Abstract
Background
Autonomic ganglionated plexi (GPs) play a significant role in the initiation and maintenance of atrial fibrillation (AF). GPs are key targets for a maze procedure. The purpose of this study was to identify the location of the left atrial GPs based on dense epicardial mapping during a maze procedure in patients with concomitant AF.
Methods
Sixteen patients (age, 68 ± 10 years; 11 males, 69%) with heart failure and concomitant AF (duration 55 ± 86 months) underwent intraoperative epicardial electrophysiological mapping and a GP ablation using the maze procedure at our institution. Twenty-four-site, high-frequency stimulation (1,000/min; output, 18 V; pulse width, 0.75 ms) was performed by placing tweezers directly onto the potential GP sites on the left atrial epicardium.
Results
Active GPs were found in 13 (81%) of the 16 patients, and 12 (92%) of 13 patients had active GPs between the right pulmonary veins (PVs) and the interatrial groove. For those patients with active locations, a 7-day event-loop recording demonstrated that 12 (92%) of 13 patients were maintained in sinus rhythm 3 months after the operation.
Conclusion
Dense epicardial mapping in the potential GP areas identified active GP locations in a high percentage of patients. GPs between the PVs and interatrial groove have a high potential as ablation targets for treatment of concomitant AF.
Keywords: autonomic nervous system, ganglionated plexi, maze procedure, atrial fibrillation
Introduction
The intrinsic cardiac autonomic nervous system, which is composed of axons and ganglionated plexi (GPs) within the epicardial fat pads of the heart, has great plasticity in the initiation and maintenance of atrial fibrillation (AF).1–3
In human hearts, there are five left atrial GP areas located around the antrum of the pulmonary veins (PVs) and the interatrial groove.4 A new strategy for anatomically based GP ablation has recently been proposed for the treatment of paroxysmal and even long-standing persistent AF.5–8 The combination of a minimally invasive maze procedure with a GP ablation may decrease AF recurrences in lone AF patients at least in the short term.9–12 On the other hand, to the best of our knowledge, there have been no reports on dense epicardial mapping of GPs with an open surgical approach for patients undergoing concomitant AF ablation. Ware et al. treated concomitant AF patients with a GP ablation and a maze procedure; however, the mapping sites for the GPs were limited.13 Moreover, although GPs, especially in the posterior areas, require a highly technical approach to ablate them and have only a moderate probability of being identified as being active, some GPs in high-potential and readily approachable areas could be missed.
The purpose of this study was to identify the location of the left atrial GPs based on dense epicardial mapping during a maze procedure in patients with concomitant AF.
Materials and Methods
Patient Population
After providing written informed consent, 16 consecutive patients underwent treatment for concomitant AF that included GP mapping, ablation, and a valve repair or replacement through an open surgical approach. All patients had experienced failure or intolerance, or refused medical therapy for heart failure associated with AF. The exclusion criteria were patients over 80 years old and evidence of a thrombus in the heart.
Electrophysiological Mapping
The patients underwent intraoperative epicardial electrophysiological mapping at the time of the operation to determine the location of the active GPs. A previous study showed a schematic diagram of the epicardial mapping locations around the bilateral PVs and interatrial groove.9–12 We constructed a modified diagram of the possible GP locations for high-resolution mapping that were considered to be easily accessible (Fig. 1). In brief, the autonomic GPs were identified by rapid atrial pacing via a temporary pacemaker (Osypka Medical, Berlin, Germany) instead of the use of a specific stimulator after the manual removal of the fatty epicardial tissue on the epicardial surface. The maximum output of this particular temporary pacemaker was limited to 18 V.
Figure 1.
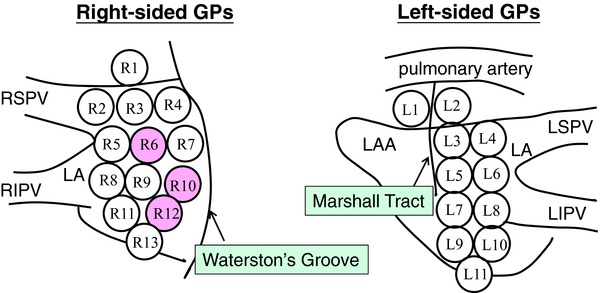
New diagram of the epicardial mapping locations. Active GPs were predominantly identified in the anterior and inferior right region of the left atrium between the right PVs and the interatrial groove (pink circle). GPs = ganglionated plexi; LA = left atrium; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
High-frequency stimulation (HFS) at a rate of 1,000 beats/min, output of 18 V, and pulse duration of 0.75 ms was achieved by placing tweezers directly on the left atrial epicardium. The electrocardiogram was monitored, and the locations where the stimulation site resulted in ventricular slowing with doubling of the electrocardiographic R-R interval, were identified as active GPs. All stimulation and mapping procedures were performed before any dissection or ablation. First, electrophysiology mapping on the right side was performed, followed by a right-sided GP ablation. Then, mapping on the left side was obtained, and subsequently followed by a GP ablation on the left.
GP Ablation
Radiofrequency GP ablation was performed by applying a dry bipolar pen probe as shown in Figure 2 (Atricure, West Chester, OH, USA). The cardiac response to the GP stimulation was eliminated with the radiofrequency ablation. After the procedure, the GPs were restimulated to confirm their ablation (Fig. 3). If the GPs remained active, the radiofrequency delivery was repeated. This entire process was duplicated until the responses of the GPs were completely eliminated. The GP ablation was performed and then followed by another radiofrequency procedure for AF, a maze IV procedure, and valve operation.
Figure 2.
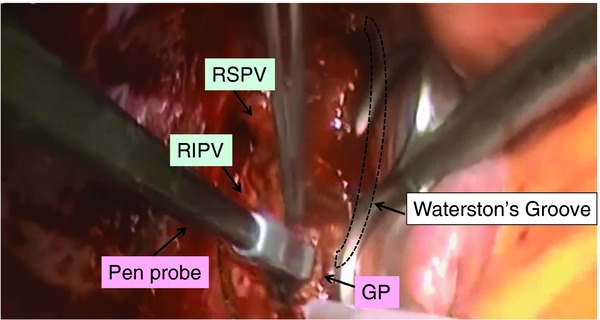
Radiofrequency ganglionated plexi (GP) ablation in the left atrium with a dry bipolar pen probe. RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Figure 3.
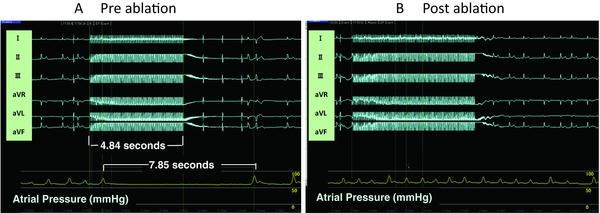
Parasympathetic response elicited during a 4.85 second burst of high-frequency stimulation (HFS) applied to the ganglionated plexi (GP). (A) An atrioventricular block lasting 7.85 seconds was induced by HFS. The ventricular conduction quickly returned after the HFS was terminated. (B) The cardiac response to the GP stimulation was eliminated by radiofrequency ablation.
Detection of AF Recurrence
Prolonged monitoring was required to evaluate the therapeutic interventions for AF. Therefore, an external loop recorder was used in this study. The SpiderFlash-t A-fib (Sorin Biomedical, Saluggia, Italy) is the only noninvasive event-loop recorder (ELR) that is capable of accurately detecting AF by a dedicated and AF-specific algorithm. This AF algorithm is independent of the main arrhythmia-detection algorithm embedded in the Spiderflash-t. Detection of irregular rhythms in general and those of AF in particular is based on an embedded moving histogram of 30 seconds. In addition, the dedicated AF algorithm evaluates the variance dispersion of the R-R intervals and identifies AF episodes as short as 10 seconds. Three months after surgery, all patients were fitted with this ELR and monitored for a 7-day period for continuous detection of any AF recurrences. All documented episodes of AF lasting ≥30 seconds were considered to be recurrences.
Statistical Analysis
All variables are reported as means ± standard deviations. Continuous variables were compared with a Student's t-test. All P values were two-sided, and statistical significance was established at a P < 0.05.
Results
Study Population
Sixteen patients (age, 68 ± 10; 11 males, 69%; left atrial diameter, 53 ± 8 mm; and left ventricular ejection fraction, 57 ± 12%) were enrolled in this study. The mean duration of AF was 55 ± 86 months; AF was paroxysmal in four patients (25%). All patients underwent intraoperative epicardial electrophysiological mapping and a GP ablation in addition to a maze IV procedure at our institution between April 2011 and August 2012. The clinical and electrophysiological characteristics of the study population are shown in Table 1.
Table 1.
Clinical and Electrophysiological Characteristics of the Study Population
| Patient | Age | Sex | Active GPs on the Right Side | Active GPs on the Left Side | LAD | EF | Valve Operation | AF |
|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 6,9,12 | None | 61 | 72 | MVP/TAP/CABG | Chronic |
| 2 | 16 | M | None | None | 50 | 59 | MVP/TAP | Chronic |
| 3 | 73 | F | 10 | None | 52 | 54 | AVR/TAP | Chronic |
| 4 | 73 | M | 6, 9,12 | None | 64 | 62 | MVP/TAP | Chronic |
| 5 | 69 | M | 10,12 | None | 43 | 57 | AVR/MV | Paroxysmal |
| 6 | 73 | F | 10 | 6 | 44 | 66 | MVP/TAP | Chronic |
| 7 | 73 | M | 7,10 | None | 62 | 49 | MVR/TAP/CABG | Chronic |
| 8 | 38 | M | 1,4,6,10 | None | 46 | 63 | MVP | Paroxysmal |
| 9 | 64 | M | I,4,7,10,12 | None | 40 | 67 | AVR | Paroxysmal |
| 10 | 78 | F | None | None | 54 | 34 | AVR/MVP | Chronic |
| 11 | 75 | M | 6,9 | 3 | 41 | 39 | AVR/TAP/CABG | Chronic |
| 12 | 48 | M | 1,3,6,7,10,11 | 1 | 50 | 38 | ASDC/TAP | Chronic |
| 13 | 73 | M | 9,12 | None | 58 | 60 | MVP/CABG | Chronic |
| 14 | 72 | F | None | None | 62 | 57 | MVP/TAP | Paroxysmal |
| 15 | 61 | M | 4,10,12 | 1 | 59 | 73 | MVP/CABG | Chronic |
| 16 | 64 | M | 3 | 9,10 | 56 | 65 | MVP | Chronic |
ASDC = atrial septal defect closure; AVR = aortic valve replacement; CABG = coronary artery bypass graft; EF = ejection fraction; F = female; LAD = left atrial diameter; M = male; MV = mitral valvuloplasty; MVR = mitral valve replacement; TAP = Tricuspid annuloplasty.
Activity of the Left Atrial GPs
The mean number of active GPs identified in the patients was 2.2 on the right side and 0.4 on the left side. The number of active GPs on the right side was much greater than that on the left side (P < 0.01). Thirteen (81%) of the 16 patients were found to have active GPs, which were mainly mapped between the right PVs and interatrial groove on the inferior right region of the left atrium (6, 10, and 12; Fig. 1 [pink circle]). The probability of active GPs on the left side was extremely low. Active GPs were found bilaterally in five patients and unilaterally in eight patients with only right-sided activity. The incidence of activity according to the epicardial location is presented in Table 2.
Table 2.
The Incidence of Activity according to the Epicardial Location
| Location | Right | Left |
|---|---|---|
| 1 | 3/16 (19%) | 2/16 (13%) |
| 2 | 0/16 (0%) | 0/16 (0%) |
| 3 | 2/16 (13%) | 1/16 (6%) |
| 4 | 3/16 (19%) | 0/16 (0%) |
| 5 | 0/16 (0%) | 0/16 (0%) |
| 6 | 5/16 (31%) | 1/16 (6%) |
| 7 | 3/16 (19%) | 0/16 (0%) |
| 8 | 0/16 (0%) | 0/16 (0%) |
| 9 | 4/16 (25%) | 1/16 (6%) |
| 10 | 8/16 (50%) | 1/16 (6%) |
| 11 | 1/16 (6%) | 0/16 (0%) |
| 12 | 6/16 (38%) | |
| 13 | 0/16 (0%) |
GP Ablation
The cardiac response to GP stimulation was eliminated with radiofrequency ablation in all patients. Nine patients required only a single procedure, whereas four patients needed additional procedures.
Follow-Up
AF recurrence was confirmed by an ELR in all patients. Thirteen (81%) of the 16 patients were maintained in sinus rhythm 3 months after the operation. For those patients with active GP locations, 12 (92%) of 13 patients were maintained in sinus rhythm. In contrast, one of three patients without any active GPs (patient 10, see Table 1) was maintained in sinus rhythm. AF was sustained in patients 2 and 14 without any active GPs. Atrial tachycardia (AT) was sustained in patient 16 and the gaps in the lesions were found on the roof and bottom of the PVs. The patient regained sinus rhythm after an endocardial radiofrequency catheter ablation. There were no deaths or complications during the study period.
Discussion
This study was intended to identify the location of left atrial GPs based on dense epicardial mapping during a maze IV procedure through an open surgical approach in patients not with lone AF but with concomitant AF. The patient population in our study consisted of patients with rather more severe heart conditions requiring valve treatment. In contrast, the patient populations in the previous studies had relatively smaller left atrial diameters and lone AF treated by a minimally invasive maze procedure.9,12,14
The left atrial epicardial fat pads can be visualized directly to some extent (Fig. 4). The GPs are clustered around the PVs, interatrial groove, and ligament of Marshall, and the cardiac response to GP stimulation can be eliminated with a bipolar radiofrequency isolation. A previous study defined the five major left atrial GP areas as follows: superior left GP, inferior left GP, Marshall-tract GP, anterior right GP, and inferior right GP.4 A recent report showed that autonomic GPs were identified by a 20-point rapid atrial pacing method, and active GPs were identified dominantly on the right side of the left atrium (a mean of 5.0 GPs on the right and 2.7 on the left), especially between the right PVs and interatrial groove.9 We revised the diagram of the epicardial mapping locations by attempting to increase the number of points between the right PVs and interatrial groove. The 24-site HFS was delivered to the specific left atrial areas, as depicted in the epicardial diagram, by a temporary pacemaker and tweezers; it was a simpler and more economical method than using a specific stimulator and catheter.
Figure 4.
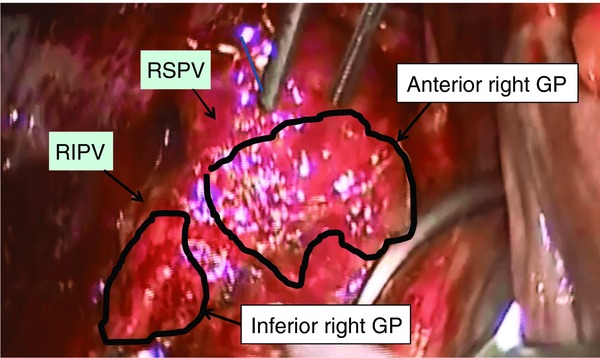
The fat pads on the right side of the left atrium.
A higher voltage for the HFS may be required to elicit a response of the GPs that were not active. However, the safety of a higher voltage for the HFS has not been demonstrated sufficiently and the ganglion axons are not tolerable to higher voltages, which would lead to a negative GP response. That would limit the margin of available values of the HFS.
Similar to the previous studies, the GPs were mainly mapped between the right PVs and interatrial groove (Table 2), whereas the probability of a GP on the left side was extremely lower than in those studies.9 We simply removed the epicardial fatty tissue manually. Our results suggest that manual removal of the fatty tissue prior to the ablation could be effective in reducing the GP activity. The major left atrial GPs are known to be connected to the atrioventricular node (AVN). A recent report described the atrial neural network.15,16 The inferior right GP area is expected to function as the gateway for the intrinsic cardiac autonomic nervous system for the modulation of the AVN. In this study, the right-sided GP areas were stimulated first. Therefore, the right-sided HFS might markedly attenuate the ventricular slowing response induced by the left-sided HFS. Our data supported the observation that the inferior right GPs were an important pathway between the other GPs and the AVN. Furthermore, not only did it isolate the local excitation of the GPs, but we believed that the ablation of this pathway was also effective in treating AF.
Clinical Implications
The GP ablation with a maze IV procedure was successful in maintaining sinus rhythm 3 months after the operation. Further, sinus rhythm was restored by endocardial radiofrequency catheter ablation in the patient who had AT around the right PVs. These results of successfully regaining sinus rhythm suggest that GPs are highly associated with the initiation and maintenance of AF.
An important finding from this study is that the inferior and anterior right GPs between the right PVs and the interatrial groove were significant targets for the treatment in most AF patients.
Study Limitations
This study included a small number of patients and the follow-up period was short. In addition, a clear limitation was the order of the procedure. All GP mapping and ablation procedures were performed on the right side first, followed by the left side. It was possible that a hemodynamic change could have been caused by the exposure of the surface of the left atrium. Because drainage of the blood was required, a heart-lung machine should have been prepared during the procedure on the left side to correct any hemodynamic instability. The posterior area of the left atrium was not accessible for the procedure through an open surgical approach.
Conclusions
The dense epicardial mapping in the potential GP areas identified active GPs in a high percentage of patients. Active GPs were predominantly identified in the anterior and inferior right region of the left atrium between the right PVs and interatrial groove. The maze procedure combined with a targeted partial GP ablation was an efficacious approach for the treatment of conco-mitant AF.
References
- 1.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- 2.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 3.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6(Suppl 12):S26–S34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Pokushalov E, Turov A, Shugayev P, Artyomenko S, Romanov A, Shirokova N. Catheter ablation of left atrial ganglionated plexi for atrial fibrillation. Asian Cardiovasc Thorac Ann. 2008;16:194–201. doi: 10.1177/021849230801600304. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2008;102:330–334. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shugayev P, Shirokova N, Katritsis DG. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010;12:342–346. doi: 10.1093/europace/euq014. [DOI] [PubMed] [Google Scholar]
- 8.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shirokova N, Katritsis DG. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:1231–1238. doi: 10.1111/j.1540-8159.2010.02800.x. [DOI] [PubMed] [Google Scholar]
- 9.Mehall JR, Kohut RM, Jr, Schneeberger EW, Taketani T, Merrill WH, Wolf RK. Intaraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg. 2007;83:538–541. doi: 10.1016/j.athoracsur.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Edgerton JR, Brinkman WT, Weaver T, Prince SL, Culica D, Herbert MA, Mack MJ. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg. 2010;140:823–828. doi: 10.1016/j.jtcvs.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 11.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: New approach to treat atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1289–1295. doi: 10.1111/j.1540-8167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 12.Beyer E, Lee R, Lam BK. Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: Early multicenter results. J Thorac Cardiovasc Surg. 2009;137:521–526. doi: 10.1016/j.jtcvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Ware AL, Suri RM, Stulak JM, Sundt TM, 3rd, Schaff HV. Left atrial ganglion ablation as an adjunct to atrial fibrillation surgery in valvular heart disease. Ann Thorac Surg. 2011;91:97–102. doi: 10.1016/j.athoracsur.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz A, Geuzebroek GS, Van Putte BP, Boersma LV, Sonker U, De Bakker JM, Van Boven WJ. Completely thoracoscopic pulmonary vein isolation with ganglionic plexus ablation and left atrial appendage amputation for treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2010;38:356–360. doi: 10.1016/j.ejcts.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 15.Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, et al. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Scherlag BJ, Niu G, Lu Z, Patterson E, Liu S, Lazzara R, et al. Autonomic elements within the ligament of Marshall and inferior left ganglionated plexus mediate functions of the atrial neural network. J Cardiovasc Electrophysiol. 2009;20:318–324. doi: 10.1111/j.1540-8167.2008.01315.x. [DOI] [PubMed] [Google Scholar]


