Abstract
Objective
Patients with rheumatoid arthritis (RA) are at increased risk for herpes zoster (HZ) (i.e., shingles). The aim of this study was to determine whether treatment with tofacitinib increases the risk of HZ in patients with RA.
Methods
HZ cases were identified as those reported by trial investigators from the databases of the phase II, phase III, and long-term extension (LTE) clinical trials in the Tofacitinib RA Development Program. Crude incidence rates (IRs) of HZ per 100 patient-years (with 95% confidence intervals [95% CIs]) were calculated by exposure group. Logistic regression analyses were performed to evaluate potential risk factors for HZ (e.g., age, prednisone use).
Results
Among 4,789 participants, 239 were identified as having tofacitinib-associated HZ during the phase II, phase III, and LTE trials, of whom 208 (87%) were female and whose median age was 57 years (range 21–75 years). One HZ case (0.4%) was multidermatomal; none of the cases involved visceral dissemination or death. Twenty-four patients with HZ (10%) permanently discontinued treatment with tofacitinib, and 16 (7%) were either hospitalized or received intravenous antiviral drugs. The crude HZ IR across the development program was 4.4 per 100 patient-years (95% CI 3.8–4.9), but the IR was substantially higher within Asia (7.7 per 100 patient-years, 95% CI 6.4–9.3). Older age was associated with HZ (odds ratio 1.9, 95% CI 1.5–2.6), and IRs for HZ were similar between patients receiving 5 mg tofacitinib twice daily (4.4 per 100 patient-years, 95% CI 3.2–6.0) and those receiving 10 mg twice daily (4.2 per 100 patient-years, 95% CI 3.1–5.8). In the phase III trials among placebo recipients, the incidence of HZ was 1.5 per 100 patient-years (95% CI 0.5–4.6).
Conclusion
In the Tofacitinib RA Development Program, increased rates of HZ were observed in patients treated with tofacitinib compared with those receiving placebo, particularly among patients within Asia. Complicated HZ among tofacitinib-treated patients was rare.
The reactivation of varicella zoster virus (VZV), also known as herpes zoster (HZ) or shingles, is of substantial public health importance, as up to one-third of adults in the US will develop HZ within their lifetime (1–4). Nearly 100% of individuals in the US age 40 years or older have been exposed to this virus, and its reactivation is related to diminished VZV-specific cell-mediated immunity associated with increasing age and immunosuppressive conditions. Among patients who develop HZ, postherpetic neuralgia occurs in ∼15%, causing long-term disability. More rarely, disseminated forms of HZ occur, potentially leading to encephalitis and death. In the US, the annual incidence rates (IRs) of HZ vary between 0.4 and 1.1 per 100 patient-years in patients ages 50–80 years, with the highest rates seen in women (1). In patients with rheumatoid arthritis (RA), the risk of HZ is elevated 2–3-fold (5,6).
Although certain disease-modifying therapies, such as anti–tumor necrosis factor α (anti-TNFα) therapies, have been linked to further increases in risk, studies evaluating this have produced conflicting results. To date, only prednisone has been consistently shown to increase the risk of shingles by an additional 1.5–2-fold (7). Furthermore, although disseminated HZ is much more common in immunosuppressed populations, it is unclear whether RA and/or its disease-modifying therapies increase the risk (7,8).
Tofacitinib is an oral JAK inhibitor for the treatment of RA. Tofacitinib preferentially inhibits JAK-3 and/or JAK-1, modulating the immune response via down-regulation of several cytokines (e.g., interleukins 2, 4, 7, 9, 15, and 21) that are integral to lymphocyte development and function (9). Given its mechanism of action and the increased baseline risk of HZ among patients with RA, an increased risk of HZ is a theoretical concern. Accordingly, we undertook a retrospective evaluation of HZ cases as reported within the Tofacitinib RA Development Program, with the objectives of describing the outcomes of and identifying risk factors for HZ among tofacitinib-treated patients, as well as evaluating the relative incidence of HZ in tofacitinib-treated patients as compared with those receiving placebo.
PATIENTS AND METHODS
Development program
The Tofacitinib RA Development Program was a large, global clinical study in patients with RA. We evaluated the data from 6 phase II studies (10–15), 5 phase III studies (16–20), and 2 open-label long-term extension (LTE) studies (21,22), comprising a total of 4,789 treated patients and 5,651 patient-years of tofacitinib exposure across 44 nations worldwide (as of March 2011). The phase III trials included patients who were treated with tofacitinib at a dosage of either 5 mg or 10 mg twice daily or patients who received placebo. One of the trials included an adalimumab active control arm (n = 204). In the phase III studies of 6 months' duration, patients receiving placebo were advanced to the tofacitinib treatment arm at month 3, receiving a dosage of either 5 mg or 10 mg twice daily. In the phase III studies of 12 months' duration, nonresponding patients receiving placebo were advanced to the tofacitinib treatment arm, at a dosage of either 5 mg or 10 mg twice daily, at month 3, while all remaining patients receiving placebo were advanced to the tofacitinib arm at month 6.
All phase II, phase III, and LTE studies were conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the regulations of the relevant local countries. All enrolled patients provided informed consent, and all participating institutions provided institutional review board approval prior to participation.
For each study, adverse event (AE) data were reported by site investigators and entered within the Pfizer clinical database. Standard Medical Dictionary for Regulatory Activities (MedDRA) codes were used to categorize AEs, and infectious AEs such as HZ were graded “serious” if they required hospitalization or the use of parenteral therapy.
Identification and description of HZ cases
For the phase II, phase III, and open-label LTE studies, we searched the databases for all preferred and low-level MedDRA terms that contained text consistent with a potential diagnosis of HZ, as reported by the site investigators for each treated patient; a data cutoff date of March 29, 2011 was used. These terms included “Herpes zoster” (MedDRA code 10019974), “Herpes zoster disseminated” (MedDRA code 10065038), “Herpes zoster infection neurological” (MedDRA code 10061208), “Herpes zoster multi-dermatomal” (MedDRA code 10058428), “Herpes zoster ophthalmic” (MedDRA code 10019983), “Herpes zoster oticus” (MedDRA code 10063491), “Varicella” (MedDRA code 10046980), and “Varicella post vaccine” (MedDRA code 10063522). For cases classified by investigators as “serious,” we reviewed reports on serious AEs to validate this designation and to describe the treatment and outcomes.
For all HZ cases occurring in the Tofacitinib RA Development Program, we collected descriptive clinical and epidemiologic information on each patient at the time of randomization (baseline), including age, sex, race, site of enrollment, corticosteroid use (limited by protocol to ≤10 mg/day prednisone or equivalent), concomitant use of nonbiologic disease-modifying antirheumatic drugs (DMARDs), comorbidities (e.g., smoking and diabetes), body mass index (BMI), measures of RA severity (Disease Activity Score in 28 joints 23 utilizing the C-reactive protein level), and disease duration (years since diagnosis), as well as baseline lymphocyte counts.
Nested case–noncase study
To evaluate risk factors for HZ among tofacitinib-treated patients, we conducted a formal case–noncase study among all tofacitinib-treated patients from the phase II, phase III, and LTE trials; 15 patients with incomplete records were excluded from the case–noncase analysis. Demographic variables, medical comorbidities, known risk factors for HZ (i.e., age, prednisone use), and other collected covariates (as described above) and their association with the outcome of HZ were evaluated, first, using univariate logistic regression. Age, baseline BMI, RA severity, RA duration, and lymphocyte counts were evaluated as continuous variables. The associations between HZ and these covariates were checked one at a time. In addition, a stepwise logistic regression was performed, with a significance level of <0.05 for entry and elimination. The covariates selected by the stepwise algorithm were confirmed by testing whether their apparent associations remained when combined with the other selected covariates in a linear logistic model in which inclusion was forced. We conducted all analyses using SAS statistical software.
Calculation of the HZ IR and evaluation of comparative risk
Across the tofacitinib development program, HZ events were attributed to either tofacitinib, placebo, or adalimumab, based on the treatment exposure at the time of the event. We calculated crude IRs for HZ per 100 patient-years (with 95% confidence intervals [95% CIs]) in tofacitinib-treated patients, with the rates stratified by race, age, baseline DMARD use, and other factors. For those phase III studies in which only the tofacitinib dosage groups of 5 mg or 10 mg twice daily were evaluated, we separately calculated crude IRs for each study arm (tofacitinib 5 mg twice daily, tofacitinib 10 mg twice daily, placebo, and adalimumab), and Kaplan-Meier curves for the phase III studies were constructed, depicting time-to-event for all randomized groups. To further evaluate comparative risk by exposure, we conducted a formal cohort study in the first 3 months after the start of treatment (given that nonresponding patients could advance to receive tofacitinib for the first time at month 3). In this study, patients were censored at the time of the HZ event, death, study discontinuation, or 3 months, whichever came first. Crude HZ incidence in this time interval was calculated and compared between each treatment group.
RESULTS
Incidence of HZ
Among 4,789 patients treated with tofacitinib in the phase II, phase III, and LTE studies, we identified 239 reported cases of HZ (5% of patients; crude IR 4.4 per 100 patient-years, 95% CI 3.8–4.9). Of these, 16 (7%) were cases of serious HZ; all patients with serious HZ were either hospitalized or received intravenous antiviral agents (for nonserious cases, antiviral use was not systematically reported by the investigators). Among the 16 serious cases, most patients were receiving 5 mg tofacitinib twice daily (n = 12) and most were female (n = 15). The serious cases occurred in Japan (n = 10), Korea (n = 2), and 1 each in Argentina, the Czech Republic, Croatia, and Finland. Among all 239 of the serious and nonserious HZ cases, no patient died or experienced visceral HZ. One multidermatomal case and 2 ocular HZ cases were reported.
For most of the patients who developed HZ (89%), either temporary discontinuation or no discontinuation of the study drug was reported; in the remaining 10% treatment with tofacitinib was permanently discontinued due to the HZ event. Among patients treated with tofacitinib, crude IRs for HZ were lowest for those lacking concomitant treatment with both methotrexate and prednisone (IR 3.4 per 100 patient-years, 95% CI 2.4–4.7), but the rates were not significantly different in patients who received both methotrexate and prednisone (IR 4.3 per 100 patient-years, 95% CI 3.5–5.4), those who received prednisone without methotrexate (IR 5.7 per 100 patient-years, 95% CI 4.4–7.3), or those who received methotrexate without prednisone (IR 4.2 per 100 patient-years, 95% CI 3.3–5.4). With regard to the possibility of an effect of prednisone according to age, there was a trend toward higher IRs for HZ in patients receiving prednisone compared with those not receiving prednisone, regardless of age. The crude IR for HZ in patients ≥60 years of age receiving prednisone was 6.3 per 100 patient-years (95% CI 4.8–8.2), compared with a crude IR of 5.4 per 100 patient-years (95% CI 4.0–7.2) in those without prednisone use, while in patients <60 years of age, the crude IRs were 4.2 per 100 patient-years (95% CI 3.4–5.2) and 3.1 per 100 patient-years (95% CI 2.4–4.1) among those with and those without prednisone use, respectively.
The results of univariate analysis demonstrated that patients who developed HZ were significantly more likely to be older or to be enrolled from Asian study sites. A trend toward an increased risk of HZ was observed in patients reporting a history of oral corticosteroid use at baseline, those with lower lymphocyte counts at baseline, or those with longer durations of RA at baseline (Table 1). In multivariate analysis, only older age and having Asia as the site of enrollment were independently associated with the risk of developing HZ (Table 1).
Table 1.
Univariate and multivariate analyses of the baseline factors assessed for associations with HZ among tofacitinib-treated patients in the phase II, phase III, and long-term extension studies*
| HZ cases (n = 239) | Non-HZ cases (n = 4,550) | Univariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|---|---|
| Age, median (range) years | 57 (21–75) | 54 (18–86) | 1.7 (1.3–2.3)† | 1.9 (1.5–2.6) |
| Female, no. (%) | 208 (87) | 3,795 (84) | 1.3 (0.9–1.9) | – |
| Race, no. (%) | ||||
| White | 114 (48) | 2,756 (61) | – | – |
| Black | 3 (1) | 136 (3) | – | – |
| Asian | 107 (45) | 1,217 (27) | 2.2 (1.7–2.9) | 2.4 (1.9–3.2) |
| Duration of RA, mean years | 9.9 | 8.9 | 1.2 (1.0–1.6)† | – |
| Diabetes mellitus, no. (%) | 21 (9) | 351 (8) | 1.1 (0.7–1.8) | – |
| COPD, no. (%) | 18 (7.5) | 341 (7.5) | 1.0 (0.6–1.6) | – |
| Smoking, no. (%) | 77 (32) | 1,530 (34) | 0.7 (0.5–1.1) | – |
| BMI, mean kg/m2 | 25.8 | 26.8 | 0.7 (0.5–1.0)† | – |
| Absolute lymphocyte count, mean | 1.5 | 1.7 | 1.3 (1.0–1.7)† | – |
| Severity of RA, mean DAS28-ESR | 5.2 | 5.3 | 0.8 (0.6–1.1)† | – |
| Concomitant DMARDs, no. (%) | ||||
| Methotrexate | 144 (60) | 2,900 (64) | 0.9 (0.7–1.1) | – |
| Leflunomide | 7 (3) | 202 (4.5) | 0.6 (0.3–1.4) | – |
| Hydroxychloroquine | 19 (8) | 309 (7) | 1.2 (0.7–1.9) | – |
| Baseline corticosteroids‡ | 140 (59) | 2,419 (53) | 1.2 (1.0–1.6) | – |
95% CI = 95% confidence interval; RA = rheumatoid arthritis; COPD = chronic obstructive pulmonary disease; BMI = body mass index; DAS28-ESR = Disease Activity Score in 28 joints (4-variable) using the erythrocyte sedimentation rate; DMARDs = disease-modifying antirheumatic drugs.
Odds ratios (ORs) calculated for continuous variables were the factor by which the odds of herpes zoster (HZ) being present increased in response to an increase of 2 standard deviations in a given variable.
Per protocol, patients were allowed to take corticosteroids with a prednisone or equivalent dosage of ≤10 mg daily.
Comparative risk cohort studies
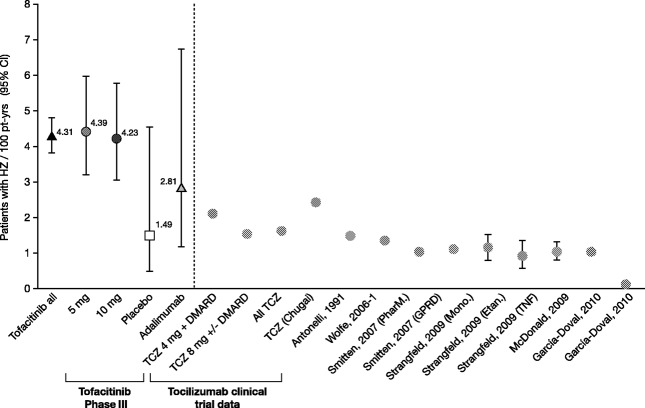
Within the phase III studies, patients in the tofacitinib 5 mg twice daily, tofacitinib 10 mg twice daily, placebo, and adalimumab exposure groups were similar with regard to known HZ risk factors such as age, sex, and corticosteroid use (Table 2). Overall, the crude IR for HZ in tofacitinib-treated patients was 4.3 per 100 patient-years (95% CI 3.4–5.4), with rates being similar between the tofacitinib 5 mg twice daily group and tofacitinib 10 mg twice daily group. A trend toward a lower incidence of HZ was noted in patients treated with adalimumab (2.8 per 100 patient-years, 95% CI 1.2–6.8) and patients receiving placebo (1.5 per 100 patient-years, 95% CI 0.5–4.6) (5,24–30) (Figure 1).
Table 2.
Baseline characteristics of the patients entering the phase III tofacitinib trials, by treatment exposure group*
| Tofacitinib | Placebo (n = 681) | Adalimumab (n = 204) | ||
|---|---|---|---|---|
| 5 mg BID (n = 1,216) | 10 mg BID (n = 1,214) | |||
| Age, median (range) years | 54 (19–86) | 54 (18–86) | 54 (18–82) | 54 (24–78) |
| Female, no. (%) | 1,027 (84) | 1,031 (85) | 553 (81) | 162 (79) |
| Race, no. (%) | ||||
| White | 737 (61) | 741 (61) | 439 (64) | 148 (72) |
| Black | 45 (4) | 35 (3) | 24 (3) | 3 (1) |
| Asian | 327 (27) | 314 (26) | 166 (24) | 29 (14) |
| Other | 107 (9) | 124 (10) | 52 (8) | 24 (12) |
| Duration of RA, mean years | 8.7 | 9.1 | 9.3 | 8.1 |
| Diabetes mellitus, no. (%) | 108 (9) | 103 (9) | 48 (7) | 16 (8) |
| COPD, no. (%) | 104 (9) | 105 (9) | 64 (9) | 11 (5) |
| Smoking history, no. (%) | 408 (34) | 406 (33) | 254 (37) | 71 (35) |
| BMI, mean (range) kg/m2 | 27 (14–71) | 27 (14–58) | 27 (15–55) | 27 (14–46) |
| Severity of RA, mean DAS28-CRP | 5.4 | 5.3 | 5.3 | 5.3 |
| Concomitant DMARDs, no. (%) | ||||
| Methotrexate | 903 (74) | 899 (74) | 520 (76) | 199 (97) |
| Leflunomide | 91 (7) | 84 (7) | 34 (5) | 0 (0) |
| Hydroxychloroquine | 115 (9) | 104 (9) | 51 (7) | 2 (<1) |
| Baseline corticosteroids | 700 (58) | 675 (56) | 385 (57) | 116 (57) |
BID = twice daily; RA = rheumatoid arthritis; COPD = chronic obstructive pulmonary disease; BMI = body mass index; DAS28-CRP = Disease Activity Score in 28 joints (3-variable) using the C-reactive protein level; DMARDs = disease-modifying antirheumatic drugs.
Figure 1.
Crude incidence rates of herpes zoster (HZ) in the Tofacitinib Rheumatoid Arthritis (RA) Development Program (left of broken line) and in published studies of patients with RA treated with nonbiologic and biologic disease-modifying agents (right of broken line) (see refs.5 and24–30). HZ incidence rates (with 95% confidence intervals [95% CIs]) are expressed per 100 patient-years of exposure. The Tofacitinib all group comprises data from tofacitinib-treated patients in the phase II, phase III, and long-term extension studies of the development program. The Tofacitinib phase III group comprises data from the phase III studies in the 5 mg tofacitinib twice daily, 10 mg tofacitinib twice daily, placebo, and adalimumab exposure groups. The Tocilizumab (TCZ) clinical trial data group comprises data from patients treated with 4 mg TCZ plus disease-modifying antirheumatic drugs (DMARDs), those treated with 8 mg TCZ with or without DMARDs, all TCZ-treated patients, and TCZ-treated patients reported in the US Food and Drug Administration documents submitted by Chugai (29,30). PharM. = PharMetrics database; GPRD = General Practice Research Database; Mono. = monoclonal anti–tumor necrosis factor α (anti-TNFα) antibodies (adalimumab and infliximab); Etan. = etanercept.
Within the first 3 months after start of treatment, the incidence of HZ was 2.5-fold higher in patients receiving tofacitinib at a dosage of 10 mg twice daily (IR 5.2 per 100 patient-years, 95% CI 3.1–8.6) compared with patients receiving tofacitinib at a dosage of 5 mg twice daily (IR 2.1 per 100 patient-years, 95% CI 0.9–4.6), although the rates were not statistically significantly different between exposure groups given the limited number of events and the exposure time (Table 3). By 6 months after study start, the risk estimates for HZ were similar between the 5 mg twice daily and 10 mg twice daily exposure groups. Similar trends were seen among patients advancing from the placebo group to the 5 mg or 10 mg twice daily tofacitinib groups at months 3 and 6, in which the incidence of HZ trended higher within the first several months in those whose treatment was advanced to 10 mg twice daily compared with those whose treatment was advanced to 5 mg twice daily (Figure 2).
Table 3.
Crude incidence rates of HZ by treatment exposure group within the pooled phase III tofacitinib studies in the first 3 months after start of treatment
| HZ events | Patient-years of exposure | HZ incidence rate (95% CI)* | |
|---|---|---|---|
| Tofacitinib 5 mg BID (n = 1,216)† | 6 | 290 | 2.1 (0.9–4.6) |
| Tofacitinib 10 mg BID (n = 1,214)† | 15 | 290 | 5.2 (3.1–8.6) |
| Placebo (n = 681) | 2 | 156 | 1.3 (0.3–5.1) |
| Adalimumab (n = 204) | 0 | 49 | 0 (0–7.5) |
The crude incidence rates of herpes zoster (HZ) events, with 95% confidence intervals (95% CIs), are expressed per 100 patient-years.
BID = twice daily.
Figure 2.
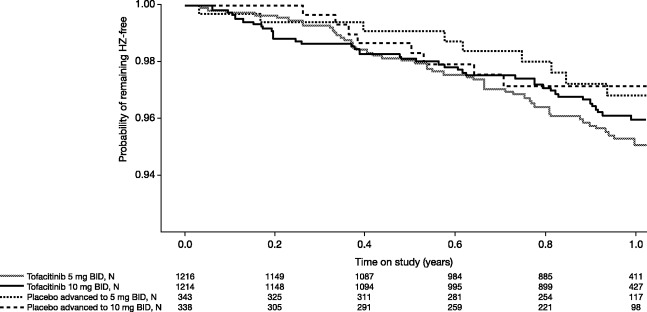
Kaplan-Meier curves depicting time to herpes zoster (HZ) development within the phase III tofacitinib studies, by treatment group. Note that the placebo-treated patients were advanced to the tofacitinib dosage groups of 5 mg or 10 mg twice daily (BID) at 3 months or 6 months during these trials. The numbers of patients in each treatment group are shown below each time point.
Across all phase II, phase III, and LTE studies, the crude IRs varied strongly by region of enrollment and by race. Although Asian race was a risk factor for HZ, all but 19 Asian patients were enrolled in Asia, making it impossible to evaluate race separately from region of enrollment. The crude rates of HZ within Asia were more than double those seen in North America, Europe, and Latin American regions, and within Asia, rates were significantly higher within some subregions compared with others. The highest rates of HZ were observed in Japan and Korea, whereas lower rates were seen in Southeast Asia (Table 4). When considering all sites of enrollment outside of Asia, the crude rates of HZ were similar between tofacitinib-treated patients (range of IRs 2.7–3.7 per 100 patient-years) (Table 4) and adalimumab-treated patients (5 cases in 175 subjects; IR 3.26 per 100 patient-years, 95% CI 1.4–7.8). The studies that included adalimumab did not include Asian patients, and therefore we were unable to ascertain whether the higher rates observed in Asian patients receiving tofacitinib could be attributed to race or to an effect of tofacitinib among patients of Asian race.
Table 4.
Crude incidence rates of HZ overall and by geographic region of enrollment in the phase II, phase III, and long-term extension studies
| HZ events | Patient-years of exposure | HZ incidence rate (95% CI)* | |
|---|---|---|---|
| Global rheumatoid arthritis program | 239 | 5,482 | 4.4 (3.8–4.9) |
| By region | |||
| US/Canada/Australia | 40 | 1,216 | 3.3 (2.4–4.5) |
| Western Europe | 12 | 450 | 2.7 (1.5–4.7) |
| Eastern Europe | 43 | 1,425 | 3.0 (2.2–4.1) |
| Latin America | 37 | 991 | 3.7 (2.7–5.2) |
| Asia | 107 | 1,388 | 7.7 (6.4–9.3) |
| Within Asian countries | |||
| Japan/Korea | 85 | 920 | 9.2 (7.5–11.4) |
| India | 8 | 90 | 8.9 (4.4–17.7) |
| Thailand/Malaysia/Philippines | 3 | 137 | 2.2 (0.7–6.8) |
| China/Taiwan | 11 | 241 | 4.6 (2.5–8.2) |
The crude incidence rates of herpes zoster (HZ) events, with 95% confidence intervals (95% CIs), are expressed per 100 patient-years.
DISCUSSION
The current studies are the largest analyses to date that have examined the risk of HZ in patients with RA treated with tofacitinib, a JAK inhibitor for RA. Within the Tofacitinib RA Development Program, we documented relatively high rates of HZ among patients treated with tofacitinib, those treated with adalimumab, and those receiving placebo, although IRs were higher among tofacitinib-treated patients compared with placebo recipients. Enrollment site was the most important risk factor for HZ, since high rates were observed within a subset of Asian countries. HZ rates were similar between the low- and high-dosage tofacitinib groups, although in the first several months after drug start, a somewhat higher risk was observed in the 10 mg twice daily group. Importantly, no patients with HZ who were being treated with tofacitinib developed visceral disease. Our data suggest that preventive strategies for HZ should be developed for patients with RA, given the high rate of HZ-related morbidity observed regardless of RA therapy.
Patients with RA are known to be at increased risk for HZ and this risk can be further exacerbated by certain RA therapies. Prednisone raises this risk 1.5-fold to 2-fold, in a dose-dependent manner, and biologic therapies such as TNFα antagonists have been suggested, in some but not all studies, to also increase this risk (24,25,31). Our data suggest that tofacitinib might also increase the risk of HZ, with higher IRs observed in tofacitinib-treated patients compared with those receiving placebo. A trend toward higher risk was also observed in tofacitinib-treated patients when compared with those receiving adalimumab, although the rates of HZ were similar between tofacitinib- and adalimumab-treated patients outside of Asia.
Whether there is a potential for increased risk of HZ with tofacitinib treatment compared with adalimumab or other TNF antagonists is unclear, since our study was not intended to evaluate this question and we lacked statistical power to evaluate risk differences between these groups. Regardless, evidence of any association between TNF blockade and HZ risk is conflicting to date. Some studies have suggested that there is a relatively higher risk of HZ with monoclonal antibody therapies, i.e., adalimumab and infliximab, when compared with etanercept, whereas another study suggested that there is a lower risk with adalimumab relative to etanercept (7). A more recent, large observational study within the US failed to show any increased risk associated with TNF blockade, since the reported rates of HZ were between 1.2 and 1.3 per 100 patient-years for patients with RA who had started treatment with either TNF blockers or nonbiologic DMARDs (8). Furthermore, similar to the reported experience with tofacitinib, patients who develop HZ under conditions of TNF blockade appear no more likely to develop disseminated infection (7).
A biologic mechanism has yet to be proposed or understood with regard to how TNF antagonists could raise the risk of HZ. T cell–mediated immune responses against VZV-specific antigens, as measured by interferon-γ (IFNγ) production from VZV-stimulated lymphocytes, are correlated with HZ immunity (32–34). Reactivation of VZV is more likely when such responses have waned (35). TNF antagonists have been shown to down-regulate the antigen-stimulated production of IFNγ in vitro with tuberculosis antigens and other antigens, although to our knowledge, such experiments with VZV-specific antigens and TNF antagonists have not been performed. Furthermore, it is unknown whether such a suppressed response would even promote the reactivation of VZV or would diminish control of infection by the host.
Similarly, understanding of the biologic mechanism that could explain a potential increased risk of VZV reactivation with tofacitinib is currently lacking. Human antiviral defense hinges upon intact type I (IFNα and IFNβ) and type II (IFNγ) responses (36), both of which signal via the JAK-1 receptor. Given that tofacitinib inhibits signaling through this receptor, it is possible that such a mechanism is related to an increased risk of HZ. Furthermore, tofacitinib could theoretically inhibit the development and/or maintenance of VZV-specific memory T cells (37). However, similar to the situation with TNF blockade, it is unclear how these potential mechanisms promote VZV reactivation, and our observations to date suggest that patients who do develop HZ while receiving tofacitinib are no more likely to have disseminated or multidermatomal disease.
Although the rates of HZ were similar between patients treated with 5 mg tofacitinib twice daily and those treated with 10 mg tofacitinib twice daily, there was a trend toward a higher event rate shortly after commencement of the drug at the higher dosage. This is consistent with the idea that there exists a pool of patients with waning cell-mediated immunity who are at higher risk of VZV reactivation, such that a dose-dependent effect could be seen for a short time period prior to depletion of such susceptible individuals following initiation of therapy. Further research should be undertaken to evaluate the mechanisms by which tofacitinib and/or TNF antagonists could promote VZV reactivation.
There was a large disparity in HZ rates within the tofacitinib development program based upon region of enrollment. Whereas the rates within North America and Europe were ∼1.5-fold to 2-fold higher than those observed within the clinical trial experiences with tocilizumab and various observational studies of RA populations (5,24–30) (Figure 1), the observed tofacitinib-associated rates of HZ within some regions of Asia were 2-fold to 3-fold higher than those observed within North America and Western Europe. Furthermore, within Asia, there were large differences by country and subregion, such that some rates within Asian areas were similar to those observed within North America and Western Europe. The reasons for this are unknown, and it is unclear whether it is due to race, genetic predisposition, or cultural or medical differences in the diagnosis of HZ between regions. Results of prior studies have suggested that the incidence of HZ can vary by race and region (38). Unfortunately, in our study very few Asian patients were enrolled outside of Asia, and therefore we were unable to examine race separately from region of enrollment as a risk factor for HZ. We explored factors such as BMI and concomitant medications (e.g., prednisone, methotrexate) as potential explanations for the differential rates observed, but no such factors were significantly associated with HZ development overall, nor did they account for the higher rates observed within Asia. Furthermore, there is no indication that the background incidence of HZ is higher in such regions (39,40). Interestingly, outside of Asia, the rates of HZ were similar between adalimumab- and tofacitinib-treated patients. Further research is warranted to clarify whether subsets of Asian patients are truly at higher risk for HZ during tofacitinib use and what mechanisms might explain such risk.
HZ is a preventable disease, and its prevention is of great interest among physicians treating patients with RA and other autoimmune diseases. Currently, the American College of Rheumatology recommends administration of Zostavax (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.), a live attenuated VZV vaccine, prior to initiation of biologic therapy in patients with RA age 60 years and older (41). Despite these recommendations and despite studies showing patients with RA to be at high risk for HZ, <5% of patients with RA have been vaccinated with Zostavax (42). While the barriers to such vaccination are not well defined, it is likely that contraindications against live vaccinations during biologic therapy preclude many physicians and patients from using this vaccine (43). Currently, the necessity of this contraindication is unclear, and limited specific data are available regarding the safety of this vaccine when used concomitantly with biologic agents, nonbiologic DMARDs, or prednisone.
One recent observational study suggested that a large number of patients being treated with biologic agents (n = 652, 551 of whom were taking anti-TNF agents) had been inadvertently vaccinated while actively receiving such therapies, without any resulting adverse sequelae or harm (44). That study also suggested that vaccinated patients had less subsequent risk of HZ, with a demonstrable vaccine efficacy similar to that observed in a large shingles prevention study (45). More recently, the vaccine was licensed for use in individuals age 50 years and older, although there is not currently a formal recommendation by professional organizations for use in this age group. Given the higher rates of HZ among patients with RA within this age group, it is likely that patients with RA between the ages of 50 years and 60 years would also benefit from vaccination. At this time, however, safety and efficacy information on Zostavax within RA populations is lacking. Studies should be undertaken to evaluate the utility of this vaccine among patients with RA who are receiving various immunosuppressant regimens, including tofacitinib.
In summary, within the tofacitinib development program for RA, higher-than-expected rates of HZ were documented, particularly within certain subregions of Asia. While a defined mechanism for JAK inhibition and HZ development has yet to be elucidated, further research should be conducted to identify potential explanations for these observations. Furthermore, and of utmost clinical importance, studies evaluating the safety and efficacy of Zostavax in the prevention of HZ among patients with RA are greatly needed.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Winthrop and coauthors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Winthrop, Valdez, Chew, Krishnaswami, Kawabata, Riese.
Acquisition of data. Yamanaka, Chew, Krishnaswami, Kawabata, Riese.
Analysis and interpretation of data. Winthrop, Yamanaka, Valdez, Mortensen, Chew, Krishnaswami, Kawabata, Riese.
ROLE OF THE STUDY SPONSOR
Pfizer Inc. facilitated the phase II, phase III, and LTE clinical trials in the Tofacitinib RA Development Program. The LTE studies included in this analysis (ClinicalTrials.gov Identifiers NCT00413699 and NCT00661661) are still ongoing; data derived from these studies are therefore interim results from an unlocked database that is still undergoing cleaning. Drs. Valdez, Mortensen, Chew, Krishnaswami, Kawabata, and Riese are employees of Pfizer Inc. For this retrospective analysis, the analysis plan was conceived by Dr. Winthrop and study team, with analysis conducted using a Pfizer statistician, Dr. Chew. The manuscript was drafted by Dr. Winthrop, with all authors providing subsequent critical revision. All authors interpreted the results, approved the final draft, and had the final decision to submit the manuscript for publication. Pfizer Inc. did not control the analysis or interpretation of the study results. Pfizer Inc. provided editorial assistance (performed by Jason Gardner and Martin Goulding at Complete Medical Communications, funded by Pfizer Inc.). Publication of this article was not contingent upon approval by Pfizer Inc.
Acknowledgments
We wish to thank Jennifer Ku (Oregon Health & Science University) for assistance in formatting the manuscript.
REFERENCES
- 1.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 2.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–4. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–6. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 5.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–8. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 6.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop KL, Furst DE. Rheumatoid arthritis and herpes zoster: risk and prevention in those treated with anti-tumour necrosis factor therapy. Ann Rheum Dis. 2010;69:1735–7. doi: 10.1136/ard.2010.133843. [DOI] [PubMed] [Google Scholar]
- 8.Winthrop KL, Baddley JW, Chen L, Liu L, Grijalva CG, Delzell E, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309:887–95. doi: 10.1001/jama.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 2010;7:41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Takeuchi T, Yamanaka H, Suzuki M, Nakamura H, Toyoizumi S, et al. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week phase IIb study. Arthritis Rheum. 2011;63(Suppl 10):S854–5. [abstract] [Google Scholar]
- 12.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Yamanaka H, Nakamura H, Takeuchi T. Study results of oral JAK inhibitor tofacitinib in patients with active RA [abstract]. Presented at the 55th Annual Scientific Meeting of the Japan College of Rheumatology; Kobe, Japan, July 17–20. 2011. URL: http://www.jcr2011.com. [Google Scholar]
- 14.Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 15.McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis. 2014;73:124–31. doi: 10.1136/annrheumdis-2012-202442. [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 17.Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four–month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 18.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 19.Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 20.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic DMARDs in patients with active rheumatoid arthritis: a randomized controlled trial. Ann Intern Med. 2013;159:253–61. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 21.Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, long-term extension studies. J Rheumatol. 2014;41:837–52. doi: 10.3899/jrheum.130683. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka H, Tanaka Y, Takeuchi T, Suzuki M, Nakamura H, Komuro Y, et al. Tofacitinib (CP,690,550), an oral Janus kinase inhibitor, as monotherapy or with background methotrexate in Japanese patients with rheumatoid arthritis: a phase 2/3 long-term extension study. Arthritis Rheum. 2011;63(Suppl 10):S473. [abstract] [Google Scholar]
- 23.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-α agents. JAMA. 2009;301:737–44. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–71. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonelli MA, Moreland LW, Brick JE. Herpes zoster in patients with rheumatoid arthritis treated with weekly, low-dose methotrexate. Am J Med. 1991;90:295–8. [PubMed] [Google Scholar]
- 27.Garcia-Doval I, Perez-Zafrilla B, Descalzo MA, Rosello R, Hernandez MV, Gomez-Reino JJ, et al. Incidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonists. Ann Rheum Dis. 2010;69:1751–5. doi: 10.1136/ard.2009.125658. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti–tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–34. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Drug approval package, Actemra (tocilizumab) injection: medical review, Table 4 and Table 6. 2010. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/125276s000MedR.pdf.
- 30.US Food and Drug Administration. Arthritis Advisory Committee meeting to discuss BLA 125276 for tocilizumab for the treatment of moderately to severely active rheumatoid arthritis (RA) 2008. URL: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4371b1-01-FDA.pdf.
- 31.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35:387–93. [PubMed] [Google Scholar]
- 32.Berger R, Florent G, Just M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32:24–7. doi: 10.1128/iai.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 34.Sadaoka K, Okamoto S, Gomi Y, Tanimoto T, Ishikawa T, Yoshikawa T, et al. Measurement of varicella-zoster virus (VZV)-specific cell-mediated immunity: comparison between VZV skin test and interferon-γ enzyme-linked immunospot assay. J Infect Dis. 2008;198:1327–33. doi: 10.1086/592219. [DOI] [PubMed] [Google Scholar]
- 35.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–54. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 37.Paniagua R, Si MS, Flores MG, Rousvoal G, Zhang S, Aalami O, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80:1283–92. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 38.Nagasako EM, Johnson RW, Griffin DR, Elpern DJ, Dworkin RH. Geographic and racial aspects of herpes zoster. J Med Virol. 2003;70(Suppl 1):S20–3. doi: 10.1002/jmv.10315. [DOI] [PubMed] [Google Scholar]
- 39.Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–51. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyama N, Shiraki K. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81:2053–8. doi: 10.1002/jmv.21599. [DOI] [PubMed] [Google Scholar]
- 41.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13:R174. doi: 10.1186/ar3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American College of Rheumatology. Update on herpes zoster (shingles) vaccine for autoimmune disease patients. 2013. URL: http://www.rheumatology.org/publications/hotline/2012_09_12_herpeszoster.asp.
- 44.Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43–9. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]