Abstract
OBJECTIVE
To analyze vaccination coverage and factors associated with a complete immunization scheme in children < 5 years old.
METHODS
This cross-sectional household census survey evaluated 1,209 children < 5 years old living in Bom Jesus, Angola, in 2010. Data were obtained from interviews, questionnaires, child immunization histories, and maternal health histories. The statistical analysis used generalized linear models, in which the dependent variable followed a binary distribution (vaccinated, unvaccinated) and the association function was logarithmic and had the children’s individual, familial, and socioeconomic factors as independent variables.
RESULTS
Vaccination coverage was 37.0%, higher in children < 1 year (55.0%) and heterogeneous across neighborhoods; 52.0% of children of both sexes had no immunization records. The prevalence rate of vaccination significantly varied according to child age, mother’s level of education, family size, ownership of household appliances, and destination of domestic waste.
CONCLUSIONS
Vulnerable groups with vaccination coverage below recommended levels continue to be present. Some factors indicate inequalities that represent barriers to full immunization, indicating the need to implement more equitable policies. The knowledge of these factors contributes to planning immunization promotion measures that focus on the most vulnerable groups.
Keywords: Immunization Coverage, Socioeconomic Factors, Integrated Management of Childhood Illness, Child Health, Health Inequalities, Health Surveys
Abstract
OBJETIVO
Analisar a cobertura vacinal e os fatores associados ao esquema vacinal completo em crianças menores de cinco anos.
MÉTODOS
Inquérito domiciliar seccional e censitário com 1.209 crianças até cinco anos de idade, de Bom Jesus, Angola, em 2010. Entrevistas, questionários, carteiras de vacinação infantil e de saúde materna foram fontes de dados. A análise estatística utilizou modelos lineares generalizados em que a variável dependente segue distribuição binária (vacinados; não vacinados) e a função de ligação é logarítmica, tendo como variáveis independentes os fatores individuais, familiares e socioeconômicas das crianças.
RESULTADOS
Registrou-se cobertura vacinal de 37,0%, maior em menores de um ano de idade (55,0%), heterogênea entre os bairros; 52,0% das crianças, de ambos os sexos, não tinha carteira de vacinas. A razão de prevalência da situação vacinal mostrou diferença significativa para a idade da criança, grau de conhecimento das mães, tamanho da família, posse de eletrodomésticos e destino de lixo.
CONCLUSÕES
Persistiram grupos vulneráveis com cobertura vacinal aquém da preconizada. Alguns fatores expressam desigualdades que representam barreiras à vacinação completa, o que indica a necessidade de reforçar políticas mais equitativas. O conhecimento desses fatores contribui para o planejamento de medidas de promoção vacinal ajustadas aos grupos mais vulneráveis.
INTRODUCTION
Childhood vaccination is the most effective and efficient activity in public health. Immunization programs and epidemiological surveillance are two fundamental components for the control of transmissible diseases. 11 , 13 However, there is a gap between the potential use of this practice and its real contribution to child survival. Approximately three million children die annually worldwide and several others become disabled as a result of vaccine-preventable diseases. 11 , a Lifesaving vaccines remain inaccessible to approximately 24 million children who are exposed to a greater risk of illnesses and death. 12
Vaccination coverage is the percentage of a target population that receives the full schedule of vaccinations. The schedule includes all the vaccinations recommended by the National Immunization Program (NIP), applied at the correct ages (epidemiological adequacy) and correct intervals (immunological adequacy). Combined institutional activities organized by the public sector at various levels are required to achieve adequate vaccination coverage. Knowledge of vaccination coverage facilitates monitoring the volume of susceptible individuals in the population as well as the identification of factors related to child health and service performance, supporting the planning and restructuring of vaccination programs. 11
Investigations into the reasons for nonadherence to vaccination programs are proposed by experts in the medical field and aim to guide interventions to reverse this situation and ensure greater protection for the populations at greater risk. 3 , 5
The factors that interfere with vaccination coverage can be grouped into four areas: immunization system (policy), i.e., structure for vaccine distribution; parent knowledge and attitudes about the vaccination programs; communication and information; and family characteristics. 7 , 9 , b This latter area involves low-income status, residence in rural areas, extremes of maternal age, high parity, low maternal education level, larger families, residence in the area for < 1 year, mother working outside the home, lack of knowledge about vaccine-preventable diseases, transportation difficulties, labor disputes around workdays lost to care for children, lack of health insurance, and presence of disease among the children. 1 , 11 , 13
The calculation of vaccination coverage can have biases in the numerator or denominator. In the first case, the results may not express the total number of vaccinated children living in the area, instead using the number of doses applied. This calculation does not consider avoiding immunization services to obtain them in other regions, and includes individuals who come from other regions to be vaccinated. Another calculation bias is related to the live birth records because vaccination coverage is calculated on the basis of data obtained from registry offices, which may not include the live births not registered in the period. When live births are calculated from other data sources, the rates may differ because of data adjustment in accordance with delayed reporting of birth records. These limitations can underestimate the target population. 1 , 13 , c Results from population surveys are more reliable because they are not influenced by movement to avoid or obtain vaccinations because the numerator values are present in the denominator. 1 3 , c
Since the 1970s, several countries have begun to use computerized immunization records (linked or not to electronic health records) as a strategy for making immunization programs more effective. Such records are used to schedule vaccinations, identify and search for unvaccinated individuals, and monitor vaccination coverage. 9 , 13 , c
Vaccine-preventable diseases are responsible for 15.0% of all deaths among children < 5 years of age in Angola. Vaccines have been used as a control measure since the nineteenth century. However, the universal and free NIP was established only in 1979 and has contributed to the reduction of social and regional inequalities by allowing all children to be vaccinated. Furthermore, NIP includes staff training and motivation, community activities to promote awareness of the importance of the problem, routine vaccination, and campaigns planned for the population groups who cannot use regular immunization services. d , e The NIP has established the following aims: vaccination coverage ≥ 90.0% vaccination coverage for against Bacillus Calmette-Guerin (BCG) and tuberculosis and neonatal tetanus (TT), vaccination coverage ≥ 95.0% vaccination coverage for diphtheria, tetanus, and pertussis (DTP); oral polio vaccine (OPV); hepatitis B (HB); Haemophilus influenzae type B (Hib); and monovalent measles vaccine, as well as full vaccination against yellow fever (YF). 3 , e , f In 2009, vaccination coverage in Angola reached 83.0% for BCG, 77.0% for measles, 73.0% for DPT, 78.0% for OPV, 23.0% for HB and Hib, 40.0% for YF, and 78.0% for TT; this indicates that major challenges remain to be overcome. 17 , f
The aim of this study was to assess vaccination coverage and the factors associated with a complete immunization schedule in children < 5 years of age.
METHODS
This cross-sectional household census survey evaluated 1,209 children aged ≤ 5 years living in Bom Jesus, in the Province of Luanda, Angola, in 2010.
The community of Bom Jesus has 6,794 inhabitants and a poor infrastructure for public services and assets. The health network comprises a health care center in the urban area and a health care unit in the rural area. The health care center has one doctor, four nurses (including the NIP coordinator), two nursing assistants, three outpatient care assistants, and two general assistants. The health care unit has two nursing assistants. The survey was conducted in 10 residential neighborhoods (three in urban areas and seven in rural areas). The population comprised three ethnolinguistic groups (Kimbundu, Umbundu, and Bakongo) that conducted agricultural, industrial, and commercial activities, and 21.0% of the population was < 5 years of age. b Vaccination status was monitored using child vaccination cards, vaccination records obtained from the health facilities, and reports issued by the municipal health services.
The following instruments were used for analysis: household survey through interviews with parents or caregivers using a questionnaire prepared to obtain data on socioeconomic, familial, and individual characteristics; child hood vaccination history, to obtain data on vaccination status; and health history of the mother, to obtain data on the doses of tetanus toxoid.
The fieldwork involved the preparation and execution of the survey, which was conducted between April 10 and June 30, 2010. The preparation included presentation of the teams and immunization programs to the local health authorities, training interviewers on the objectives, methodology, and survey instruments, classroom simulations, and household interviews. The survey was implemented by two physicians, nine students in their sixth year of a public medical school, two health care technicians from the local vaccination program, and two second year high school students who lived in the community. The researchers were organized into seven teams, coordinated by the physicians, and worked 10h daily shifts from Monday to Saturday. Weekly meetings were conducted to evaluate the activities. Traditional leaders in each neighborhood mobilized activities in the community to facilitate respondent participation. The households were visited and children < 5 years were surveyed. For this purpose, a spreadsheet form using the same format as the immunization history cards was used to facilitate copying immunization data of the mothers and children onto the form. The vaccination dates for BCG (one dose), measles (one dose and one reinforcement), HB, Hib, DTP, and OPV (three doses), YF (one dose), and TT were recorded. Oral information was marked with a special code in the absence of immunization history. In the case of BCG, the interviewers were instructed to look for the scar in the deltoid region of the right arm. In addition, the dates of polio vaccination campaigns were recorded. Children were considered vaccinated if they received the number of doses recommended by Angolan vaccination program. Children were considered protected against neonatal tetanus if their mothers had received at least two doses of tetanus vaccine and the last dose was received within the last five years before the child was born.
Vaccination status was the outcome variable. The independent variables were: a) Individual factors for children: sex, age (in years: ≤ 1, 1, > 1), birth order (up to the fourth; after the fourth), presence of civil birth certificate (yes, no), awareness of the mother or caregiver of available health care services(yes, no); b) Family factors: family size (2-3 people, 4-5 people, ≥ 6 people), head of household (mother, father, or others), number of living children (≤ 4, > 4), age of the mother (15-29 years, ≥ 30 years), mother’s occupation (housewife, not a housewife), mother’s level of education (illiterate; ≤ 4 years, > 4 years), and mother’s language (Kimbundu, others); c) Socioeconomic factors: residential area (urban, rural), home ownership (yes, no), type of lighting in the home (public network, generator, lamp, others); type of water treatment (boiling, chemical treatment, untreated; occasional treatment); disposal of household waste (public waste collection, burning, burying, others); household appliances (radio, TV, radio and TV, others, none); wage (≤ 5 minimum wage equivalents, > 5 minimum wage equivalents); and head of household’s level of education (no schooling, with education).
EpiInfo version 3.5.2 software was used to construct the database; R i3863.0.0 and SAS version 9 were used for data processing.
The statistical analysis used generalized linear models where the dependent variable followed a binary distribution (vaccinated, unvaccinated), and the association function was logarithmic. This model is known as the log-binomial model. 2 Its advantage over logistic regression is that the parameters are interpreted as prevalence ratios (PR) and not odds ratios. This regression model considers that the data are arranged by family groups. Parameters were estimated based on the technique known as Generalized Estimating Equations (GEE) to treat data regarding possible correlations among individuals belonging to the same family group. These models allowed the estimation of PR for the variables of interest and their respective confidence intervals (95%CI). A confidence interval that did not contain the value 1 suggested a correlation between the respective variable and vaccination (similar to p < 0.05). The GENMOD procedure of SAS version 9 was used for the computational implementation of these models. Spiegelman & Hertzmark 21 warned that estimating the parameters of a log-binomial model using the SAS program could lead to problems regarding convergence of the numerical algorithm and other computational instabilities. However, no convergence problems were observed.
The log-binomial model was individually adjusted for each variable of interest and gross PR estimates were obtained along with their respective 95%CI. The independent variables were classified into three hierarchical groups: on a more comprehensive level, socioeconomic variables were selected to construct multiple models; the intermediate level considered family variables; and the primary level considered variables related to the individuals. A first multiple regression model was adjusted considering all variables for the more comprehensive level (socioeconomic), a second model comprised all variables for the intermediate level and the socioeconomic variables, and a third model comprised variables of all three hierarchical groups. Independent qualitative variables were inserted into the model as indicator variables (dummy variables). Discreet variables were included in the model and grouped by classes of interest as dummy variables.
A total of 1,596 households were visited. The urban area comprised 1st neighborhood, 2nd neighborhood, and Honga Samba corresponded to 56.0% of the households; 90.0% of houses were built with poor materials, 67.0% had one or two divisions; 2.0% had ≥ 8 divisions (mean of 4, median of 2, minimum of 1, and maximum of 10), 61.0% had children aged ≤ 5 years, 51.0% had public electricity, 36.0% had drinking water, 38.0% had access to public waste collection, and 76.0% had appliances.
Among the 6,794 inhabitants interviewed, 65.0% lived in urban areas, 82.0% owned their own home (of these, 51.0% were women), 18.0% were aged < 5 years, 45.0% were aged < 15 years, 5.0% were aged ≥ 60 years; the average number of family members was 6 (median of 5, minimum of 2, and maximum of 12) and the average number of children per family was 3 (median of 2). A total of 1,209 children aged < 5 years were evaluated; of these 71.0% lived in urban areas, 52.0% were female, 75.0% were aged ≥ 12 months, 24.0% were firstborn, 66.0% belonged to Kimbundu families and the remainder belonged to migrant families, 81.0% had no birth records; 40.0% of the caregivers were aged 15-24 years. With regard to the mothers, 64.0% had 2-4 children, 22.0% were illiterate, 76.0% had 1-7 years of schooling, and 53.0% were housewives. Among the children evaluated, 78.0% had the father as family head, and 18.0% of these were illiterate and 53.0% earned less than five minimum wages (Table 1).
Table 1. Sociodemographic profile of Bom Jesus, Province of Luanda, Angola, 2010. (N = 1,596).
| Households | n | % |
|---|---|---|
| Urban zone | 901 | 56.5 |
| Rural zone | 695 | 3.5 |
| Traditional housing | 1,443 | 90.4 |
| Brick/Concrete housing | 153 | 9.6 |
| Number of divisions | ||
| 1 to 2 | 1,063 | 67.6 |
| 3 to 7 | 504 | 32.6 |
| ≥ 8 | 29 | 1.8 |
| With electricity | 815 | 51.1 |
| Without electricity | 781 | 48.9 |
| With potable water | 573 | 35.9 |
| Without potable water | 1,023 | 64.1 |
| With public waste collection service | 602 | 37.7 |
| Without public waste collection service | 994 | 62.3 |
| Owned house | 1,297 | 81.3 |
| Rented house | 299 | 19.7 |
| With appliances | 1,092 | 68.4 |
| Without appliances | 504 | 31.6 |
| Population | ||
| Urban area | 4,255 | 62.6 |
| Rural area | 2,539 | 37.4 |
| Men | 3,342 | 49.2 |
| Women | 3,452 | 50.8 |
| 15 to 29 years | 4,886 | 71.9 |
| ≥ 30 years | 1,908 | 28.1 |
| Kimbundu ethnicity | 4,483 | 66.0 |
| Umbundu ethnicity | 2,258 | 33.2 |
| Kikongo and others | 53 | 0.8 |
| < 5 years old | ||
| ≤ 1 month | 54 | 4.5 |
| 2 to 11 months | 250 | 20.7 |
| 12 to 59 months | 905 | 74.9 |
| Urban zone | 846 | 70.0 |
| Rural zone | 363 | 30.0 |
| Male | 583 | 48.2 |
| Female | 626 | 51.8 |
| Kimbundu ethnicity | 802 | 66.3 |
| Umbundu ethnicity | 391 | 32.4 |
| Kikongo and others | 16 | 1.3 |
| Birth order | ||
| 1 | 287 | 23.7 |
| 2 to 4 | 704 | 58.3 |
| > 5 | 218 | 18.0 |
| Presence of birth certificate | ||
| Yes | 225 | 18.6 |
| No | 984 | 81.4 |
| Number of children | ||
| ≤ 4 | 939 | 77.7 |
| > 4 | 270 | 22.3 |
| Head of household’s salary (minimum wage equivalents) | ||
| ≤ 5 | 704 | 58.2 |
| > 5 | 505 | 41.8 |
Source: Bom Jesus Immunization survey, 2010.
% N Vac: percentage of unvaccinated children; % Vac: percentage of vaccinated children
The study was approved by the Research Ethics Committee of the Universidade Agostinho Neto de Angola (Process 26, DPDB72009) and by the Center for School Health of the Faculdade de Medicina de Ribeirão Preto of the Universidade de São Paulo (Opinion 272,439 from 4/9/2013).
RESULTS
Of the 1,209 children evaluated, 52.0% did not have an immunization history, 37.0% had completed the vaccination schedule, and 55.0% of this subgroup were aged < 1 year. No differences were observed between sexes; 38.0% children were Kimbundu and 34.0% belonged to other ethnic groups. Vaccine coverage was 90.0% for BCG, 72.0% for TT, 70.0% for OPV, 48.0% for DPT, 47.0% for monovalent measles, 43.0% for YF, and 14.0% for HB and Hib.
Coverage for all vaccines was inferior to the levels established by NIP and national averages, with the exception of BCG. The specific coverage for each vaccine was superior to vaccination coverage for the complete vaccination schedule, except for Hib and HB.
Coverage decreased in proportion to child age (70.0% in the first month to 30.0% after the first year).
Coverage was spatially heterogeneous and decreased from the periphery to the central areas of the community, where local administration services and health care units were concentrated. The highest vaccination coverage levels were observed in the neighborhoods of Matabuleiro and Coxe, in the peripheral, rural, and poorer areas, and the lowest vaccination coverage occurred in the Honga Samba neighborhood in the central urban, area (Figure).
Figure. Distribution of vaccination coverage among children < 5 years old in residential neighborhoods in Bom Jesus, Province of Luanda, Angola, 2010.
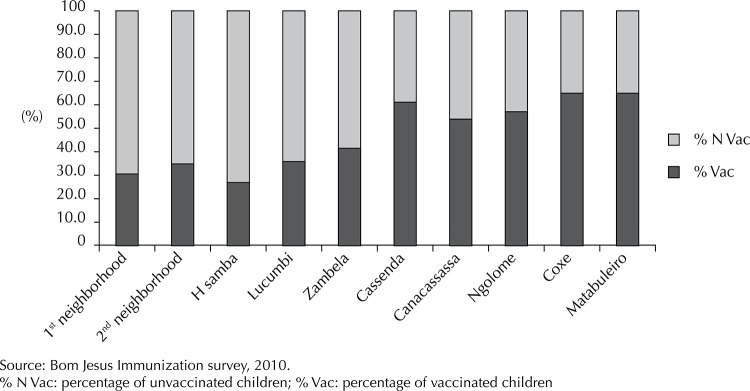
Child age was associated with knowledge about health care services (Table 2). Considering that gross PR were similar to the adjusted values, no potential confounding effects due to other variables considered in the model were observed. The percentage of vaccinated children < 1 year of age corresponded to approximately 1.8 times the rate for children > 1 year.
Table 2. Individual factors and vaccination status according to a log-binomial analysis, estimated prevalence ratios, and a 95% confidence interval. Bom Jesus, Province of Luanda, Angola, 2010.
| Variable | Total | n | % | PRraw | 95%CI | PRadjusted | 95%CIa |
|---|---|---|---|---|---|---|---|
| Order | |||||||
| > 4 | 219 | 68 | 31.1 | 1 | 1 | ||
| ≤ 4 | 990 | 372 | 37.6 | 1.21 | 0.97;1.51 | 0.95 | 0.64;1.39 |
| Birth certificate | |||||||
| Yes | 222 | 75 | 33.8 | 1 | 1 | ||
| No | 980 | 362 | 36.9 | 1.09 | 0.88;1.35 | 1.00 | 0.81;1.24 |
| Age (years) | |||||||
| > 1 | 849 | 247 | 29.1 | 1 | 1 | ||
| ≤ 1 | 360 | 193 | 53.6 | 1.84 | 1.59;2.13 | 1.78 | 1.53;2.07 |
| Knowledge of immunization programs | |||||||
| No | 258 | 75 | 29.1 | 1 | 1 | ||
| Yes | 951 | 365 | 38.4 | 1.32 | 1.06;1.64 | 1.32 | 1.07;1.63 |
Adjusted for other variables of the same level and for familial and socioeconomic variables.
With regard to the influence of family factors, family size was statistically associated with immunization status, although maternal age and number of children were analytical important (Table 3).
Table 3. Familial factors and vaccine coverage according to log-binomial analysis, estimated prevalence ratios, and 95% confidence interval. Bom Jesus, Province of Luanda, Angola, 2010.
| Variable | Total | Vaccinated | % | PRraw | 95%CI | PRadjusted | 95%CIa |
|---|---|---|---|---|---|---|---|
| Family size | |||||||
| 2 to 3 | 121 | 56 | 46.3 | 1.40 | 1.11;1.76 | 1.34 | 1.05;1.71 |
| 4 to 5 | 489 | 186 | 38.0 | 1.15 | 0.96;1.37 | 1.06 | 0.86;1.29 |
| ≥ 6 | 599 | 198 | 33.1 | 1 | 1 | ||
| Head of household | |||||||
| Mother | 112 | 37 | 33.0 | 1 | 1 | ||
| Father or other | 1,097 | 403 | 36.7 | 1.11 | 0.82;1.50 | 1.07 | 0.80;1.44 |
| Age of the mother (years) | |||||||
| 15 to 29 | 754 | 300 | 39.9 | 1.31 | 1.09;1.56 | 1.21 | 0.98;1.48 |
| ≥ 30 | 455 | 139 | 30.6 | 1 | 1 | ||
| Mother works as housewife | |||||||
| Yes | 644 | 244 | 37.9 | 1.09 | 0.92;1.29 | 1.00 | 0.83;1.18 |
| No | 565 | 196 | 34.7 | 1 | 1 | ||
| Level of education of the mother (years) | |||||||
| Illiterate | 206 | 68 | 33.0 | 1 | 1 | ||
| ≤ 4 | 594 | 209 | 35.2 | 1.07 | 0.84;1.35 | 1.07 | 0.84;1.35 |
| > 4 | 409 | 163 | 39.9 | 1.21 | 0.94;1.54 | 1.12 | 0.87;1.44 |
| Mother’s language | |||||||
| Kimbundo | 802 | 305 | 38.0 | 1.15 | 0.95;1.37 | 1.15 | 0.96;1.38 |
| Others | 407 | 135 | 33.2 | 1 | 1 | ||
| Number of children | |||||||
| ≤ 4 | 940 | 361 | 38.4 | 1.31 | 1.05;1.62 | 1.07 | 0.82;1.41 |
| > 4 | 269 | 79 | 29.4 | 1 | 1 | ||
Adjusted for other variables of the same level and for socioeconomic variables.
In the adjusted analysis, waste destination and household appliance ownership were found to be associated with a higher percentage of children completing the vaccination schedule (Table 4). The percentage of fully vaccinated children whose families had access to public waste collection or who buried the waste corresponded to two times the percentage of children whose families burned waste.
Table 4. Socioeconomic factors and vaccination status according to a log-binomial analysis, estimated prevalence ratios, and 95% confidence interval. Bom Jesus, Province of Luanda, Angola, 2010.
| Variable | Total | n | % | PRraw | 95%CI | PRadjusted | 95%CIa |
|---|---|---|---|---|---|---|---|
| Area | |||||||
| Urban | 266 | 83 | 31.2 | 1 | 1 | ||
| Rural | 943 | 357 | 37.9 | 1.21 | 0.97;1.51 | 1.19 | 0.95;1.47 |
| Head of household’s salary (minimum wage equivalents) | |||||||
| ≤ 5 | 954 | 345 | 36.2 | 1 | 1 | ||
| > 5 | 255 | 95 | 37.2 | 1.03 | 0.84;1.25 | 0.97 | 0.80;1.19 |
| Head of household’s education (years) | |||||||
| Illiterate | 99 | 30 | 30.3 | 1 | 1 | ||
| ≤ 4 | 398 | 126 | 31.7 | 1.04 | 0.75;1.45 | 0.99 | 0.71;1.36 |
| > 4 | 712 | 284 | 39.9 | 1.32 | 0.97;1.78 | 1.30 | 0.96;1.76 |
| Owned house | |||||||
| Yes | 938 | 347 | 37.0 | 1.08 | 0.88;1.31 | 1.09 | 0.90;1.33 |
| No | 271 | 93 | 34.3 | 1 | 1 | ||
| Appliances | |||||||
| Radio | 115 | 50 | 43.5 | 1.40 | 1.02;1.91 | 1.45 | 1.05;1.99 |
| TV | 273 | 85 | 31.1 | 1 | 1 | ||
| Radio and TV | 653 | 253 | 38.7 | 1.24 | 0.99;1.55 | 1.14 | 0.91;1.43 |
| Radio, TV, and others | 166 | 52 | 31.3 | 1.00 | 0.74;1.35 | 0.97 | 0.69;1.36 |
| None | 2 | 0 | – | – | |||
| Water treatment method | |||||||
| Boiling | 20 | 9 | 45.0 | 1.73 | 0.89;3.36 | 1.47 | 0.77;2.80 |
| Chemical treatment | 36 | 10 | 27.8 | 1.07 | 0.51;2.21 | 0.99 | 0.49;2.02 |
| Never treat water | 1,080 | 402 | 37.2 | 1.43 | 0.97;2.10 | 1.42 | 0.96;2.10 |
| Sometimes treat water | 73 | 19 | 26.0 | 1 | 1 | ||
| Lighting | |||||||
| Public | 772 | 287 | 37.2 | 1.07 | 0.84;1.36 | 1.03 | 0.77;1.37 |
| Generator | 209 | 73 | 34.9 | 1.01 | 0.74;1.36 | 0.91 | 0.65;1.27 |
| Lamp | 156 | 54 | 34.6 | 1 | 1 | ||
| Others | 72 | 26 | 36.1 | 1.04 | 0.70;1.55 | 0.99 | 0.68;1.45 |
| Waste disposal | |||||||
| Public collection | 109 | 50 | 45.9 | 1.97 | 1.11;3.48 | 2.26 | 1.26;4.07 |
| Burning | 43 | 10 | 23.3 | 1 | 1 | ||
| Burying | 242 | 100 | 41.3 | 1.78 | 1.02;3.09 | 1.99 | 1.12;3.54 |
| Others | 815 | 280 | 34.4 | 1.48 | 0.86;2.53 | 1.71 | 0.97;3.00 |
Adjusted for the other variables of the same level.
DISCUSSION
Analysis of the child and immunization history cards indicated overall vaccination coverage of 37.0%, revealing non adherence to the goals proposed by the Angolan Ministry of Health (> 80.0%) and those proposed by the WHO/Africa Regional Committee (90.0%-100%).
Vaccination coverage is an important indicator of population health and the quality of health care provided by health services. In addition to revealing aspects of child health and health care services, the study of this indicator subsidizes the immunization planning process, particularly the restructuring of immunization activities. 8 , 11
Communities on the periphery of Bom Jesus, which were more distant from the local health care unit, had higher vaccination coverage rates than more central neighborhoods, and the distribution of children who completed the current vaccination scheme was heterogeneous. The neighborhoods of Coxe and Matabuleiro attained a vaccination coverage of 65.0%, two times the rate of children from more central neighborhoods. This contradicts the results of previous studies regarding the likelihood of increased vaccination coverage in areas closer to immunization services. 11
This heterogeneity, which is not explained by the distance to immunization services, may be influenced by several factors, including the intensification of vaccination campaigns in rural areas, high degree of social cohesion in these peripheral, traditional communities that are monolithic from an ethnolinguistic and cultural point of view, where traditional leaders play an important role in connecting the community with local health care services; varied ethnic composition of inhabitants in more central areas who have specific cultural traits that may influence the demand for and use of immunization services; and passive attitudes of health professionals in relation to spontaneous demand, resulting in the increased number of missed opportunities for vaccination.
Researchers question the role of social and cultural elements underlying the acceptance of vaccination by the population to understand the extent to which this acceptance is permanent, and it goes beyond the social legitimacy that vaccination has acquired. There has been considerable diversity of responses, depending on the cultural and social elements of the population and how researchers approach the problem. 14 Full explanation will probably require studies and analyses that go beyond factorial associations.
Several children are not vaccinated for reasons ranging from the cultural and economic level of the parents to causes related to beliefs, superstitions, myths, and religious creeds. 9 , 20
Only the BCG vaccine attained the established NIP and regional target rates. vaccination coverage for the remaining vaccines was < 70.0%, and Hib and HB reached only 14.0% of the target group. The latter two had the lowest rates probably because they were included in the program only in 2007.
Dropout rates for the program were estimated at 32.0% for DTP, 33.0% for polio, and 25.0% for both HB and Hib, and were higher than the goals established by WHO. These aspects suggest that children have access to vaccination but health care monitoring remains limited.
Some authors report that single dose vaccines have high vaccination coverage, in contrast to the low coverage of multiple dose vaccines, which may be a result of shortages and delays in meeting the immunization schedule. The existence of children prone to vaccine-preventable diseases poses a risk to individuals and the population at large. This facilitates the introduction and development of infectious agents. 4 , 9
The effect of these factors on increased or decreased coverage varies by region and period in which the surveys are conducted, and depends on the conceptual, methodological, and operational strategies adopted. 14
In the present study, the unadjusted analysis suggests that increased vaccination coverage is correlated with the head of household’s education level, child caretaker’s education level, and the condition of belonging to the Kimbundu ethnic group (autochthonous).
A higher level of education and increased parental awareness would facilitate increased vaccination coverage because they ensure increased awareness of health issues and provide individuals with increased access to services, information, and the skills to interact with professionals and health care services. In contrast, belonging to groups with limited social inclusion (emigrant minority groups and others) increases the probability of nonaderence to the vaccination scheme because of the presence of mechanisms that limit access to goods and services. 8 , 18
There is a tendency for vaccination coverage to increase as the child’s age, family size, number of children, and maternal age increase.
Factors, such as low education level of the mother, low family income, large families, high parity, lack of knowledge about vaccination, and poor communication and information, lead to behaviors where preventive activities are not considered family priorities, and these factors positively correlate with the lack of vaccination. 6 , 22 , b
Most families in Bom Jesus are large, and consequently are more likely to delay vaccination. This may be because a large number of children hinders family mobility, depending on the place of residence and access to health care services. 22
The adjusted analysis indicated increased compliance with vaccination programs among children < 1 year whose mothers were aware of the immunization services, families with up to three individuals, families with radios and TVs, and families with positive behavioral patterns related to environmental sanitation measures.
A study conducted in Iguaí and Caldeirão Grande, BA, Northeastern Brazil in 1997 indicated higher vaccination coverage among older children, 15 which was in contrast with this present study. This discrepancy could be associated with the period required to establish and maintain NIP as well as its time of existence: in Angola, NIP is more recent and more focused on younger children. Another possibility may be parents’ casual oversight; some time passes between vaccinations, which creates a psychological sense of tranquility and causes the parents to forget. 17
The results of this study showed no sex differences in vaccination coverage. This finding is in line with a study conducted in Sao Luís, MA, Northeastern Brazil, in 2006, on factors associated with incomplete basic vaccination schedules.
Approximately 16.0% of the population of Bom Jesus is illiterate and 22.0% of the mothers had no formal education. The low level of education directly affects health conditions because they remain unaware about important information including that related to child immunization, and interferes with the general outcomes associated with child health.
Consequently, improved sociodemographic patterns can positively influence the increased vaccination coverage in these communities, which is consistent with the literature. 10 , 16
In contrast, vaccination coverage decreases as indicators for socioeconomic development and human capital worsen, as observed in developed and emerging countries. 13 , 20 - 22 However, these findings are in contrast with a vaccination coverage survey conducted in Sao Paulo, SP, Southeastern Brazil in 2002, where a significant correlation was observed between increased vaccination coverage and poorer socioeconomic indicators. 10 , 22
The head of household’s monthly income, measured by the number of minimum wage equivalents, does not appear to be a predictor of increased vaccination coverage; this result is corroborated by a study conducted in northeastern Brazil in 1994. 19 However, the opposite occurs in developed countries including the USA. 4
The results of the present study underscore the importance of sociodemographic, familial, and individual factors as barriers to full immunization. Knowledge of these factors may contribute to planning immunization promotion measures which focus on the most vulnerable groups, to implement more equitable policies.
Further studies are needed to explore cultural patterns, which may affect vaccination status in this community.
Funding Statement
This study was supported by the Fuel Society of Angola (Sonangol – Letter 009/DNPQ, 2008).
Footnotes
This study was supported by the Fuel Society of Angola (Sonangol – Letter 009/DNPQ, 2008).
Bujes MK. Motivos de atraso vacinal em crianças: uma pesquisa bibliográfica [Postgraduate project] Porto Alegre: Universidade Federal do Rio Grande do Sul; 2012.
Instituto Nacional de Estatística de Angola; UNICEF. Inquérito de indicadores múltiplos, avaliando a situação das crianças e mulheres angolanas no inicio do milênio: relatório analítico. Luanda: Instituto Nacional de Estatística de Angola; 2008.
Gattás VL. Avaliação da cobertura vacinal e o uso dos serviços de saúde na região sudoeste da Grande São Paulo, 1989-1990 [Master’s dissertation]. São Paulo: Faculdade de Saúde Pública da USP; 1996.
Organização Mundial da Saúde, Comitê Regional Africano. Plano estratégico regional para o programa alargado de vacinação 2006-2009. Quinquagésima-sexta sessão; 2006 ago 28-set 1; Addis Abeba, Etiópia.
Fondo de las Naciones Unidas para la Infancia. El estado mundial de la infancia de 2013 en cifras: todos los niños y niñas cuentan. Nueva York: UNICEF; 2013.
Ministério da Saúde de Angola. Situação de programa alargado de vacinação. Luanda; 2007.
Based on the doctoral thesis by Oliveira MFS, titled: “Determinantes da Situação Vacinal de Menores de Cinco Anos em Bom Jesus, Província de Luanda, Angola”, presented to the Programa de Pós-Graduação de Saúde na Comunidade at the Faculdade de Medicina da Universidade de São Paulo, in 2014.
REFERENCES
- 1.Atkinson WL, Pickering LK, Schwartz BG, Weniger BG, Iskander JK, Watson JC. Centers for Disease Control and Prevention. General recommendations on Immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Recomm Rep. 2002;51(RR-2):1–35. [PubMed] [Google Scholar]
- 2.Blizzard L, Hosmer DW. Parameter estimation and goodness-of-fit in log binomial regression. 10.1002/bimj.200410165Biom J. 2006;48(1):5–22. doi: 10.1002/bimj.200410165. [DOI] [PubMed] [Google Scholar]
- 3.Dannetun E, Tegnell A, Normann B, Garpenholt O, Giesecke J. Influenza vaccine coverage and reasons for non-vaccination in sample of people above 65 years of age, in Sweden, 1998-2000. 10.1080/00365540310011065Scand J Infect Dis. 2003;35(6-7):389–393. doi: 10.1080/00365540310011065. [DOI] [PubMed] [Google Scholar]
- 4.Development of community- and state-based immunization registries CDC response to a report from the National Vaccine Advisory Committee. MMWR Recomm Rep. 2001;50(RR-17):1–17. [PubMed] [Google Scholar]
- 5.Egede LE, Zheng D. Racial/ethnic differences in influenza vaccination coverage in high-risk adults. 10.2105/AJPH.93.12.2074Am J Public Health. 2003;93(12):2074–2078. doi: 10.2105/ajph.93.12.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etana B, Deressa W. Factors associated with complete immunization coverage in children aged 12-23 months in Ambo Woreda, Central Ethiopia. 10.1186/1471-2458-12-566BMC Public Health. 2012;12(566) doi: 10.1186/1471-2458-12-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juliano Y, Compri PC, Almeida LR, Freire PV, Moreira FT, Vieira FHS. Segunda etapa da Campanha Nacional de Multivacinação do município de São Paulo, 2005: perfil de cobertura de diferentes Unidades Básicas de Saúde. 10.1590/S0103-05822008000100003Rev Paul Pediatr. 2008;26(1):14–19. [Google Scholar]
- 8.Lima TC, Gryschek ALFPL, Veras DDC. Nursing. 125. Vol. 11. Sao Paulo: 2008. Estado vacinal dos profissionais de uma escola de especialistas de aeronáutica; pp. 472–477. [Google Scholar]
- 9.Luhm KR, Cardoso MRA, Waldman EA. Cobertura vacinal em menores de dois anos a partir de registro informatizado de imunização em Curitiba, PR. 10.1590/S0034-89102010005000054Rev Saude Publica. 2011;45(1):90–98. [Google Scholar]
- 10.Malta RF, Mishima SM, Almeida MCP, Pereira MJB. A utilização do inquérito domiciliar como instrumento de acompanhamento de ações de saúde em microáreas: analisando a situação vacinal de menores de um ano. 10.1590/S0104-11692002000100005Rev Latino-Am Enferm. 2002;10(1):28–33. [PubMed] [Google Scholar]
- 11.Miranda AS, Scheibel IM, Tavares MRG, Takeda SMP. Avaliação da cobertura vacinal do esquema básico para o primeiro ano de vida. 10.1590/S0034-89101995000300008Rev Saude Publica. 1995;29(3):208–214. doi: 10.1590/s0034-89101995000300008. [DOI] [PubMed] [Google Scholar]
- 12.Molina AC, Godoy I, Carvalho LR, Caldas AL., Junior Situação vacinal infantil e características individuais e familiares do interior de São Paulo. Acta Sci Health Sci. 2007;29(2):99–106. [Google Scholar]
- 13.Moraes JC, Ribeiro MCSA. Desigualdades sociais e cobertura vacinal: uso de inquéritos domiciliares. 10.1590/S1415-790X2008000500011Rev Bras Epidemiol. 2008;11(Supl 1):113–124. [Google Scholar]
- 14.Nigenda-López G, Orozco E, Leyva R. Motivos de no vacunación: un análisis crítico de la literatura internacional, 1950-1990. 10.1590/S0034-89101997000300015Rev Saude Publica. 1997;31(3):313–321. doi: 10.1590/s0034-89101997000300015. [DOI] [PubMed] [Google Scholar]
- 15.Pebley AR, Goldman N, Rodríguez G. Prenatal and delivery care and childhood immunization in Guatemala: do family and community matter? 10.2307/2061874Demography. 1996;33(2):231–247. [PubMed] [Google Scholar]
- 16.Porto LA. Cobertura vacinal nos municípios de Iguaí e Caldeirão Grande, Bahia, em 1997. Inf Epidemiol SUS. 1998;7(4):7–24. [Google Scholar]
- 17.Ramos CF, Paixão JGM, Donza FCS, Silva AMP, Caçador DF, Dias VDV, et al. Cumprimento do calendário de vacinação de crianças em uma unidade de Saúde da Família. Rev Pan-Amaz Saude. 2010;1(2):55–60. [Google Scholar]
- 18.Rocha R, Sampaio MJ, Pereira CA, Liberal I. Factores associados ao não cumprimento do Programa Nacional de Vacinação e das vacinas pneumocócica conjugada heptavalente e contra o rotavírus. Acta Pediatr Port. 2010;41(5):195–200. [Google Scholar]
- 19.Silva AAM, Gomes UA, Tonial SR, Silva RA. Cobertura vacinal e fatores de risco associados a não-vacinação em localidade urbana do Nordeste brasileiro, 1994. 10.1590/S0034-89101999000200006Rev Saude Publica. 1999;33(2):147–156. doi: 10.1590/s0034-89101999000200006. [DOI] [PubMed] [Google Scholar]
- 20.Silveira ASA, Silva BMF, Peres EC, Meneghin P. Controle de vacinação de crianças matriculadas em escolas municipais da cidade de São Paulo. 10.1590/S0080-62342007000200018Rev Esc Enferm USP. 2007;41(2):299–305. doi: 10.1590/s0080-62342007000200018. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. 10.1093/aje/kwi188Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 22.Tertuliano GC, Stein AT. Atraso vacinal e seus determinantes: um estudo em localidade atendida pela Estratégia Saúde da Família. 10.1590/S1413-81232011000200015Cienc Saude Coletiva. 2011;16(2):523–530. doi: 10.1590/s1413-81232011000200015. [DOI] [PubMed] [Google Scholar]
- 23.Yokokura AVCP, Silva AAM, Bernardes ACF, Lamy F, Filho, Alves MTSSB, Cabra NAL, et al. Cobertura vacinal e fatores associados ao esquema vacinal básico incompleto aos 12 meses de idade, São Luís, Maranhão, Brasil, 2006. 10.1590/S0102-311X2013000300010Cad Saude Publica. 2013;29(3):522–534. doi: 10.1590/s0102-311x2013000300010. [DOI] [PubMed] [Google Scholar]


