Abstract
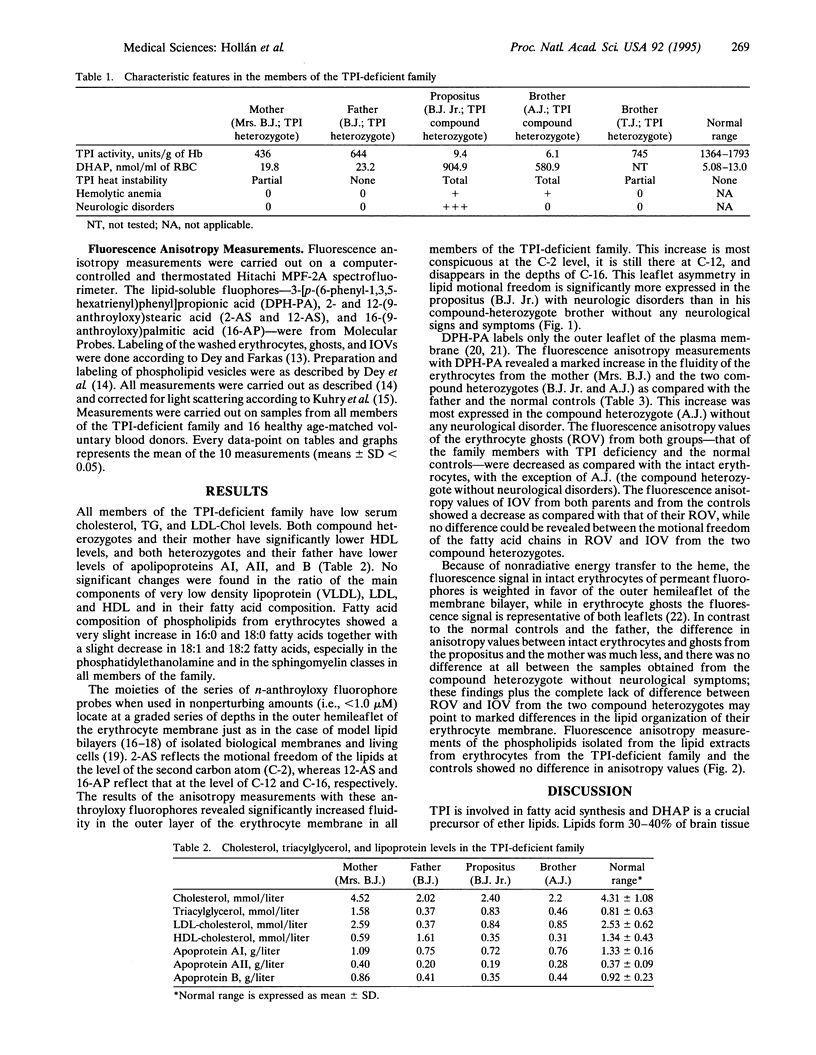
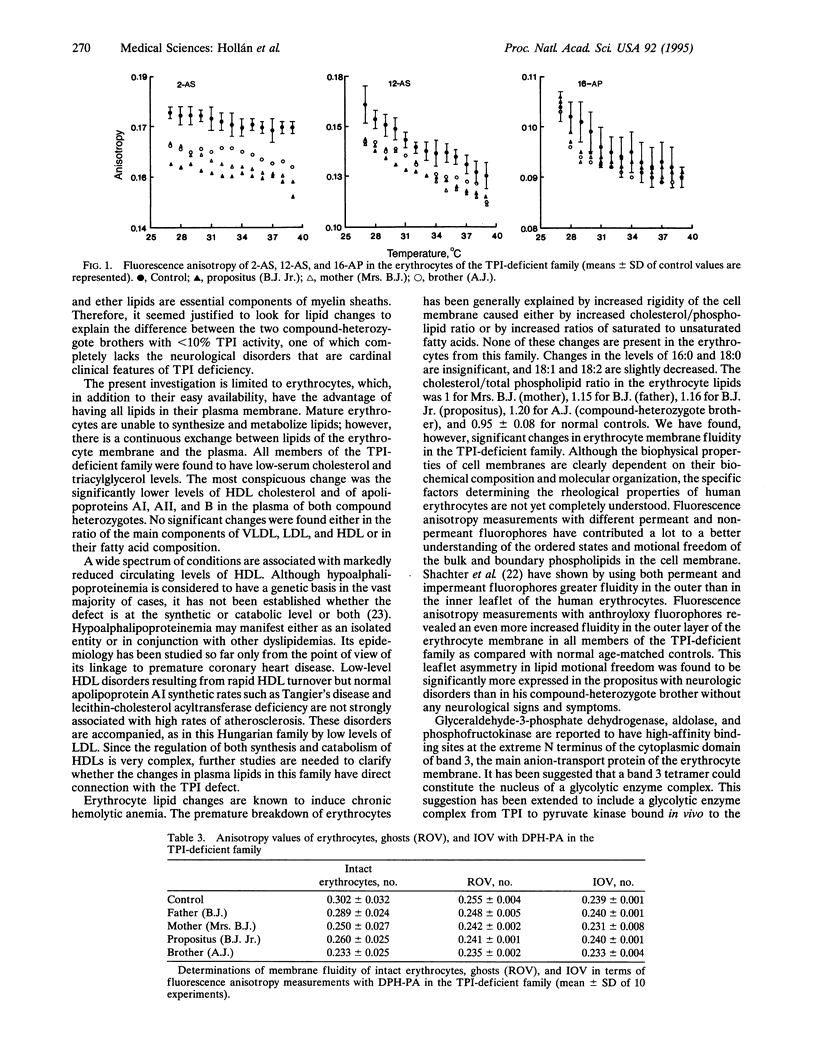
Marked hypoalphalipoproteinemia was found together with relatively low serum cholesterol, triacylglycerol, and LDL levels in a triose-phosphate isomerase (TPI; D-glyceraldehyde-3-phosphate ketol-isomerase, EC 5.3.1.1)-deficient Hungarian family, especially in the two compound-heterozygote brothers. Apart from a slight increase in palmitic and stearic acids together with a slight decrease in oleic and linoleic acids, no other changes were found in the fatty acid composition of the erythrocyte phospholipids. Anisotropy measurements with n-(9-anthroyloxy) stearic and -palmitic acid fluorophores revealed increased motional freedom of the fatty acid chains in the external lipid layers of the intact erythrocytes from all members of the TPI-deficient family as compared with normal age-matched controls. This asymmetric increase in membrane fluidity was found to be significantly higher in the propositus than in his compound-heterozygote brother without any neurological disorders. The change in membrane fluidity may result from as-yet-unresolved aspects of the lipid composition of the plasma membrane. Our findings that the differences between the TPI-deficient individuals and normal controls and the differences between the two compound-heterozygote brothers were all absent in the phospholipid extracts of the same erythrocytes favor the assumption that the increased motional freedom of the fatty acid chains in the external surface of the bilayer is caused by the binding of the mutant TPI molecule to the N-terminal sequence of band 3 protein.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang M. L., Artymiuk P. J., Wu X., Hollán S., Lammi A., Maquat L. E. Human triosephosphate isomerase deficiency resulting from mutation of Phe-240. Am J Hum Genet. 1993 Jun;52(6):1260–1269. [PMC free article] [PubMed] [Google Scholar]
- Chatelier R. C., Sawyer W. H. The transverse organisation of ubiquinones in mitochondrial membranes as determined by fluorescence quenching. Evidence for a two-site model. Eur Biophys J. 1985;11(3):179–185. doi: 10.1007/BF00257396. [DOI] [PubMed] [Google Scholar]
- Cheng J., Mielnicki L. M., Pruitt S. C., Maquat L. E. Nucleotide sequence of murine triosephosphate isomerase cDNA. Nucleic Acids Res. 1990 Jul 25;18(14):4261–4261. doi: 10.1093/nar/18.14.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. M., Grogan W. M. Comparisons of steady-state anisotropy of the plasma membrane of living cells with different probes. Biochim Biophys Acta. 1991 Aug 26;1067(2):171–176. doi: 10.1016/0005-2736(91)90040-f. [DOI] [PubMed] [Google Scholar]
- Dey I., Buda C., Wiik T., Halver J. E., Farkas T. Molecular and structural composition of phospholipid membranes in livers of marine and freshwater fish in relation to temperature. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7498–7502. doi: 10.1073/pnas.90.16.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein C., Fredenrich C., Schuelke J. C., Jensen W. E., Sitrin M., Iverius P. H., Scanu A. M. Hypoalphalipoproteinemia: postprandial response of subjects with preprandial normotriglyceridemia and hypertriglyceridemia to various diets. Metabolism. 1993 Feb;42(2):247–257. doi: 10.1016/0026-0495(93)90043-n. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Firestone D., Horwitz W. IUPAC gas chromatographic method for determination of fatty acid composition: collaborative study. J Assoc Off Anal Chem. 1979 Jul;62(4):709–721. [PubMed] [Google Scholar]
- Fossel E. T., Solomon A. K. Ouabain-sensitive interaction between human red cell membrane and glycolytic enzyme complex in cytosol. Biochim Biophys Acta. 1978 Jun 16;510(1):99–111. doi: 10.1016/0005-2736(78)90133-5. [DOI] [PubMed] [Google Scholar]
- Higashi T., Richards C. S., Uyeda K. The interaction of phosphofructokinase with erythrocyte membranes. J Biol Chem. 1979 Oct 10;254(19):9542–9550. [PubMed] [Google Scholar]
- Hollán S., Fujii H., Hirono A., Hirono K., Karro H., Miwa S., Harsányi V., Gyódi E., Inselt-Kovács M. Hereditary triosephosphate isomerase (TPI) deficiency: two severely affected brothers one with and one without neurological symptoms. Hum Genet. 1993 Nov;92(5):486–490. doi: 10.1007/BF00216456. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Matsubayashi M., Kotani K., Usui K., Kametani F. Asymmetry of membrane fluidity in the lipid bilayer of blood platelets: fluorescence study with diphenylhexatriene and analogs. J Membr Biol. 1991 Feb;119(3):221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- Kuhry J. G., Duportail G., Bronner C., Laustriat G. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim Biophys Acta. 1985 Apr 22;845(1):60–67. doi: 10.1016/0167-4889(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Low P. S., Rathinavelu P., Harrison M. L. Regulation of glycolysis via reversible enzyme binding to the membrane protein, band 3. J Biol Chem. 1993 Jul 15;268(20):14627–14631. [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Maretzki D., Reimann B., Rapoport S. M. A reappraisal of the binding of cytosolic enzymes to erythrocyte membranes. Trends Biochem Sci. 1989 Mar;14(3):93–96. doi: 10.1016/0968-0004(89)90128-x. [DOI] [PubMed] [Google Scholar]
- Miwa I., Fukatsu H., Okuda J. Effect of mild oxidants on glycolysis in human erythrocytes. Biochem J. 1992 Apr 15;283(Pt 2):621–622. doi: 10.1042/bj2830621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. N., Liu T., Kaul R. K., Köhler H., Steck T. L. The aldolase-binding site of the human erythrocyte membrane is at the NH2 terminus of band 3. J Biol Chem. 1981 Nov 10;256(21):11203–11208. [PubMed] [Google Scholar]
- Ovádi J. Old pathway--new concept: control of glycolysis by metabolite-modulated dynamic enzyme associations. Trends Biochem Sci. 1988 Dec;13(12):486–490. doi: 10.1016/0968-0004(88)90237-x. [DOI] [PubMed] [Google Scholar]
- Podo F., Blasie J. K. Nuclear magnetic resonance studies of lecithin bimolecular leaflets with incorporated fluorescent probes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1032–1036. doi: 10.1073/pnas.74.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. Characteristics and regulation of active calcium transport in inside-out red cell membrane vesicles. Biochim Biophys Acta. 1980 May 23;598(2):326–338. doi: 10.1016/0005-2736(80)90010-3. [DOI] [PubMed] [Google Scholar]
- Schachter D., Cogan U., Abbott R. E. Asymmetry of lipid dynamics in human erythrocyte membranes studied with permanent fluorophores. Biochemistry. 1982 Apr 27;21(9):2146–2150. doi: 10.1021/bi00538a025. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Sawyer W. H. Properties and the locations of a set of fluorescent probes sensitive to the fluidity gradient of the lipid bilayer. Biochim Biophys Acta. 1978 Aug 4;511(2):125–140. doi: 10.1016/0005-2736(78)90308-5. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Paglia D. E. The primary cause of hemolysis in enzymopathies of anaerobic glycolysis: a viewpoint. Blood Cells. 1980;6(4):819–829. [PubMed] [Google Scholar]



