Abstract
Objectives
Sleep disorders are common in patients with HIV/AIDS, and can lead to poor quality of life. Although many studies have investigated the aetiology of these disorders, it is still unclear whether impaired sleep quality is associated with HIV itself, social problems, or side effects of antiretroviral therapy (ART). Moreover, despite its known neurological associations, little is known about the role of the trans-activator of transcription (Tat) protein in sleep disorders in patients with HIV/AIDS. The purpose of this study was to test the hypothesis that the sleep quality of patients with HIV/AIDS affected by an altered circadian rhythm correlates with cerebrospinal HIV Tat protein concentration.
Methods
Ninety-six patients with HIV/AIDS between 20 and 69 years old completed the Pittsburgh Sleep Quality Index. Their circadian rhythm parameters of blood pressure, Tat concentration in cerebrospinal fluid, melatonin concentration, CD4 cell count and HIV RNA viral load in serum were measured.
Results
The circadian amplitude of systolic blood pressure and the score for sleep quality (Pittsburgh Sleep Quality Index) were negatively correlated with HIV Tat protein concentration, while the melatonin value was positively correlated with Tat protein concentration.
Conclusions
The HIV Tat protein affects circadian rhythmicity by interfering with the circadian system in patients with HIV/AIDS and further increases the melatonin excretion value. A Tat protein-related high melatonin value may counteract HIV-related poor sleep quality during the progression of HIV infection. This study provides the first clinical evidence offering an explanation for why sleep quality did not show an association with progression of HIV infection in previous studies.
Keywords: circadian rhythm, HIV/AIDS, sleep, Tat protein
Introduction
About 70% of adult patients with HIV/AIDS experience sleep difficulties, such as insomnia, daytime sleepiness and fragmented sleep [1–5]. It is still unclear whether poor sleep quality is associated with CD4 cell count, HIV RNA viral load, social problems or antiretroviral therapy (ART) [2,4–7]. The circadian rhythm, a major regulator of sleep timing [8,9], was found to coordinate body temperature, hormone secretion and the production of circulating immune cells abnormally in HIV-infected patients. However, the relationship between HIV viral proteins and the circadian rhythm is poorly understood.
The HIV trans-activator of transcription (Tat) protein, which is released from HIV-infected glial cells and macrophages in the brain, plays a pivotal role in HIV replication. HIV Tat is known to directly affect the mammalian master circadian pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN), which underlies lentiviral circadian rhythm dysfunction [10]. This circadian rhythm dysfuntion may result from Tat protein-induced activation of N-methyl-d-aspartate receptors and alteration of the light entrainment pathway. Recently, chronic Tat exposure in the brain has been found to decrease locomotor activity and the amplitude of its circadian rhythm in Tat transgenic mice [11]. In the light of these previous findings, we elected to study the relationships between circadian rhythm-related poor sleep quality and HIV Tat protein concentrations in HIV-infected patients. We therefore utilized a clinical epidemiology approach to assess the circadian amplitudes of blood pressure and heart rate, Pittsburgh Sleep Quality Index score, melatonin value and Tat protein concentration in patients with HIV/AIDS.
Methods
Subjects and setting
Ninety-six patients with HIV/AIDS between 20 and 69 years old were recruited for the study from Chengdu Infectious Diseases Hospital, Chengdu, China. Exclusion criteria were as follows: (1) a previous diagnosis or history of obstructive sleep apnoea; (2) a family history of sleep disorders; (3) a change in ART within the past 30 days; (4) being currently on any medication, such as sedating or stimulant drugs, other than anti-infective medications; (5) use of any recreational drugs in the past 30 days; (6) a history of neurological disorders or diseases impacting sleep.
Design and procedures
After the patients had signed informed consent forms, their blood pressure and heart rate were recorded using ambulatory blood pressure monitors to assess circadian rhythms of systolic blood pressure, diastolic blood pressure and heart rate in Chengdu Infectious Diseases Hospital over the course of 3 days. On day 1 of the hospital stay, the Pittsburgh Sleep Quality Index questionnaire was filled out by patients, and on day 2, at between 7:00 and 8:00 am, cerebrospinal fluid or blood was drawn from each patient for determination of HIV Tat protein concentration, melatonin concentration, CD3/CD4/CD8 cell count, HIV RNA viral load and IgG/IgA/IgM concentration.
Statistical analysis
To determine MESOR (M), amplitude (A) and acrophase (φ), serial data for blood pressure and heart rate for each patient over time were analysed with the least-squares fit of a 24-hour period cosine function, f(t) = M + Acosin(ωt + φ), i.e. a single cosinor analysis [12,13]. Briefly, the data were analysed as described [12,13] to yield the estimated parameters of circadian rhythm: M [midline estimating statistic of rhythm (MESOR)], A (amplitude) and φ (acrophase). Of note, the relative amplitude was computed as A/M to account for inter-individual differences in the MESOR.
A linear regression analysis was used to calculate the correlation of HIV Tat protein concentration with the A/M of blood pressure and heart rate, Pittsburgh Sleep Quality Index, melatonin value, and HIV RNA viral load, respectively. The data distribution was analysed using the D'Agostino−Pearson normality test. A P value < 0.05 was considered statistically significant, and P < 0.01 highly significant.
Ethics statement
This study was approved by the authorities of the ethics committee of Chengdu Infectious Diseases Hospital according to the principles of clinical research enacted by the China Health Ministry. All participants provided informed consent prior to the study.
Results
A total of 96 HIV-infected patients were recruited for our clinical study. The mean age of our recruited patients with HIV/AIDS was 38.55 (± 10.62, mean ± SD) years; the mean HIV RNA viral load was 57426 (± 153634, mean ± SD) HIV-1 RNA copies/mL; the mean CD4 count was 89.16 (± 114.24, mean ± SD) cells/μL; the mean melatonin value was 66.67 (± 55.75, mean ± SD) pg/ml; and the mean score on the Pittsburgh Sleep Quality Index was 11.97 (± 4.87, mean ± SD) (Table 1).
Table 1.
Baseline characteristics of patients with HIV/AIDS
| Variable | Mean ± SD |
|---|---|
| Age (years) | 38.55 ± 10.62 |
| Viral load (copies/ml) | 57426 ± 153634 |
| CD4 count (cells/μL) | 89.16 ± 114.24 |
| CD3 count (cells/μL) | 654.58 ± 570.93 |
| CD8 count (cells/μL) | 541.07 ± 484.22 |
| Melatonin (pg/ml) | 66.67 ± 55.75 |
| Pittsburgh Sleep Quality Index score | 11.97 ± 4.87 |
| IgG (g/L) | 16.74 ± 6.08 |
| IgA (g/L) | 4.66 ± 2.63 |
| IgM (g/L) | 1.69 ± 1.83 |
The values given are mean ± standard deviation (SD). Ig, immunoglobulin.
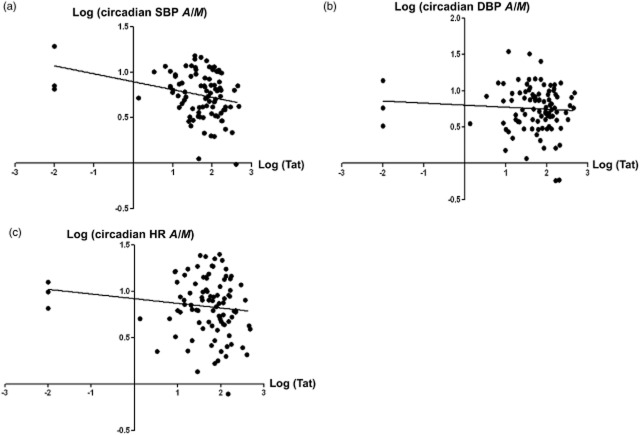
The demographic characteristics of three patients with Tat concentration outliers were representative of those of the entire sample. Thus, we analysed our data with and without the Tat concentration outliers. As shownin Fig. 1, we found that, when the data were analysed with the outliers, the Tat protein concentration was negatively associated with the circadian parameter A/M of systolic blood pressure (Fig. 1a; R2 = 0.081; F = 8.37; P = 0.0047). There was not a statistically significant correlation between Tat protein level and the A/M of diastolic blood pressure (Fig. 1b; R2 = 0.0053; F = 0.504; P = 0.479) or the A/M of heart rate (Fig. 1c; R2 = 0.0331; F = 4.504; P = 0.200), but there were clear negative trends. The analyses without the outliers revealed the same trends for the A/M of systolic blood pressure (Fig. S1a; R2 = 0.067; F = 6.61; P = 0.0012), the A/M of diastolic blood pressure (Fig. S1b; R2 = 0.0069; F = 0.63; P = 0.42) and the A/M of heart rate (Fig. S1c; R2 = 0.014; F = 1.230; P = 0.260). As Tat protein concentration indirectly reflects HIV viral reproduction in the central nervous system, these data suggest that circadian parameters exhibited a stage-dependent decrease in patients with HIV/AIDS.
Fig 1.
Correlation of trans-activator of transcription (Tat) protein concentration with circadian parameters [amplitude/midline estimating statistic of rhythm (A/M)] in patients with HIV/AIDS, including Tat concentration outliers. (a) The Tat protein level was negatively associated with the A/M of systolic blood pressure (SBP) in patients with HIV/AIDS (R2 = 0.081; F = 8.37; P = 0.0047). (b) The association between Tat protein concentration and the A/M of diastolic blood pressure (DBP) in patients with HIV/AIDS was not statistically significant (R2 = 0.0053; F = 0.504; P = 0.479), but showed a negative trend. (c) The association between Tat protein concentration and the A/M of heart rate (HR) was not statistically significant (R2 = 0.0331; F = 4.504; P = 0.200), but showed a negative trend.
A total of 93 patients with HIV/AIDS completed the Pittsburgh Sleep Quality Index questionnaire. Interestingly, the collected data unexpectedly indicated that patients with HIV/AIDS with higher Tat protein concentrations had better sleep quality (Fig. 2a; R2 = 0.0912; F = 11.272; P = 0.001). We also found that secretion of melatonin from the pineal gland was greater when more Tat protein was present in the cerebrospinal fluid, when we analysed the data with (Fig. 2b; R2 = 0.29; F = 38.17; P < 0.001) or without (Fig. S1d; R2 = 0.359; F = 51.12; P < 0.001) Tat concentration outliers. Of note, the Tat concentration, the A/M ratios of circadian parameters, and melatonin were all converted into their logtransforms, because the D'Agostino−Pearson normality test suggested that our data were not normally distributed.
Fig 2.
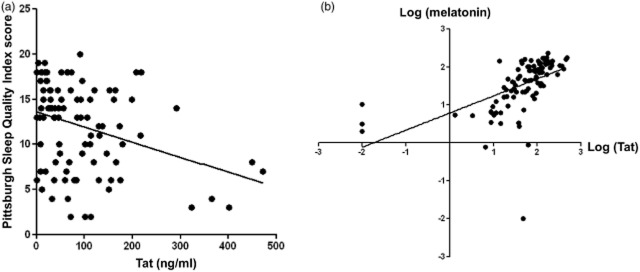
The relationship between trans-activator of transcription (Tat) protein concentration, sleep quality and melatonin levels in HIV-infected patients. (a) Tat protein level was negatively associated with sleep quality in HIV-infected patients, as indicated by the Pittsburgh Sleep Quality Index (R2 = 0.0912; F = 11.272; P = 0.001). (b) Tat protein level was positively associated with melatonin level (R2 = 0.29; F = 38.17; P < 0.001).
Discussion
A total of 96 patients with HIV/AIDS were enrolled in the study following the principles of clinical epidemiology. These patients with HIV/AIDS had high HIV RNA viral loads and CD4 counts < 200 cells/μL, and were considered to have advanced AIDS. We demonstrated that, in these patients with HIV/AIDS, HIV Tat protein disrupts the circadian rhythm, as indicated by the decrease in the A/M of blood pressure with increasing Tat concentration. In contrast, the results from the Pittsburgh Sleep Quality Index questionnaire showed that patients with HIV/AIDS with higher Tat protein concentrations had better sleep quality. Furthermore, we also detected increased melatonin secretion from the pineal gland correlated with increasing Tat protein expression in the cerebrospinal fluid.
Our finding that HIV Tat protein concentration was negatively correlated with the patients' circadian amplitude of blood pressure is consistent with the results of animal experiments carried out by Duncan et al. [11], which demonstrated that transgenic mice with SCN Tat expression showed a decrease in the amplitude of the circadian rhythm, suggesting that the expression of the Tat protein in the brain affects the circadian rhythm. We also observed trends for negative correlations between Tat protein concentration and the A/M of diastolic blood pressure and the A/M of heart rate. The statistical nonsignificance of these data may be attributable to the small sample size. Moreover, other studies found that both adult patients with HIV/AIDS and mammals infected with related lentiviruses had irregularities in physiological functions related to circadian rhythms, such as locomotor activity, immune cell levels and body temperature [11,14–17]. However, these studies were focused on the effects of HIV itself or cytokines that interrupt circadian modulation of the SCN. This study, for the first time, determined the relationship between the HIV Tat protein and the circadian rhythm in HIV-infected patients in a clinical study. Determining which circadian clock genes are affected by the Tat protein will be the objective of future studies.
Sleep disorders are one of the most common symptoms in patients with HIV/AIDS. Unexpectedly, the Pittsburgh Sleep Quality Index scores suggested that HIV-infected patients with higher HIV Tat protein concentrations had better sleep quality. This contrasts with the findings of previous studies which suggested poor sleep quality in HIV-infected patients [2,4–7,17–19]. However, Fig. 2b shows that the melatonin value was significantly positively associated with Tat protein level, indicating that more melatonin is secreted from the pineal gland when Tat protein levels increase. A limitation here could be that melatonin in blood is usually elevated at night. Melatonin is usually increased at around 9 pm and stays elevated for about 12 h until dawn, when it falls back to low daytime values by about 9 am. In our study, melatonin values were measured in the early morning to avoid interfering with patients' sleep. Even though we measured patients' melatonin values before they fell to low daytime values, this may not have been the optimal time to determine melatonin levels. Melatonin, secreted from the pineal gland as a hormone, not only has a function involving coordination of the circadian rhythm, but also has the effect of promoting sleep and controlling the sleep−wake cycle [20–22]. Circadian melatonin secretion is a well-known output signal from the circadian pacemaker in the SCN. Through a polysynaptic projection, the SCN functionally inhibits the activity of the superior cervical ganglion (SCG), which supplies the pineal gland with an excitatory, noradrenaline (NA)-containing input. This allows light to suppress the production and release of melatonin from the pineal gland but to induce melatonin secretion during dark periods. Melatonin reciprocally activates neurons in the SCN through activation of melatonin 1 (MT1) and MT2 receptors, and serotonergic input from the raphe nucleus modulates the SCN via interactions with the serotonin [also known as 5-hydroxytryptamine (5-HT)] receptor 5-HT2C and other classes of 5-HT receptor [23–27]. As the master clock located in the SCN of the hypothalamus coordinates circadian rhythms, Tat protein-induced SCN dysfunction may interfere with SCN function in inhibiting SCG. Thus, increased melatonin secretion from the pineal gland in patients with HIV/AIDS further affects their sleep quality. Previous studies suggested that the poor sleep quality of HIV-infected patients was associated with HIV and changes in the condition of the immune system, but sleep quality is not significantly associated with the progression of HIV infection [2,28–30]. Our unexpected finding represents important evidence offering an explanation for why HIV-related clinical variables (e.g. duration of HIV infection, CD4 count and viral load) were not significantly associated with sleep quality in those studies: Tat protein-related high melatonin values may counteract the poor sleep quality caused by HIV during the progression of HIV infection.
In this study, we have concluded that the HIV Tat protein impairs the circadian rhythm in patients with HIV/AIDS, by decreasing the circadian amplitudes of blood pressure and heart rate. However, this impairment of the circadian rhythm also increases melatonin secretion levels from the pineal gland to further affect sleep quality in patients with HIV/AIDS.
Acknowledgments
We thank Yanling Guo for assisting with data collection and JunJie Ying for assisting with data analysis. This study was funded by the National Nature Science Foundation of China (grant no. 41074131 to ZW).
Conflicts of interest
The authors have no conflicts of interest to report.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site
Correlation of trans-activator of transcription (Tat) protein concentration with circadian parameters [amplitude/midline estimating statistic of rhythm (A/M)] in patients with HIV/AIDS, without Tat concentration outliers. (a) Tat protein level was negatively associated with the A/M of systolic blood pressure (SBP) in patients with HIV/AIDS (R2 = 0.067; F = 6.61; P = 0.0012). (b) The association between Tat protein concentration and the A/M of diastolic blood pressure (DBP) in patients with HIV/AIDS was not statistically significant (R2 = 0.0069; F = 0.63; P = 0.42), but showed a negative trend. (c) The association between Tat protein concentration and the A/M of heart rate (HR) was not statistically significant (R2 = 0.014; F = 1.230; P = 0.260), but showed a negative trend. (d) Tat protein level was positively associated with melatonin level in HIV-infected patients (R2 = 0.359; F = 51.12; P < 0.001).
References
- 1.Gamaldo CE, Spira AP, Hock RS, et al. Sleep, function and HIV: a multi-method assessment. AIDS Behav. 2013;17:2808–2815. doi: 10.1007/s10461-012-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:260–265. doi: 10.1097/00042560-199811010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low Y, Preud'homme X, Goforth HW, Omonuwa T, Krystal AD. The association of fatigue with depression and insomnia in HIV-seropositive patients: a pilot study. Sleep. 2011;34:1723–1726. doi: 10.5665/sleep.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JL, Darko DF, Brown SJ, et al. Early central nervous system response to HIV infection: sleep distortion and cognitive-motor decrements. AIDS. 1995;9:1043–1050. doi: 10.1097/00002030-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosom Med. 2005;67:260–269. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- 7.Wagner GJ, Rabkin R. Effects of dextroamphetamine on depression and fatigue in men with HIV: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2000;61:436–440. doi: 10.4088/jcp.v61n0608. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 9.Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007;8(Suppl 3):27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Clark JP, 3rd, Sampair CS, Kofuji P, Nath A, Ding JM. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol. 2005;289:R656–R662. doi: 10.1152/ajpregu.00179.2005. [DOI] [PubMed] [Google Scholar]
- 11.Duncan MJ, Bruce-Keller AJ, Conner C, et al. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1680–R1687. doi: 10.1152/ajpregu.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen G, Bakken E, Delmore P, et al. From various kinds of heart rate variability to chronocardiology. Am J Cardiol. 1990;66:863–868. doi: 10.1016/0002-9149(90)90369-c. [DOI] [PubMed] [Google Scholar]
- 14.Bourin P, Mansour I, Doinel C, Roué R, Rouger P, Levi F. Circadian rhythms of circulating NK cells in healthy and human immunodeficiency virus-infected men. Chronobiol Int. 1993;10:298–305. doi: 10.1080/07420529309059712. [DOI] [PubMed] [Google Scholar]
- 15.Burudi EM, Fox HS. Simian immunodeficiency virus model of HIV-induced central nervous system dysfunction. Adv Virus Res. 2001;56:435–468. doi: 10.1016/s0065-3527(01)56035-2. [DOI] [PubMed] [Google Scholar]
- 16.Martini E, Muller JY, Doinel C, et al. Disappearance of CD4-lymphocyte circadian cycles in HIV-infected patients: early event during asymptomatic infection. AIDS. 1988;2:133–134. doi: 10.1097/00002030-198804000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Swoyer J, Rhame F, Hrushesky W, et al. Circadian rhythm alteration in HIV infected subjects. Prog Clin Biol Res. 1990;341A:437–449. [PubMed] [Google Scholar]
- 18.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 19.Hand GA, Phillips KD, Dudgeon WD. Perceived stress in HIV-infected individuals: physiological and psychological correlates. AIDS Care. 2006;18:1011–1017. doi: 10.1080/09540120600568376. [DOI] [PubMed] [Google Scholar]
- 20.Wurtman RJ, Zhdanova I. Improvement of sleep quality by melatonin. Lancet. 1995;346:1491. doi: 10.1016/s0140-6736(95)92509-0. [DOI] [PubMed] [Google Scholar]
- 21.Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE. 2013;8:e63773. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- 23.de Bodinat C, Guardiola-Lemaitre B, Mocaër E, Renard P, Muñoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9:628–642. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- 24.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- 25.Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9:5–9. doi: 10.1016/j.smrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Perreau-Lenz S, Kalsbeek A, Garidou ML, et al. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- 27.Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Jean-Louis G, Weber KM, Aouizerat BE, et al. Insomnia symptoms and HIV infection among participants in the Women's Interagency HIV Study. Sleep. 2012;35:131–137. doi: 10.5665/sleep.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breitbart W, McDonald MV, Rosenfeld B, Monkman ND, Passik S. Fatigue in ambulatory AIDS patients. J Pain Symptom Manage. 1998;15:159–167. doi: 10.1016/s0885-3924(97)00260-1. [DOI] [PubMed] [Google Scholar]
- 30.Cohen FL, Ferrans CE, Vizgirda V, Kunkle V, Cloninger L. Sleep in men and women infected with human immunodeficiency virus. Holist Nurs Pract. 1996;10:33–43. doi: 10.1097/00004650-199607000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of trans-activator of transcription (Tat) protein concentration with circadian parameters [amplitude/midline estimating statistic of rhythm (A/M)] in patients with HIV/AIDS, without Tat concentration outliers. (a) Tat protein level was negatively associated with the A/M of systolic blood pressure (SBP) in patients with HIV/AIDS (R2 = 0.067; F = 6.61; P = 0.0012). (b) The association between Tat protein concentration and the A/M of diastolic blood pressure (DBP) in patients with HIV/AIDS was not statistically significant (R2 = 0.0069; F = 0.63; P = 0.42), but showed a negative trend. (c) The association between Tat protein concentration and the A/M of heart rate (HR) was not statistically significant (R2 = 0.014; F = 1.230; P = 0.260), but showed a negative trend. (d) Tat protein level was positively associated with melatonin level in HIV-infected patients (R2 = 0.359; F = 51.12; P < 0.001).