.
Schematic representation of the potential causes of chronic immune activation in HIV-infected patients, its impact on clinical end-points, and strategies of interventions tested in recently completed and ongoing clinical trials.
Strategies to reduce persistent immune activation in HIV-infected patients
Pharmacological agents
Multiple clinical trials have been completed (Table 1) [32–76], or are in development (Table 2) [27,77–86], to reduce immune activation in HIV-infected patients and have also been recently reviewed in [87]. Potential drivers of immune activation include microbial translocation which occurs due to persistent dysfunction in the gut-associated lymphoid tissue (GALT), persistent HIV infection, coinfections with cytomegalovirus (CMV) and hepatitis C virus (HCV), aberrant activation of plasmacytoid dendritic cells (pDC), and altered ratio of Tregs and Th17 cells. Immune activation, though significantly reduced, persists even in patients receiving suppressive combination anti-retroviral therapy (cART) and leads to increased lymph node fibrosis and T-cell exhaustion, which affects CD4 T-cell recovery. Chronic immune activation also activates monocytes, which drives local inflammation in tissues and leads to the development of various end-organ damage and non-AIDS-defining illnesses including cardiovascular disease (CVD). Various treatment strategies to attenuate immune activation or its effects have recently been trialed and are labeled A to F. These strategies include (A) agents that promote mucosal repair in the GALT (bovine serum colostrum, micronutrient supplementation, probiotics and prebiotics); (B) cART treatment intensification (maraviroc and raltegravir); (C) treatment of coinfections (valgancyclovir, interferon-α, and ribavirin); (D) agents that reduce pDC activation (chloroquine and hydroxychloroquine); (E) agents that reduce transforming growth factor-β1 (TGF-β1)-mediated lymph node fibrosis (pirfenidone); and (F) immunomodulators [HMG CoA reductase inhibitors, minocycline, selective cyclooxygenase-2 inhibitors, leflunomide, and intravenous immunoglobulin (IVIG)]. Modified from [12].
Table 1.
Therapeutic agents/biologicals that have been evaluated in HIV-infected patients for their effects on immune activation and associated morbidities.
| Drug name | Immune activation/ inflammatory markers |
Clinical outcomes
|
Ref | |||||
|---|---|---|---|---|---|---|---|---|
| T-cell activation (coexpression of HLA-DR and CD38)a |
Soluble activation markers |
Other markers |
CD4 T-cell counts |
HIV RNA levels [assay detection limit, copies/ml]b/ markers of viral persistence |
AIDS- defining illness |
All-cause mortality |
||
| HMG CoA reductase inhibitors | ||||||||
| Statin use | - | - | - | - | - | - | ↓ | [37] |
| - | - | - | - | - | ↓(NHL) | - | [38] | |
| - | - | - | ↔ | - | - | - | [36] | |
| Atorvastatin | ↓: CD8+ | - | - | ↔ | ↔ | - | - | [32] |
| ↓: CD8+ (CD38+) | ↔hsCRP | - | ↔ | - | - | - | [33] | |
| ↑: CD8+ (CD38+) | - | - | ↓ | ↔ | - | - | [67] | |
| Rosuvastatin/Pravastatin | - | ↔: sTNFR ↓: hsCRP |
- | - | - | - | - | [34] |
| Pravastatin | - | ↔: hsCRP, PAI-1 | ↔: P-selectin | - | - | - | - | [35] |
| Chloroquine | ↓: CD8+ | - | ↔: Ki-67 expression in CD4+ and CD8+ T cells | - | ↔ | - | - | [39] |
| Hydroxychloroquine | ↓: CD4+ | ↓: IL-6 | - | ↔ | - | - | - | [41] |
| ↔: CD8+ | ↔: TNF-a | |||||||
| ↔: CD8+, CD4+ | ↔: IL-6, D-dimer | ↔: Ki-67 expression in CD4+ and CD8+ T cells | ↓ | ↑ | - | - | [40] | |
| Selective cyclooxygenase-2 inhibitors | ||||||||
| Celecoxib/rofecoxib | ↓: CD8+c (HLA-DR+ and CD38+) | - | - | ↑d | - | - | - | [42] |
| Celecoxib | ↓: CD8+ (CD38+) | - | - | ↔ | - | - | - | [43] |
| Leflunomide | ↓: CD8+ ↔: CD4+ |
↔: D-dimer, CRP, sCD14 | ↓: BrDU incorporation in CD4+ T cells | ↔ | ↔ | [44] | ||
| Intravenous immunoglobulin (IVIG) | ↔: CD4+, CD8+ | ↔: CRP | ↔: Ki-67 expression in CD4+ and CD8+ T cells | ↔ | ↔ | - | - | [68] |
| ↔: CD4+, CD8+ | - | - | - | ↔[<2] | - | - | [69] | |
| Minocycline | ↔: CD8+(blood & CSF) | ↔: CCL2 (CSF), neopterin (blood & CSF) | ↔: CD16+ (blood & CSF) | ↓ | ↔ (blood & CSF) | - | - | [70] |
| Bovine colostrum | - | - | - | ↑ | ↔ | - | - | [71] |
| - | - | - | ↑e | - | - | - | [45,47] | |
| ↔: CD4+, CD8+ | ↔: sCD14, LPS | ↔: 16srDNA | ↔ | [52] | ||||
| Micronutrient supplementation | - | - | - | ↔ | ↔ | - | ↓ | [72] |
| - | - | - | ↔ | - | - | ↓ | [73] | |
| - | - | - | ↑ | ↓ | - | - | [48] | |
| - | - | - | ↑ | - | - | - | [49] | |
| Probiotics | ||||||||
| Bifidobacterium bifidum, Streptococcus thermophiles | - | - | - | ↑ | - | - | - | [50] |
| Lactobacillus rhamnosus/Lactobacillus reuteri | - | - | - | ↑ | - | - | - | [46] |
| - | - | - | ↔ | - | - | - | [74] | |
| Lactobacillus rhamnosus | - | - | - | ↑ | - | - | - | [51] |
| Prebiotics | ||||||||
| Oligosaccharide mixturef | ↔: CD8+ (CD38+) | ↓: sCD14 | ↓: CD4+CD25+ T cells | ↔ | - | - | - | [75] |
| Raltegravir treatment intensification | ↔: CD8+, CD4+ | - | - | ↑ | ↔: SCA, 2-LTR, HIV DNA | - | - | [53,76] |
| ↔: CD8+ | - | - | ↔ | ↔: Usen HIV RNA, cell-associated HIV RNA, HIV DNA | - | - | [54] | |
| ↓: CD8+g | - | - | ↔ | ↔: SCA, HIV DNA ↑: 2-LTRg |
- | - | [59] | |
| ↔: CD8+ | ↔: sCD14, LPS | ↔: 16srDNA | ↔ | ↔: Usen HIV RNA | - | - | [52] | |
| ↓: CD8+ (blood & GI tract) | - | - | ↑(tissue)/↔ (blood) | ↓: US HIV RNA(tissue) ↔: US HIV RNA, Usen HIV RNA, HIV DNA (blood & tissue) |
- | - | [55] | |
| ↔: CD8+
(blood & CSF), CD4+
(CSF) ↑: CD4+ (blood) |
↔: neopterin (CSF & blood) | - | - | ↔: SCA (CSF & blood) | - | - | [56] | |
| - | - | - | ↔ | ↔: HIV DNA (tissue & blood) | - | - | [57] | |
| - | ↔: β2- microglobulin (blood & CSF), neopterin (blood & CSF) | - | ↔ | ↔: HIV RNA [<20] (CSF & blood) | - | - | [58] | |
| ↓: CD8+ ↔: CD4+ |
↓: LPS ↔: sCD14 |
- | ↔ | ↓: IUPM in memory CD4+
T cells ↔: SCA, 2-LTR |
- | - | [60] | |
| Maraviroc treatment intensification | ↓: CD4+, CD8+ | ↑: sCD14, LPS | - | ↔ | ↔: SCA,2-LTR | - | - | [61] |
| ↑: CD8+ (blood & tissue) | ↓: LPS ↑: sCD14 |
- | ↔ | ↔SCA | - | - | [64] | |
| ↓: CD4+, CD8+ | - | ↑: CD57+ ↓: caspase3+, Bcl-2 |
↔ | - | - | - | [62] | |
| ↓: CD8+ | - | - | ↑ | ↔: HIV DNA, Usen HIV RNA | [63] | |||
| Valgancyclovir | ↔: CD4+ ↓: CD8+ |
↔: hsCRP, IL-6, D-dimer, sCD14, cystatin C | - | ↔ | ↔ | - | - | [65] |
| IFN-α + ribavirin | ↓: CD8+ (CD38+), CD4+ (CD38+) | - | - | ↓h | - | - | - | [66] |
CRP, C-reactive protein; CSF, cerebrospinal fluid; LPS, lipopolysaccharide; LTR, long-terminal repeat; NHL, non-Hodgkin lymphoma; PAI-1, plasminogen activator inhibitor-1; SCA, single-copy assay; TNF, tumor necrosis factor; US, unspliced; Usen, ultrasensitive. Bold fonts indicate studies that were done in cART-treated patients, whereas normal text indicates studies that were performed in treatment-naive HIV-infected individuals.
T-cell activation markers represent coexpression of CD38+HLA-DR+ on T cells, unless otherwise specified.
Assay detection limit <50 copies/ml, unless otherwise specified.
Only in viremic patients.
Only in aviremic patients.
Concomitant reduction in HIV-associated diarrhea.
Mixture of short-chain galacto-oligosaccharides, long-chain fructo-oligosaccharides, pectin-hydrolysate-derived acidic oligosaccharides.
In a subset of patients.
Transient.
Table 2.
Therapeutic agents currently in or being considered for clinical trial to reduce immune activation levels in HIV.
| Drug name/compounds (trial number)a | Proposed mechanism of action (references) | Target group | Primary end-point studied |
|---|---|---|---|
| Rifaximin (NCT01466595) | Poorly absorbed antibiotic shown to reduce bacterial load in the gastrointestinal tract [77]. In combination with sulfasalazine (non-absorbable anti-inflammatory agent), shown to reduce markers of microbial translocation, immune activation, inflammation, and coagulation; viral load and mucosal CD4 T-cell depletion in acute SIV infection of pigtail macaques [78] | cART-treated patients with suboptimal CD4 T-cell recovery | Change in CD8+ T-cell activation at 4 weeks from baseline |
| Pyridostigmine (NCT 00518154) | Acetylcholine esterase inhibitor shown to reduce T-cell activation, proliferation, and IFN-γ production [79]. | cART-treated patients with suboptimal CD4 T-cell recovery | Change in CD4+ T-cell counts at 12 weeks from baseline |
| Sevelemer carbonate (NCT01543958) | Non-calcium phosphate binder shown to reduce endotoxin-driven production of IL-6, hsCRP, LPS, and sCD14 levels [80] | cART-naive patients | Change in sCD14 and endotoxin levels at 8 weeks from baseline |
| Meselamine (NCT 01090102) | Poorly absorbed anti-inflammatory agent shown to reduce non-infectious colitis [81]. | cART-treated patients | Change in CD8+ T-cell activation at 12 weeks vs. placebo |
| Lisinopril (NCT01535235) | Angiotensin-converting enzyme inhibitor shown to reduce markers of inflammation (hsCRP, TNF-α) [82] and inhibit TGF-β1-mediated fibrosis [83] | cART-treated patients | Change in HIV RNA (copies/million CD4) and mean baseline GALT RNA at 24 weeks vs. placebo |
| Methotrexate (NCT00000834) | Immunosuppressive agent used in the treatment of autoimmune diseases including rheumatoid arthritis. | Antiretroviral (zidovudine and lamivudine)-treated patients | Phase I study to determine safety profile in HIV-infected patients |
| Pirfenidone (not in clinical trial) | Shown to reduce TGF-β1 signaling pathway and collagen production [27]. | - | - |
| Sifalimumab (not in clinical trial) | Anti-IFN-α monoclonal antibody for the treatment of systemic lupus erythematosus (SLE) has been shown to reduce type-I IFN mRNAs (IL-10, TNF-α, IL-1β, GM-CSF) [84–86]. | - | - |
cART, combination antiretroviral therapy; GM-CSF, granulocyte-macrophage colony-stimulating factor; hsCRP, highly-sensitive C-reactive protein; IFN, interferon; LPS, lipopolysaccharide; SIV, simian immunodeficiency virus; TNF, tumor necrosis factor; TGF, transforming growth factor.
Active clinical studies referenced from clintrials.gov.
Statins
The use of statins in HIV-infected patients on and off cART has reported variable changes in T-cell activation and highly sensitive C-reactive protein (hsCRP) levels [32–35] but no effect on CD4 T-cell counts [32,33,36]. However, in two large observational studies of cART-treated patients, the use of statins was associated with reduced mortality [37] and reduced incidence of non-Hodgkin lymphoma (NHL) [38]. No immunological correlates were assessed in these two studies and a greater understanding of the mechanisms underlying the benefits of statins is needed.
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine inhibit endosomal acidification in plasmacytoid dendritic cells (pDCs) and Toll-like receptor 7 (TLR-7) signaling by HIV-1 single stranded (ss)RNA and also inhibit IFN-α production [88,89]. In vitro, chloroquine inhibited pDC activation and maturation, reduced IFN-α-mediated CD8 T-cell activation, and downmodulated indolamine 2–3 dioxygenase (IDO) and PD-L1 expression on pDCs, which are negative regulators of T-cell responses [90].
A recent randomized controlled trial (RCT) in cART-naive patients (n =13) found chloroquine was associated with decreased memory CD8 T-cell activation, CD4 and CD8 T-cell proliferation, and LPS levels compared to baseline but there were no changes in plasma HIV RNA [39]. In contrast, a RCTof hydroxychloroquine in cART-naive patients demonstrated no change in CD8 and CD4 T-cell activation and proliferation, an increase in HIV RNA, and decrease in CD4 T-cell counts [40]. In a small nonrandomized study (n =20), administration of hydroxychoroquine to patients receiving suppressive cARTwas associated with a reduction in multiple markers of immune activation but no significant increase in CD4 T-cell recovery [41]. Given these promising findings, numerous clinical trials are currently being conducted with choloroquine (NCT00819390) and hydroxychloroquine (NCT01232660).
Selective cyclooxygenase-2 inhibitors
Selective cyclooxygenase-2 (COX-2) inhibitors are anti-inflammatory agents that modulate T-cell activation via inhibition of prostaglandin E2 and the cyclic adenosine 3′,5′-monophosphate (cAMP)-protein kinase A pathway ([91,92], reviewed in [93]). In cART-treated patients, selective COX-2 inhibitors were associated with increased T-cell proliferation [94], a nonsignificant reduction in T-cell activation, and increased perforin-containing CD8 T cells [42]. A recent RCT of high-dose celecoxib in untreated HIV-infected patients (n = 31) reported a significant reduction in immune activation levels [43].
Leflunomide
A77 1726, the active metabolite of the antirheumatoid arthritis agent leflunomide, has anti-HIVactivity [95,96], inhibits pyrimidine synthesis [96,97], and reduces proliferation of activated T cells in vitro [98]. A small RCT in cART-naive patients (ALETHIA, A Study of Leflunomide to Target Immune Activation in HIV; n =16), found no significant change in CD4 and CD8 T-cell counts or HIV RNA levels in patients treated with leflunomide compared to placebo [44]. Furthermore, more grade 1 and 2 adverse events were reported with leflunomide. However, short-term leflunomide use was associated with reduced T-cell cycling and activation. It is currently unclear whether similar immunological effects will be seen in patients receiving cART.
Biological agents
Bovine colostrum, micronutrients, and prebiotics/probiotics
Multiple approaches are now being taken to directly reduce microbial load and translocation in HIV patients. These include supplementation with micronutrients, bovine colostrum, probiotics, and prebiotics, all of which have previously been shown to reduce HIV-associated diarrhea [99–101,45,46]. These strategies may also alter the composition of gut microflora, which may be important in modulating microbial translocation-driven immune activation [102,103]. Most of these studies have been in cART-naive patients and some have reported increases in CD4 T-cell counts [45,46,47–51] (Table 1). In a RCT of orally administered hyperimmune bovine colostrum (that contains antibodies to LPS), there was no effect on immune activation or CD4 T-cell recovery in patients receiving suppressive cART [52].
Antiretroviral intensification
Chronic immune activation in patients receiving cART may also be driven by low-level HIV viremia [15,16,104,105]. In multiple observational and RCT studies, the addition of raltegravir to suppressive cART resulted in no significant immune activation reduction in plasma, cerebrospinal fluid, or tissue [52,53–58] nor any change in endothelial function, a surrogate marker of cardiovascular disease [106]. There have, however, been two studies that have shown that the addition of raltegravir led to a significant reduction in T-cell activation markers in a subset of patients and a reduction in reservoir size [59,60]. Further larger randomized studies are still needed to definitively determine the impact of raltegravir intensification on immune activation.
Several studies of maraviroc intensification have shown a reduction in immune activation [61–63]; however, one study reported an unexpected increase in immune activation [64] (Table 1). CCR5 antagonists inhibit the binding and signaling of CCR5 ligands (including CCL3, CCL4, and CCL5) leading to an increase in their plasma concentration. This increase could potentially activate monocytes/macrophages via CCR1 [62] and/or increase antigen-specific T-cell and antibody responses, which has been observed in some [107] but not all studies [108]. Further studies are needed to better characterize the immunological changes associated with maraviroc use.
The timing of cART initiation may be an important parameter that influences immune activation. Studies of patients treated during chronic infection have demonstrated persistently elevated immune activation levels post-cART compared to uninfected controls [2–4]. A recent prospective study of cART initiated during acute infection demonstrated reduced immune activation to normal levels after 48 weeks [109]. Prospective or randomized trials need to be performed to determine the effect of early versus delayed cARTon immune activation in patients with chronic infection.
Treatment of coinfections
Anti-cytomegalovirus treatment: valgancyclovir
Increased CMV-specific antibodies and/or T cells have been associated with atherosclerosis [110,111] and impaired CD4 T-cell reconstitution [112] in HIV-infected patients on cART, suggesting that CMV coinfection may be a driver of persistent immune activation. A RCTwith valgancyclovir in CMV-seropositive cART-treated patients (n = 30) found that both CMV DNA and expression of CD38+HLA DR+ on T cells declined significantly during valgancyclovir therapy [65]. It is currently unclear whether this approach will translate to clinical benefits and the feasibility of prolonged administration of valgancyclovir may be limited by significant toxicities of the drug.
Anti-hepatitis C virus treatment: interferon alpha and ribavirin
HCV-specific treatment with IFN-α and ribavirin in HIV/HCV coinfected patients receiving cART has been associated with a significant reduction in markers of T-cell activation [66] and endothelial dysfunction [113]; however, its impact on clinical end-points is currently unknown.
Other strategies including treatment with intravenous immunoglobulin (IVIG) and minocycline have also been trialed in small studies but have yielded negative results (see Table 1).
Challenges in designing clinical trials to reduce immune activation
There are multiple challenges in designing clinical trials to reduce chronic immune activation in patients receiving suppressive cART. First, these studies will require patients who are otherwise clinically well to take an additional drug(s) that may be associated with toxicities. Therefore, the risks and benefits need to be carefully assessed. Second, there are multiple markers of immune activation and inflammation that have been studied and it is currently unclear which best predicts AIDS and non-AIDS-related morbidities in patients receiving suppressive cART. Biomarkers such as IL-6, D-dimer, and sCD14 show promise as they are relatively easy to standardize from measurements in plasma but whether they are indeed robust markers for predicting clinical outcomes following specific interventions needs further evaluation. Finally, given that clinical events are rare in patients on suppressive cART, relatively large samples sizes will be required to demonstrate a clinically relevant impact of any intervention to reduce immune activation.
Conclusion
To date, most studies aimed at reducing immune activation have only included a small number of patients and/or shown an effect on biomarkers of immune activation and have not had the power to assess any effects on clinical outcomes. Given that there are several candidate approaches that have shown promise in small proof-of-concept trials, these compounds warrant evaluation in larger randomized clinical trials that systematically evaluate both immune activation biomarkers and clinical outcomes.
Acknowledgments
R.R. and S.L. wrote the article. G.K., A.K., M.F., and P.U.C. provided helpful comments and edited the article.
R.R. and A.K. receive funding from the HIR/MOHE grant (UM.C/625/1/HIR/MOHE/MED01), R.R. from UMRG (RG377/11HTM) and S.R.L. from the National Institutes of Health (U19 AI096109 and 1R56AI095073-01A1. S.R.L. is an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellow. G.K. is a recipient of a NHMRC postgraduate scholarship.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: the ‘Mortalite 2000 and 2005’ surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 2.Chege D, Sheth PM, Kain T, Kim CJ, Kovacs C, Loutfy M, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS. 2011;25:741–749. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 3.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 4.Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24:1991–2000. doi: 10.1097/QAD.0b013e32833c93ce. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tincati C, Bellistri GM, Casana M, Merlini E, Comi L, Bai F, et al. CD8+ hyperactivation and senescence correlate with early carotid intima-media thickness in HIV+ patients with no cardiovascular disease. J Acquir Immune Defic Syndr. 2009;51:642–644. doi: 10.1097/QAI.0b013e3181add695. [DOI] [PubMed] [Google Scholar]
- 8.Carsenti-Dellamonica H, Vassallo M, Pradier C, Durant J, Harvey-Langton A, Lebrun-Frenay C, et al. LPS may be a predictive factor even in mild forms of HIV-associated neurocognitive impairment: sub-analysis of the Neuradapt Study. 18th Conference on Retroviruses and Opportunistic Infections; Boston. 27 Feb to 2 Mar 2011; Paper# 404. [Google Scholar]
- 9.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzola L, Ballistri G, Bai F, Savoldi A, Bini T, Marchetti G, et al. Higher CD4+and CD8+T-cell activation are associated factors of impaired bone mineral density in HIV infected patients. In. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. 2012. [Google Scholar]
- 11.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 12.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavigner M, Delobel P, Cazabat M, Dubois M, L’Faqihi-Olive FE, Raymond S, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4:e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load <or=20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008;68:652–660. doi: 10.1111/j.1365-3083.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–1407. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120:1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedaghat AR, German J, Teslovich TM, Cofrancesco J, Jr, Jie CC, Talbot CC, Jr, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol. 2008;82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rempel H, Sun B, Calosing C, Pillai SK, Pulliam L. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS. 2010;24:1415–1423. doi: 10.1097/QAD.0b013e32833ac623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez S, Tanaskovic S, Helbig K, Rajasuriar R, Kramski M, Murray JM, et al. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J Infect Dis. 2011;204:1927–1935. doi: 10.1093/infdis/jir659. [DOI] [PubMed] [Google Scholar]
- 23.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997;2:187–228. [PubMed] [Google Scholar]
- 24.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13974–13979. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Landay AL, Sinclair E, Martinson JA, Hatano H, Emu B, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One. 2011;6:e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2012 doi: 10.1182/blood-2012-06-436436. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion and persistent decline of the antimicrobial MR1-restricted MAIT cell population in chronic HIV-1 infection. Blood. 2012 doi: 10.1182/blood-2012-07-445429. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203:756–764. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Wit S, Delforge M, Necsoi CV, Clumeck N. Downregulation of CD38 activation markers by atorvastatin in HIV patients with undetectable viral load. AIDS. 2011;25:1332–1333. doi: 10.1097/QAD.0b013e328347c083. [DOI] [PubMed] [Google Scholar]
- 34.Aslangul E, Fellahi S, Assoumou LK, Bastard JP, Capeau J, Costagliola D. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS. 2011;25:1128–1131. doi: 10.1097/QAD.0b013e328346be29. [DOI] [PubMed] [Google Scholar]
- 35.Fichtenbaum CJ, Yeh TM, Evans SR, Aberg JA. Treatment with pravastatin and fenofibrate improves atherogenic lipid profiles but not inflammatory markers in ACTG 5087. J Clin Lipidol. 2010;4:279–287. doi: 10.1016/j.jacl.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manfredi R, Calza L, Chiodo F. Long-term statin use does not act on the temporal trend of CD4 cell count in patients on virologically effective HAART. AIDS. 2006;20:455–457. doi: 10.1097/01.aids.0000199822.49488.50. [DOI] [PubMed] [Google Scholar]
- 37.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One. 2011;6:e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao C, Xu L, Abrams DI, Towner WJ, Horberg MA, Leyden WA, et al. HMG-CoA reductase inhibitors (statins) use and risk of non-Hodgkin lymphoma in HIV-positive persons. AIDS. 2011;25:1771–1777. doi: 10.1097/QAD.0b013e328349c67a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray SM, Down CM, Boulware DR, Stauffer WM, Cavert WP, Schacker TW, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84:12082–12086. doi: 10.1128/JVI.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 42.Kvale D, Ormaasen V, Kran AM, Johansson CC, Aukrust P, Aandahl EM, et al. Immune modulatory effects of cyclooxygenase type 2 inhibitors in HIV patients on combination antiretroviral treatment. AIDS. 2006;20:813–820. doi: 10.1097/01.aids.0000218544.54586.f1. [DOI] [PubMed] [Google Scholar]
- 43.Pettersen FO, Torheim EA, Dahm AEA, Aaberge IS, Lind A, Holm M, et al. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J Virol. 2011;85:6557–6566. doi: 10.1128/JVI.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read SW, DeGrezia M, Ciccone EJ, DerSimonian R, Higgins J, Adelsberger JW, et al. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One. 2010;5:e11937. doi: 10.1371/journal.pone.0011937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floren CH, Chinenye S, Elfstrand L, Hagman C, Ihse I. Colo-Plus, a new product based on bovine colostrum, alleviates HIV-associated diarrhoea. Scand J Gastroenterol. 2006;41:682–686. doi: 10.1080/00365520500380817. [DOI] [PubMed] [Google Scholar]
- 46.Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. 2008;42:239–243. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- 47.Kaducu FO, Okia SA, Upenytho G, Elfstrand L, Floren CH. Effect of bovine colostrum-based food supplement in the treatment of HIV-associated diarrhea in Northern Uganda: a randomized controlled trial. Indian J Gastroenterol. 2011;30:270–276. doi: 10.1007/s12664-011-0146-0. [DOI] [PubMed] [Google Scholar]
- 48.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretro-viral therapy: a prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr. 2006;42:523–528. doi: 10.1097/01.qai.0000230529.25083.42. [DOI] [PubMed] [Google Scholar]
- 50.Trois L, Cardoso EM, Miura E. Use of probiotics in HIV-infected children: a randomized double-blind controlled study. J Trop Pediatr. 2008;54:19–24. doi: 10.1093/tropej/fmm066. [DOI] [PubMed] [Google Scholar]
- 51.Irvine SL, Hummelen R, Hekmat S, Looman CW, Habbema JD, Reid G. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. J Clin Gastroenterol. 2010;44:e201–e205. doi: 10.1097/MCG.0b013e3181d8fba8. [DOI] [PubMed] [Google Scholar]
- 52.Byakwaga H, Kelly M, Purcell DF, French MA, Amin J, Lewin SR, et al. Intensification of antiretroviral therapy with raltegravir or addition of hyperimmune bovine colostrum in HIV-infected patients with suboptimal CD4+ T-cell response: a randomized controlled trial. J Infect Dis. 2011;204:1532–1540. doi: 10.1093/infdis/jir559. [DOI] [PubMed] [Google Scholar]
- 53.Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, Hoh R, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yukl SA, Shergill AK, McQuaid K, Gianella S, Lampiris H, Hare CB, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chege D, Kovacs C, la Porte C, Ostrowski M, Raboud J, Su D, et al. Effect of raltegravir intensification on HIV proviral DNA in the blood and gut mucosa of men on long-term therapy: a randomized controlled trial. AIDS. 2012;26:167–174. doi: 10.1097/QAD.0b013e32834e8955. [DOI] [PubMed] [Google Scholar]
- 58.Yilmaz A, Verhofstede C, D’Avolio A, Watson V, Hagberg L, Fuchs D, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–596. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 59.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 60.Vallejo A, Gutierrez C, Hernandez-Novoa B, Diaz L, Madrid N, Abad-Fernandez M, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26:1885–1894. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez C, Diaz L, Vallejo A, Hernandez-Novoa B, Abad M, Madrid N, et al. Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PLoS One. 2011;6:e27864. doi: 10.1371/journal.pone.0027864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkin TJ, Lalama CM, McKinnon J, Gandhi RT, Lin N, Landay A, et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis. 2012;206:534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuzin L, Trabelsi S, Delobel P, Barbuat C, Reynes J, Allavena C, et al. Maraviroc intensification of stable antiviral therapy in HIV-1-infected patients with poor immune restoration: MAR-IMUNO-ANRS 145 study. J Acquir Immune Defic Syndr. 2012;61:557–564. doi: 10.1097/QAI.0b013e318273015f. [DOI] [PubMed] [Google Scholar]
- 64.Hunt P, Shulman N, Hayes TL, Dahl V, Funderburg N, Adeyemi O, et al. Immunomodulatory effects of MVC intensification in HIV-infected individuals with incomplete CD4+ T cell recovery during suppressive ART. 18th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2011. [Google Scholar]
- 65.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez VD, Falconer K, Blom KG, Reichard O, Morn B, Laursen AL, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009;83:11407–11411. doi: 10.1128/JVI.01211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negredo E, Clotet B, Puig J, Perez-Alvarez N, Ruiz L, Romeu J, et al. The effect of atorvastatin treatment on HIV-1-infected patients interrupting antiretroviral therapy. AIDS. 2006;20:619–621. doi: 10.1097/01.aids.0000210617.90954.0e. [DOI] [PubMed] [Google Scholar]
- 68.Vermeulen JN, Prins JM, Bunnik E, Hack CE, Jurriaans S, Miedema F, et al. Intravenous immunoglobulin (IVIG) treatment for modulation of immune activation in human immunodeficiency virus type 1 infected therapy-naive individuals. AIDS Res Hum Retroviruses. 2007;23:1348–1353. doi: 10.1089/aid.2006.0210. [DOI] [PubMed] [Google Scholar]
- 69.Mellberg T, Gonzalez VD, Lindkvist A, Eden A, Sonnerborg A, Sandberg JK, et al. Rebound of residual plasma viremia after initial decrease following addition of intravenous immunoglobulin to effective antiretroviral treatment of HIV. AIDS Res Ther. 2011;8:21. doi: 10.1186/1742-6405-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho EL, Spudich SS, Lee E, Fuchs D, Sinclair E, Price RW. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res Ther. 2011;8:17. doi: 10.1186/1742-6405-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange JM, Gazzard B, Diaz R. Reduced CD4+ T cell decline and immune activation by NR100157: a specific multitargeted nutritional intervention in HIV-1 positive adults not on antiretroviral therapy (BITE). 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco, California. 2009. [Google Scholar]
- 72.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, et al. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS. 2003;17:2461–2469. doi: 10.1097/00002030-200311210-00008. [DOI] [PubMed] [Google Scholar]
- 73.Kelly P, Katubulushi M, Todd J, Banda R, Yambayamba V, Fwoloshi M, et al. Micronutrient supplementation has limited effects on intestinal infectious disease and mortality in a Zambian population of mixed HIV status: a cluster randomized trial. Am J Clin Nutr. 2008;88:1010–1017. doi: 10.1093/ajcn/88.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hummelen R, Changalucha J, Butamanya NL, Koyama TE, Cook A, Habbema JD, et al. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microb. 2011;2:80–85. doi: 10.4161/gmic.2.2.15787. [DOI] [PubMed] [Google Scholar]
- 75.Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the ‘COPA’ pilot randomized trial. Mucosal Immunol. 2011;4:554–563. doi: 10.1038/mi.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gandhi RT, Coombs RW, Chan ES, Bosch RJ, Zheng L, Margolis DM, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eltawil KM, Laryea M, Peltekian K, Molinari M. Rifaximin vs. conventional oral therapy for hepatic encephalopathy: a meta-analysis. World J Gastroenterol. 2012;18:767–777. doi: 10.3748/wjg.v18.i8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandrea I, Haret-Richter G, Ma D, Ribeiro RM, Nusbaum R, Trichel A, et al. Administration of rifaximin and sulfasalazine during acute SIV infection decreases microbial translocation and coagulation marker levels and significantly impacts viral replication. 19th Conference on Retrovirology and Opportunistic Infections; Seattle, Washington. 2012.. [Google Scholar]
- 79.Valdes-Ferrer SI, Crispin JC, Belaunzaran PF, Cantu-Brito CG, Sierra-Madero J, Alcocer-Varela J. Acetylcholine-esterase inhibitor pyridostigmine decreases T cell overactivation in patients infected by HIV. AIDS Res Hum Retroviruses. 2009;25:749–755. doi: 10.1089/aid.2008.0257. [DOI] [PubMed] [Google Scholar]
- 80.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Donate-Correa J, Cazana-Perez V, Garcia-Perez J. Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2272–2279. doi: 10.2215/CJN.01650211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez-Torres M, Rodriguez-Orengo JF, Rios-Bedoya CF, Fernandez-Carbia A, Salgado-Mercado R, Marxuach-Cuetara AM. Double-blind pilot study of mesalamine vs. placebo for treatment of chronic diarrhea and nonspecific colitis in immunocompetent HIV patients. Dig Dis Sci. 2006;51:161–167. doi: 10.1007/s10620-006-3102-6. [DOI] [PubMed] [Google Scholar]
- 82.Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Rhame F, et al. Angiotensin cenverting enzyme inhibitor (ACE-I) and/or HMG-CoA reductase inhibitor (’statin’) treatment as adjunct therapy for persons with HIV infection: a pilot study. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. 2012.. [Google Scholar]
- 83.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Inhibition of TGFbeta1 by anti-TGFbeta1 antibody or lisinopril reduces thyroid fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2002;169:6530–6538. doi: 10.4049/jimmunol.169.11.6530. [DOI] [PubMed] [Google Scholar]
- 84.Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, et al. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an antiinterferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 85.Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, et al. Safety profile and clinical activity of sifalimumab, a fully human antiinterferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 86.Yao Y, Higgs BW, Richman L, White B, Jallal B. Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNalpha antibody, in systemic lupus erythematosus. Arthritis Res Ther. 2010;12 (Suppl 1):S6. doi: 10.1186/ar2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.d’Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses. 2011;27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 88.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasma-cytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt B, Ashlock BM, Foster H, Fujimura SH, Levy JA. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–266. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 90.Martinson JA, Montoya CJ, Usuga X, Ronquillo R, Landay AL, Desai SN. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob Agents Chemother. 2010;54:871–881. doi: 10.1128/AAC.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aandahl EM, Aukrust P, Skalhegg BS, Muller F, Froland SS, Hansson V, et al. Protein kinase A type I antagonist restores immune responses of T cells from HIV-infected patients. FASEB J. 1998;12:855–862. doi: 10.1096/fasebj.12.10.855. [DOI] [PubMed] [Google Scholar]
- 92.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Tasken K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell Signal. 2002;14:1–9. doi: 10.1016/s0898-6568(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 93.Brudvik KW, Tasken K. Modulation of T cell immune functions by the prostaglandin E(2) - cAMP pathway in chronic inflammatory states. Br J Pharmacol. 2012;166:411–419. doi: 10.1111/j.1476-5381.2011.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johansson CC, Bryn T, Aandahl EM, Areklett MA, Aukrust P, Tasken K, et al. Treatment with type-2 selective and nonselective cyclooxygenase inhibitors improves T-cell proliferation in HIV-infected patients on highly active antiretroviral therapy. AIDS. 2004;18:951–952. doi: 10.1097/00002030-200404090-00015. [DOI] [PubMed] [Google Scholar]
- 95.Hossain MM, Margolis DM. Inhibition of HIV replication by A77 1726, the active metabolite of leflunomide, in combination with pyrimidine nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2001;28:199–201. doi: 10.1097/00042560-200110010-00016. [DOI] [PubMed] [Google Scholar]
- 96.Schlapfer E, Fischer M, Ott P, Speck RF. Anti-HIV-1 activity of leflunomide: a comparison with mycophenolic acid and hydroxyurea. AIDS. 2003;17:1613–1620. doi: 10.1097/01.aids.0000072664.21517.ad. [DOI] [PubMed] [Google Scholar]
- 97.Davis JP, Cain GA, Pitts WJ, Magolda RL, Copeland RA. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996;35:1270–1273. doi: 10.1021/bi952168g. [DOI] [PubMed] [Google Scholar]
- 98.Cherwinski HM, Cohn RG, Cheung P, Webster DJ, Xu YZ, Caulfield JP, et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther. 1995;275:1043–1049. [PubMed] [Google Scholar]
- 99.Attili SV, Gulati AK, Singh VP, Varma DV, Rai M, Sundar S. Diarrhea, CD4 counts and enteric infections in a hospital-based cohort of HIV-infected patients around Varanasi, India. BMC Infect Dis. 2006;6:39. doi: 10.1186/1471-2334-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lazzerini M, Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2008:CD005436. doi: 10.1002/14651858.CD005436.pub2. [DOI] [PubMed] [Google Scholar]
- 101.Plettenberg A, Stoehr A, Stellbrink HJ, Albrecht H, Meigel W. A preparation from bovine colostrum in the treatment of HIV-positive patients with chronic diarrhea. Clin Investig. 1993;71:42–45. doi: 10.1007/BF00210962. [DOI] [PubMed] [Google Scholar]
- 102.Ellis CL, Ma ZM, Mann SK, Li CS, Wu J, Knight TH, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr. 2011;57:363–370. doi: 10.1097/QAI.0b013e31821a603c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merlini E, Bai F, Bellistri GM, Tincati C, d’Arminio Monforte A, Marchetti G. Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS One. 2011;6:e18580. doi: 10.1371/journal.pone.0018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bastard JP, Soulie C, Fellahi S, Haim-Boukobza S, Simon A, Katlama C, et al. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther. 2012;17:915–919. doi: 10.3851/IMP2093. [DOI] [PubMed] [Google Scholar]
- 105.Steel A, Cox AE, Shamji MH, John L, Nelson M, Henderson DC, et al. HIV-1 viral replication below 50 copies/ml in patients on antiretroviral therapy is not associated with CD8+ T-cell activation. Antivir Ther. 2007;12:971–975. [PubMed] [Google Scholar]
- 106.Hatano H, Scherzer R, Wu Y, Harvill K, Maka K, Hoh R, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61:317–325. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Westrop SJ, Moyle G, Jackson A, Nelson M, Mandalia S, Imami N. CCR5 antagonism impacts vaccination response and immune profile in HIV-1 infection. Mol Med. 2012;18:1240–1248. doi: 10.2119/molmed.2012.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Canestri A, Krivine A, Assoumou L, Le Corre M, Rozenberg F, Marcelin AG, et al. Maraviroc does not affect humoral response to the pandemic influenza A-H1N1v 2009 adjuvanted vaccine in HIV-1-infected patients. AIDS. 2010;24:2887–2889. doi: 10.1097/QAD.0b013e3283402bc1. [DOI] [PubMed] [Google Scholar]
- 109.Markowitz M, Evering T, Figueroa K, Rodriguez M, La Mar M, Garmon D, et al. Very early initiation of combination anti-retroviral therapy results in normal levels of markers of immune activation. XIX International AIDS Conference; Washington DC, USA. 2012. [Google Scholar]
- 110.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 111.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, et al. Old age and anticytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 113.Guzman-Fulgencio M, Berenguer J, de Castro IF, Micheloud D, Lopez JC, Cosin J, et al. Sustained virological response to interferon-alpha plus ribavirin decreases inflammation and endothelial dysfunction markers in HIV/HCV co-infected patients. J Antimicrob Chemother. 2011;66:645–649. doi: 10.1093/jac/dkq518. [DOI] [PubMed] [Google Scholar]