Abstract
Background
The results of studies on association between ABCB1 gene polymorphisms and glucocorticoid-induced avascular necrosis of the femoral head (GANFH) are controversial. This study aimed to assess the association of ABCB1 gene polymorphisms with the risk of GANFH by conducting a meta-analysis.
Material/Methods
The PubMed, Cochrane Library, and Embase databases were searched for papers that describe the association between ABCB1 polymorphisms and GANFH risk. Summary odds ratios and 95% confidence intervals (CI) were estimated based on a fixed-effects model or random-effects model, depending on the absence or presence of significant heterogeneity.
Results
A total of 5 studies and 833 patients were included in the final analysis. Significant differences were found for rs1045642 polymorphism in the comparisons of CC vs. CT+TT (OR, 1.462; 95% CI, 1.066–2.007; P=0.019), and rs2032582 polymorphism in the comparisons of GG vs. G(TA)+(TA)(TA) (OR, 1.548; 95% CI,1.063–2.255; P=0.023).
Conclusions
The study demonstrated that the ABCB1 polymorphisms (rs1045642 and rs2032582) significantly reduced the risk of GANFH.
MeSH Keywords: ATP Binding Cassette Transporter 1, Femur Head Necrosis, Meta-Analysis
Background
Avascular necrosis of the femoral head (ANFH) is considered to be part of a multifactorial, heterogeneous group of disorders that lead to the necrosis and collapse of the femoral head during later stages [1–3]. ANFH may induce hip joint dysfunction and partial or complete loss of the ability to walk [4]. ANFH can be caused by various conditions such as trauma, glucocorticoid (GC) therapy, alcoholism [5], and storage diseases, of which GC-induced ANFH (GANFH) ranks first. The exact pathogenesis of femoral head osteonecrosis related to GC remains uncertain. There are several alternative mechanisms such as fat embolization [6], intramedullary pressure changes [7], modified artery constriction [8,9], circulatory impairment [10], coagulation disorders [11], and cell dysfunction [12,13]; however, none alone can explain the underlying mechanism. Moreover, it cannot be ignored that while some people develop osteonecrosis others do not under the same conditions. This phenomenon suggests that individual susceptibility or individual genetic factors may exist. It was recently suggested that genetic polymorphisms of the enzymes responsible for steroid metabolism, steroid receptors, and transport proteins may explain the individual differences [14,15].
The transport protein, P-glycoprotein (P-gp), which acts as an energy-dependent membrane efflux pump for a wide spectrum of therapeutic agents, including steroids, plays an important role in absorption and distribution of drugs. P-gp is encoded by the multidrug resistance gene 1 (ABCB1), also known as MDR1. The ABCB1 gene is locked on chromosomal region 7q21 and consists of 28 exons. More than 50 single-nucleotide polymorphisms (SNPs) of MDR1 have been identified, among which single-nucleotide polymorphisms in exon 21 (G2677T/A, rs2032582) and exon 26 (C3435T, rs1045642) are the most studied. Particularly, rs2032582 and rs1045642 SNPs have been found to be related with a decreased and an increased expression of P-gp, respectively [16]. However, controversy continues in clinical research. Takeshi Asano et al. [17] demonstrated the ABCB1 rs1045642 and rs2032582 polymorphism decreased the risk for GANFH after kidney transplantation. Similar results were found based on patients with systemic lupus erythematosus [18]. However, others demonstrated patients carrying rs2032582rs2032582 polymorphism had a higher risk of developing GANFH than those with wild-type genotypes [14]. Also, no significant differences were found between patients with or without rs1045642 polymorphism. Recently, another study found rs1045642 polymorphism may decrease the risk of having GANFH, but there was no relationship between the rs2032582 polymorphism and GANFH [19]. Even more, another study showed that rs1045642 polymorphism may be a risk factor for susceptibility to GANFH [20].
Thus, the aim of this study was to assess the association of the ABCB1 gene polymorphisms with the risk of GANFH by conducting a meta-analysis of eligible studies published to date.
Material and Methods
The PubMed, Cochrane Library, and Embase databases were searched independently by 2 investigators to retrieve relevant studies published before May 30, 2014. The search criteria “ABCB1”, “ATP binding cassette B1”, “multidrug resistance gene 1”, “MDR1”, and “osteonecrosis” were used in text word searches. The “related articles” function was used to broaden the search. The reference lists of the selected articles were also manually examined to find relevant studies not discovered during the database searches.
We selected any studies that investigated the relationship between ABCB1 polymorphism (rs1045642 and rs2032582) and GANFH susceptibility. All titles, abstracts, and full papers of potentially relevant studies were assessed for eligibility. When several reports from the same study were published, only the most recent or informative one was included in this meta-analysis. The language was restricted to only English.
Data extraction
The data extraction of all variables and outcomes of interest were performed independently by 2 investigators. Disagreements were resolved through discussion and consensus. Data on clinical design, country of study, number of participants, and genotyping information were extracted. If articles reported insufficient data, we contacted corresponding authors for additional information.
Quality assessment
The included studies were assessed independently by the 2 reviewers using the Newcastle-Ottawa Scale (NOS) [21,22]. The NOS employs a star rating system to assess quality from 3 broad perspectives of the study: (1) selection of the study groups, (2) comparability of the groups, and (3) identification of the exposure (for case-control studies) or outcome of interest (for cohort studies). Scores ranged from 0 to 9 stars, and studies with no less than 7 stars were considered to be of high quality.
Statistical analysis
The statistical analysis was performed using meta-analysis software called “Comprehensive Meta Analysis”. The strength of the association between gene polymorphisms and GANFH risk was calculated with the OR and respective 95% CIs. The significance of the pooled OR was determined by the Z test, and P-values of less than 0.05 were considered significant. Chi-square test was used for the Hardy-Weinberg equilibrium (HWE) of genotypes in the control group of each study. Statistical heterogeneity among studies was assessed with the I2 statistics, which ranges from 0% (complete consistency) to 100% (complete inconsistency). If the I2 -value was more than 50%, the random-effects model was chosen to calculate the pooled OR; otherwise the fixed-effects model was used. All of the results were presented as forest plots. In the sensitivity analysis, we removed each particular study and performed meta-analysis with the rest repeatedly to show how conclusions might be affected. The presence of publication bias was assessed by a visual inspection of a funnel plot and Egger’s linear regression test.
Results
Literature Search
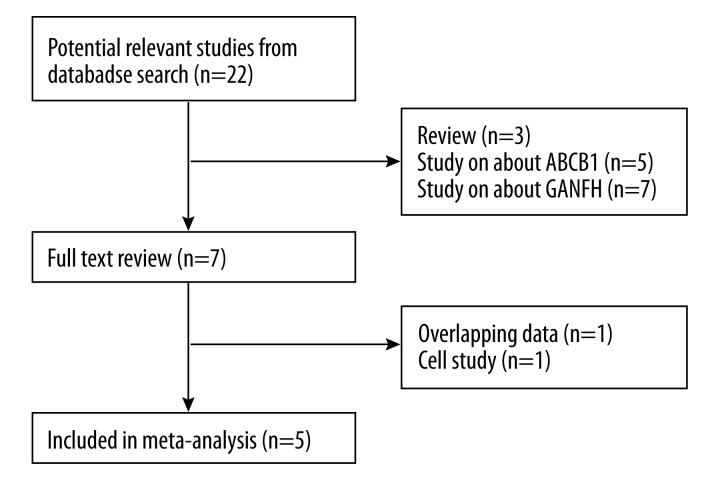
The initial literature search retrieved 22 relevant articles (duplicates were discarded) and 7 articles were excluded for not investigating the topic after carefully screening the titles and abstracts. Then, full publication review was performed, and 2 articles were excluded (1 study of overlapping data, and 1 cell study), which left 5 studies (all case-control studies) for this meta-analysis [14,17–20]. Flowcharts describing the study selection are shown in Figure 1. All included studies were in accordance with the NOS scale and were therefore defined as high-quality studies.
Figure 1.
Search strategy flow diagram.
A total of 833 patients (281 with GANFH and 552 controls) were enrolled in the studies. The key characteristics of the included studies are summarized in Table 1. A review of the data extraction revealed 100% agreement between the 2 reviewers.
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Patients | Groups | ABCB1 genotype | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C3435T,rs1045642 | G2677T/A, rs2032582 | ||||||||||||||||||
| GANFH | Control | GANFH | Control | GANFH | Control | ||||||||||||||
| CC | CT | TT | CC | CT | TT | HWE (P-Value) | GG | G (T/A) | (T/A)(T/A) | GG | G (T/A) | (T/A)(T/A) | HWE (P-Value) | ||||||
| Takeshi Asanoa | 2003 | Japan | After Kidney Transplantation | 30 | 106 | 12 | 17 | 1 | 34 | 49 | 23 | 0.501 | 9 | 17 | 4 | 21 | 50 | 35 | 0.682 |
| X. Y. Yang | 2007 | China | Systemic lupus erythematosus patients | 21 | 106 | 11 | 9 | 1 | 39 | 45 | 22 | 0.186 | 10 | 10 | 1 | 30 | 57 | 19 | 0.369 |
| Wei He | 2009 | China | With corticosteroid therapy | 31 | 17 | 24 | 7 | 0 | 14 | 3 | 0 | 0.69 | 18 | 13 | 0 | 17 | 0 | 0 | – |
| Yanqiong Zhang | 2014 | China | With corticosteroid therapy | 94 | 106 | 37 | 36 | 16 | 36 | 52 | 13 | 0.3 | 18 | 29 | 25 | 16 | 39 | 32 | 0.501 |
| Yun Xue | 2014 | China | With corticosteroid therapy | 105 | 217 | 43 | 49 | 7 | 65 | 91 | 47 | 0.17 | 24 | 49 | 27 | 36 | 109 | 64 | 0.369 |
Main analysis
Table 2 lists the main results of the meta-analysis for the relationship between gene ABCB1 polymorphism and GANFH.
Table 2.
Pooled ORs and 95% CIs of stratified meta-analysis.
| Polymorphisms | Study count | Cases/controls | AA vs. Aa | AA vs. aa | AA vs. Aa+aa | Aa vs. aa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect model | OR (95% CI) | P* values | I2 (%) | Effect model | OR (95% CI) | P* values | I2 (%) | Effect model | OR (95% CI) | P* values | I2 (%) | Effect model | OR (95% CI) | P* values | I2 (%) | |||
| RS1045642 | 5 | 270/533 | Fixed | 1.248 (0.896, 1.738) | 0.19 | 0 | Random | 2.859 (0.988, 8.273) | 0.053 | 58.6 | Fixed | 1.462 (1.066, 2.007) | 0.019 | 0 | Random | 2.381 (0.719, 7.883) | 0.155 | 67.715 |
| RS2032582 | 5 | 254/525 | Fixed | 1.41 (0.948, 2.098) | 0.09 | 35.4 | Fixed | 1.485 (0.908, 2.428) | 0.115 | 46.7 | Fixed | 1.548 (1.063, 2.255) | 0.023 | 41 | Fixed | 1.211 (0.807, 1.815) | 0.355 | 20.6 |
For convenience, we considered the major allele in the variants as “A” and the minor as “a”.
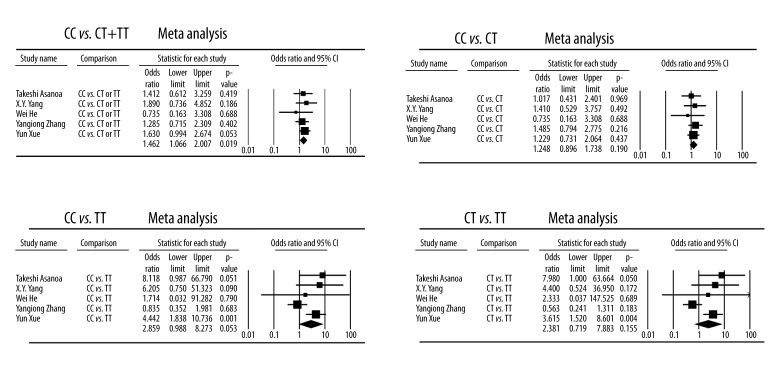
For rs1045642 polymorphism, quantitative synthesis from 5 studies showed significant differences in the comparisons of CC vs. CT+TT (Case vs. Control, OR, 1.462; 95% CI, 1.066–2.007; P=0.019). However, no significant differences were found when comparing CC vs. CT, CC vs. TT, or CT vs. TT (Figure 2).
Figure 2.
Forest plot of GANFH risk associated with the rs1045642 polymorphisms.
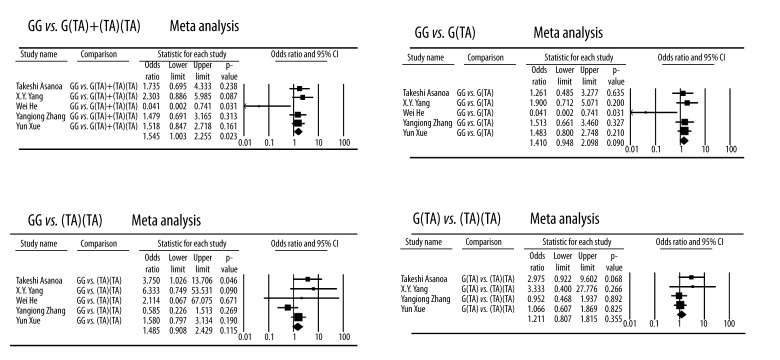
For rs2032582 polymorphism, the frequencies of G(TA) and (TA)(TA) in the Wei He et al. [14] study control groups were zero; hence, the study was excluded when we conducted meta-analysis for comparison of the G(TA) and (TA)(TA). Quantitative synthesis showed significant differences in the comparisons of GG vs. G(TA)+(TA)(TA) (Case vs. Control, OR, 1.548; 95% CI,1.063–2.255; P=0.023). However, no significant difference was found when comparing GG vs. G(TA), GG vs. (TA)(TA), or G(TA) vs. (TA)(TA) (Figure 3)
Figure 3.
Forest plot of GANFH risk associated with the rs2032582 polymorphisms.
In the sensitivity analysis, when each particular study was removed from analysis, no corresponding pooled ORs were qualitatively altered.
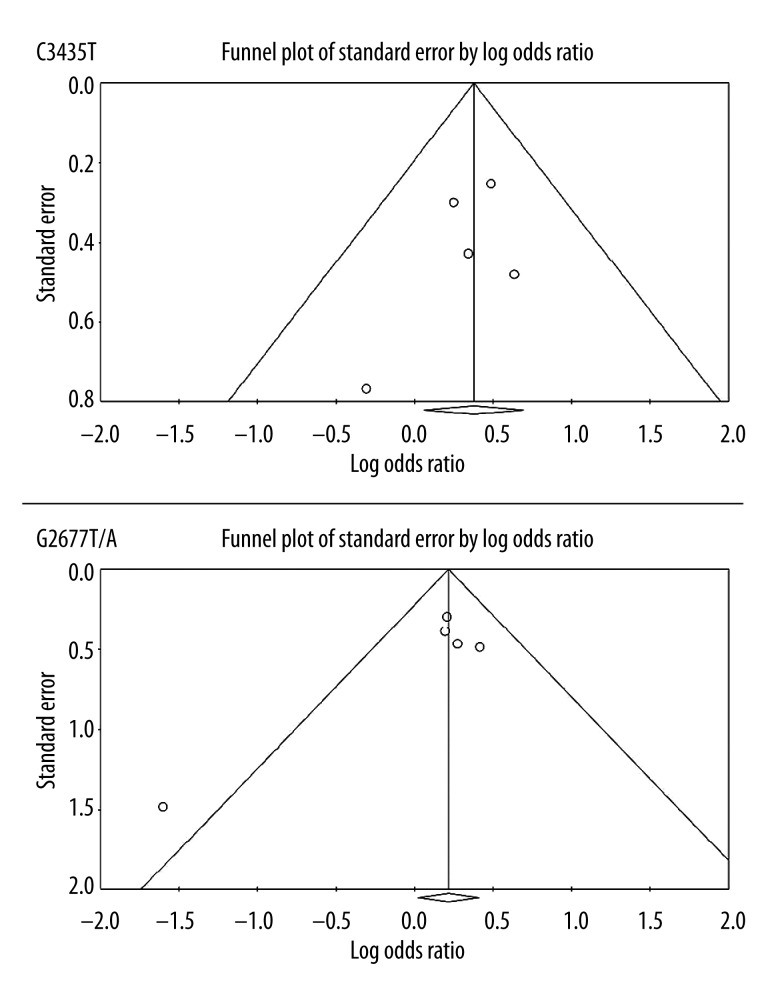
Publication bias
Funnel plots and Egger’s test were performed to assess the publication bias of studies included in this meta-analysis. The funnel plots and Egger’s test demonstrated no evidence of publication bias for CC vs. CT+TT (P=0.40) or GG vs. G(TA)+(TA)(TA) (P=0.17) (Figure 4).
Figure 4.
Funnel plots for the assessment of publication bias of studies included in this meta-analysis.
Discussion
The most important finding of this study was that the ABCB1 polymorphisms (rs1045642 and rs2032582) were found to significantly decrease GANFH susceptibility.
It is well known that glucocorticoids may induce a hypercoagulable hypofibrinolysis state in blood, which may induce thrombosis of the vessels, resulting in bone ischemia, necrosis of bone tissue, and, finally, ANFH occurs. However, the truth is that not all patients treated with virtually the same protocol for steroid administration develop ANFH, which means the individual differences in the steroid sensitivity (ability to absorb and metabolize glucocorticoids) due to genetic factors play an important role in the development of GANFH. Recently, studies found that polymorphisms of the molecules involved in steroid metabolism, steroid receptors, or drug transport may be involved in this individual difference.
The membrane transporter protein P-gp, encoded by the human ABCB1 gene, is an important determinant in drug absorption, distribution, and elimination [23]. The ABCB1 polymorphisms have been demonstrated to affect the expression and function of P-gp and therefore determine individual variability in drug resistance. Several case-control studies have examined the association between the ABCB1 polymorphisms and steroid-induced ONFH [17,18,24]. However, as discussed above, reports concerning the role of the polymorphisms in the pathogenesis of GANFH have been inconsistent. The lack of consistency across these studies may be caused by the geographic and ethnic variability of populations or the probability of a type II error resulting from small sample sizes. Thus, this meta-analysis was performed to overcome the weakness in sample size and population.
The mechanism by which these 2 SNPs decrease the risk of GANFH is still unknown. Rs2032582 of ABCB1 was recently reported to be associated with an amino acid substitution (Ala893 to Ser893 and Ala893 to Thr893), leading to an enhanced efflux transporting ability, and, finally, decreased risk of GANFH [25]. Rs1045642 SNP present in exon 26 was considered as a functional SNP, and its relationship with various diseases has been reported [26–28]. Rs1045642 SNP is a synonymous polymorphism that does not change the protein sequence, but it affects RNA stability and alters the interaction of P-gp with drugs by affecting the timing of co-translational folding [29,30]; thus, the rs1045642 SNP may decrease the amount and activity of P-gp. It is interesting that the results showed a negative association between rs1045642 and GANFH susceptibility. An explanation may be the linkage disequilibrium between rs1045642 and rs2032582, as reported in various studies [18,19].
The most important limitation of this meta-analysis is the inconsistency of the baseline characteristics (e.g., age, sex, and concomitant disease) between the case and control groups, which might increase the selection bias.
Conclusions
The results of this meta-analysis suggest that the ABCB1 polymorphisms (rs1045642 and rs2032582) significantly decrease GANFH susceptibility.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
Source of support: National Nature Science Foundation of China (NSFC 81371942.)
References
- 1.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Yang S, Duan D, Liu X, et al. A combination of granulocyte colony-stimulating factor and stem cell factor ameliorates steroid-associated osteonecrosis in rabbits. J Rheumatol. 2008;35:2241–48. doi: 10.3899/jrheum.071209. [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Wang FS, Wang CJ, et al. Increased Dickkopf-1 expression accelerates bone cell apoptosis in femoral head osteonecrosis. Bone. 2010;46:584–91. doi: 10.1016/j.bone.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Gao YS, Zhu ZH, Chen SB, et al. Injury-to-surgery interval does not affect the occurrence of osteonecrosis of the femoral head: a prospective study in a canine model of femoral neck fractures. Med Sci Monit. 2012;18(7):BR259–64. doi: 10.12659/MSM.883203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu ZH, Gao YS, Luo SH, et al. An animal model of femoral head osteonecrosis induced by a single injection of absolute alcohol: an experimental study. Med Sci Monit. 2011;17(4):BR97–102. doi: 10.12659/MSM.881708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata M, Kumagai K, Miyata N, et al. Osteonecrosis in stroke-prone spontaneously hypertensive rats: effect of glucocorticoid. J Orthop Sci. 2007;12:289–95. doi: 10.1007/s00776-007-1129-y. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CH, Chang JK, Wang YH, et al. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res. 2008;466:1047–53. doi: 10.1007/s11999-008-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drescher W, Lohse J, Varoga D, et al. Enhanced constriction of supplying arteries – a mechanism of femoral head necrosis in Wistar rats? Ann Anat. 2010;192:58–61. doi: 10.1016/j.aanat.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Drescher W, Varoga D, Liebs TR, et al. Femoral artery constriction by norepinephrine is enhanced by methylprednisolone in a rat model. J Bone Joint Surg Am. 2006;88(Suppl 3):162–66. doi: 10.2106/JBJS.F.00452. [DOI] [PubMed] [Google Scholar]
- 10.Urbaniak JR, Seaber AV, Chen LE. Assessment of ischemia and reperfusion injury. Clin Orthop Relat Res. 1997;(334):30–36. [PubMed] [Google Scholar]
- 11.Yun SI, Yoon HY, Jeong SY, Chung YS. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. J Bone Miner Metab. 2009;27:140–48. doi: 10.1007/s00774-008-0019-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Yang S, Feng Y, et al. Impairment of two types of circulating endothelial progenitor cells in patients with glucocorticoid-induced avascular osteonecrosis of the femoral head. Joint Bone Spine. 2013;80:70–76. doi: 10.1016/j.jbspin.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Varoga D, Drescher W, Pufe M, et al. Differential expression of vascular endothelial growth factor in glucocorticoid-related osteonecrosis of the femoral head. Clin Orthop Relat Res. 2009;467:3273–82. doi: 10.1007/s11999-009-1076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W, Li K. Incidence of genetic polymorphisms involved in lipid metabolism among Chinese patients with osteonecrosis of the femoral head. Acta Orthop. 2009;80:325–29. doi: 10.3109/17453670903025378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuribayashi M, Fujioka M, Takahashi KA, et al. Combination analysis of three polymorphisms for predicting the risk for steroid-induced osteonecrosis of the femoral head. J Orthop Sci. 2008;13:297–303. doi: 10.1007/s00776-008-1244-4. [DOI] [PubMed] [Google Scholar]
- 16.Hung CC, Chiou MH, Teng YN, et al. Functional impact of ABCB1 variants on interactions between P-glycoprotein and methadone. PLoS One. 2013;8:e59419. doi: 10.1371/journal.pone.0059419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asano T, Takahashi KA, Fujioka M, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2013;13:675–82. doi: 10.1097/00008571-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Yang XY, Xu DH. MDR1(ABCB1) gene polymorphisms associated with steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus. Pharmazie. 2007;62:930–32. [PubMed] [Google Scholar]
- 19.Zhang Y, Kong X, Wang R, et al. Genetic association of the P-glycoprotein gene ABCB1 polymorphisms with the risk for steroid-induced osteonecrosis of the femoral head in Chinese population. Mol Biol Rep. 2014;41:3135–46. doi: 10.1007/s11033-014-3173-y. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y, Zhao ZQ, Hong D, et al. MDR1 gene polymorphisms are associated with glucocorticoid-induced avascular necrosis of the femoral head in a Chinese population. Genet Test Mol Biomarkers. 2014;18:196–201. doi: 10.1089/gtmb.2013.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan HB, Wu L, Wu QJ, et al. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PLoS One. 2014;9:e92738. doi: 10.1371/journal.pone.0092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L, Jiang Z, Li C, Shu M. Prediction of heart rate variability on cardiac sudden death in heart failure patients: A systematic review. Int J Cardiol. 2014;174:857–60. doi: 10.1016/j.ijcard.2014.04.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jovelet C, Deroussent A, Broutin S, et al. Influence of the multidrug transporter P-glycoprotein on the intracellular pharmacokinetics of vandetanib. Eur J Drug Metab Pharmacokinet. 2013;38:149–57. doi: 10.1007/s13318-013-0123-3. [DOI] [PubMed] [Google Scholar]
- 24.Gong LL, Fang LH, Wang HY, et al. Genetic risk factors for glucocorticoid-induced osteonecrosis: a meta-analysis. Steroids. 2013;78:401–8. doi: 10.1016/j.steroids.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe M, Ieiri I, Nagata N, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43. [PubMed] [Google Scholar]
- 26.Ifergan I, Bernard NF, Bruneau J, et al. Allele frequency of three functionally active polymorphisms of the MDR-1 gene in high-risk HIV-negative and HIV-positive Caucasians. AIDS. 2002;16:2340–42. doi: 10.1097/00002030-200211220-00017. [DOI] [PubMed] [Google Scholar]
- 27.Sauer G, Kafka A, Grundmann R, et al. Basal expression of the multidrug resistance gene 1 (MDR-1) is associated with the TT genotype at the polymorphic site C3435T in mammary and ovarian carcinoma cell lines. Cancer Lett. 2002;185:79–85. doi: 10.1016/s0304-3835(02)00232-x. [DOI] [PubMed] [Google Scholar]
- 28.Siegsmund M, Brinkmann U, Schaffeler E, et al. Association of the P-glycoprotein transporter MDR1(C3435T) polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol. 2002;13:1847–54. doi: 10.1097/01.asn.0000019412.87412.bc. [DOI] [PubMed] [Google Scholar]
- 29.Fujii T, Ota M, Hori H, et al. Association between the functional polymorphism (C3435T) of the gene encoding P-glycoprotein (ABCB1) and major depressive disorder in the Japanese population. J Psychiatr Res. 2012;46:555–59. doi: 10.1016/j.jpsychires.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Sheng X, Zhang L, Tong N, et al. MDR1 C3435T polymorphism and cancer risk: a meta-analysis based on 39 case-control studies. Mol Biol Rep. 2012;39:7237–49. doi: 10.1007/s11033-012-1554-7. [DOI] [PubMed] [Google Scholar]