Abstract
Background
Although many epidemiology studies have investigated the methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and their associations with lung cancer (LC), definite conclusions cannot be drawn. To clarify the effects of MTHFR polymorphisms on the risk of LC, we performed a meta-analysis in Chinese populations.
Material/Methods
Related studies were identified from PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) until 16 February 2014. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the strength of the associations.
Results
A total of 11 studies with 2487 LC cases and 3228 controls were included in this meta-analysis. Overall, no significant association was found between MTHFR C677T polymorphism and LC risk when all studies in Chinese populations were pooled into this meta-analysis. In subgroup analyses stratified by geographical location and source of controls, significantly increased risk was found in North China (T vs. C: OR=1.28, 95% CI: 1.14–1.44; TT vs. CC: OR=1.67, 95% CI: 1.33–2.10; TT + CT vs. CC, OR=1.39, 95% CI=1.15–1.69; TT vs. CC + CT: OR=1.46, 95% CI: 1.03–2.06) and in population-based studies (TT vs. CC: OR=1.37, 95% CI: 1.14–1.65; TT vs. CC + CT: OR=1.25, 95% CI: 1.07–1.45).
Conclusions
This meta-analysis provides evidence that MTHFR C677T polymorphism may contribute to LC development in North China. Studies with larger sample sizes and wider spectrum of populations are warranted to verify this finding.
MeSH Keywords: 5,10-Methylenetetrahydrofolate Reductase (FADH2); Lung Neoplasms; Meta-Analysis
Background
Lung cancer is the most commonly diagnosed cancer and is the leading cause of cancer death in males globally, with 1.6 million newly confirmed cases and 1.4 million deaths from lung cancer annually [1]. The incidence of lung cancer is increasing significantly and constantly [2]. Although tobacco smoking has been established as the most important cause of lung cancer, not all lung cancers are due to smoking, and increasing evidence for the association between genetic factors and lung cancer risk has been identified by hundreds of studies [3,4], suggesting that genetic factors may play a very important role in the development of lung cancer.
Epidemiologic studies have provided evidence that high consumption of vegetables and fruits is associated with a reduced risk of lung cancer [5–7], and dietary folate may be one of the micronutrients that provide protection against lung carcinogenesis [8]. Biological functions of folate in so-called ‘one-carbon metabolism’ are to facilitate de-novo deoxynucleoside triphosphate synthesis and to provide methyl groups required for intracellular methylation reactions. Methylenetetrahydrofolate reductase (MTHFR) is a central regulatory enzyme in folate metabolism that catalyses the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the predominant circulating form of folate. Hence, MTHFR acts as a critical juncture in folate metabolism by directing folate metabolites toward the DNA methylation pathway and away from the DNA synthesis pathway. Two common functional polymorphisms of the MTHFR gene, C677T and A1298C, have been identified, and the variant genotypes are associated with low plasma folate levels and significantly diminish the MTHFR activity of individuals [9–11]. Therefore, polymorphisms in the MTHFR gene may contribute to genetic susceptibility to lung and other cancers [12].
A series of studies have investigated the association between the MTHFR gene polymorphisms and lung cancer susceptibility, but provided controversial or inconclusive results. To lessen the impact of different genetic background, we performed this meta-analysis to assess the relationship of MTHFR gene polymorphisms with risk of lung cancer in Chinese populations.
Material and Methods
Materials
We searched databases containing PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) up to 16 February 2014, using the following MeSH terms: (“Lung Neoplasms “ [MeSH] or “lung cancer” or “lung tumor” or “lung carcinoma” or “carcinoma of lung”) and (“MTHFR” or “methylenetetrahydrofolate reductase”). We limited the languages to English and Chinese. References from retrieved articles were also searched.
Inclusion/exclusion criteria
Studies included in this meta-analysis had to meet the following criteria: (1) case-control study or cohort study on associations between 2 functional polymorphisms (C677T and A1298C) in MTHFR gene and lung cancer susceptibility; (2) all patients with the diagnosis of lung cancer confirmed by pathological or histological examination; (3) sufficient published data about sample size, ORs, and their 95% CIs; (4) published in English or Chinese language; (5) the distribution of the genotypes in control groups was in the Hardy–Weinberg equilibrium; (6) all participants were Chinese. Studies were excluded when they were: (1) not case–control study or cohort study; (2) duplicate of a previous publication; (3) based on incomplete data; (4) meta-analyses, letters, reviews, or editorial articles.
Data extraction
Data were independently extracted by 2 reviewers using a standardized data extraction form. Discrepancies were resolved by discussion and if consensus was not achieved, the decision was made by all the reviewers. The title and abstract of all potentially relevant articles were screened to determine their relevance. Full articles were also scrutinized if the title and abstract were ambiguous. The following information was collected from each study: authors, journal and year of publication, study design, sample size, geographical location, ethnicity of subjects, source of controls, numbers of cases and controls, and genotype frequencies of MTHFR C677T and A1298C.
Statistical analysis
Statistical analysis was conducted by using STATA statistical package (version 10, STATA, College Station, TX). The distributions of genotypes in controls were tested by Hardy-Weinberg equilibrium using the chi-square test. The association of polymorphisms of MTHFR and lung cancer risk was estimated by ORs with 95% CIs. The heterogeneity was tested by the Q-statistics with P-values <0.1, and its possible sources of heterogeneity were assessed by subgroup analysis. Dependent on the results of heterogeneity test among individual studies, the fixed-effects model (Mantel–Haenszel) or random effects model (DerSimonian and Laird) was selected to summarize the combined ORs and their 95% CIs. The significance of the pooled ORs was determined by the z test. Publication bias was investigated with the funnel plot, in which the standard error (SE) of log OR of each study was plotted against its OR. Funnel-plot asymmetry was further assessed by Egger’s linear regression test. All the P values were 2-sided. P value less than 0.05 was considered statistically significant.
Results
Eligible studies
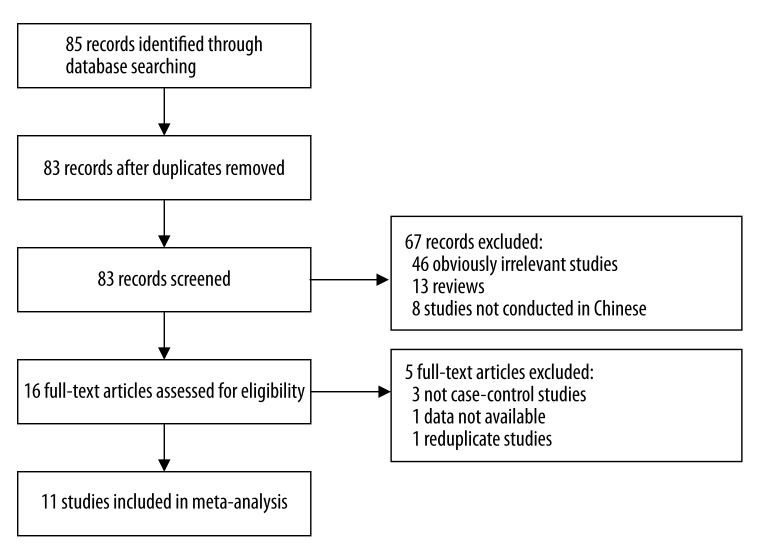
According to the inclusion criteria, 11 case-control studies [13–23] were included and 74 articles were excluded. The publication year of involved studies ranged from 2003 to 2012. The flow chart of study selection is shown in Figure 1. In total, 2487 lung cancer cases and 3228 healthy controls were involved in this meta-analysis, which evaluated the relationship between MTHFR polymorphism and lung cancer risk. The source of controls was mainly based on a healthy population. Ten of these studies were conducted for MTHFR C677T polymorphisms and 5 studies for MTHFR A1298C polymorphisms. The characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow diagram of the literature search.
Table 1.
Characteristics of studies included in the meta-analysis.
| Authors | year | Source of controls | Area | Ethnicity | Genotype | C677T | A1298C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||||||||||
| CC | CT | TT | CC | CT | TT | AA | AC | CC | AA | AC | CC | ||||||
| Jeng et al. | 2003 | PB | Taiwan | Mixed** | C677T | 36 | 22 | 1 | 123 | 95 | 14 | ||||||
| Zhang et al. | 2005 | PB | Beijing | Not stated | C677T, A1298C | 120 | 230 | 155 | 160 | 231 | 109 | 355 | 141 | 9 | 345 | 150 | 5 |
| Shen et al. | 2005 | PB | Yunnan | Not stated | C677T, A1298C | 33 | 65 | 18 | 53 | 42 | 16 | 71 | 41 | 2 | 69 | 34 | 6 |
| Jin et al. * | 2007 | PB | Guangzhou | Not stated | C677T, A1298C | 70 | 28 | 2 | 68 | 30 | 2 | ||||||
| Liu et al. | 2008 | HB | Nanjing | Han | C677T, A1298C | 157 | 245 | 98 | 149 | 265 | 103 | 341 | 141 | 18 | 364 | 142 | 11 |
| Liu et al. | 2009 | PB | Taiwan | Not stated | C677T, A1298C | 205 | 124 | 29 | 362 | 291 | 63 | 228 | 115 | 15 | 467 | 226 | 23 |
| Yao et al. | 2010 | PB | Hubei | Not stated | C677T | 27 | 46 | 20 | 36 | 51 | 19 | ||||||
| Yang et al. | 2010 | PB | Jiangxi | Han | C677T | 49 | 52 | 19 | 62 | 75 | 28 | ||||||
| Cui et al. | 2011 | PB | Shandong | Not stated | C677T | 58 | 240 | 140 | 121 | 325 | 195 | ||||||
| Cheng et al. | 2011 | PB | Henan | Han | C677T | 49 | 58 | 71 | 47 | 88 | 45 | ||||||
| Ma et al. | 2012 | HB | Yunnan | Han | C677T | 20 | 54 | 46 | 22 | 28 | 10 | ||||||
PB – population-based; HB – hospital-based.
Study excluded from the meta-analysis of MTHFR C677T; not in Hardy-Weinberg equilibrium;
Including Fukien Taiwanese, Hakka Taiwanese and Mainland Chinese.
Quantitative synthesis
The main results of this meta-analysis and the heterogeneity test were shown in Tables 2 and 3. With respect to C677T polymorphism, no significantly elevated lung cancer risk was found in overall analyses (Table 2). In the subgroup analysis by geographical locations, significantly increased risk was found in the population from North China (T vs. C: OR=1.28, 95% CI: 1.14–1.44; TT vs. CC: OR=1.67, 95% CI: 1.33–2.10; TT + CT vs. CC, OR=1.39, 95% CI=1.15–1.69; TT vs. CC + CT: OR=1.46, 95% CI: 1.03–2.06), was not found in the South. In the subgroup analysis by ethnicity, significantly increased risk was not found in Han populations. In the subgroup analysis by source of controls, significant association was found in population-based studies (TT vs. CC: OR=1.37, 95% CI: 1.14–1.65; TT vs. CC + CT: OR=1.25, 95% CI: 1.07–1.45).
Table 2.
Summary ORs and 95% CI of MTHFR C677T polymorphism and lung cancer risk.
| Analysis model | Ethnicity | OR | 95% CI (P value) | Pa |
|---|---|---|---|---|
| T vs. C | Overall | 1.15b | 0.97–1.37 (0.109) | 0.000 |
| Han | 1.20b | 0.65–2.22 (0.566) | 0.000 | |
| Not stated | 1.18b | 0.95–1.47 (0.143) | 0.002 | |
| TT vs. CC | Overall | 1.35b | 0.99–1.83 (0.058) | 0.002 |
| Han | 1.43b | 0.77–2.67 (0.259) | 0.004 | |
| Not stated | 1.42 | 1.02–1.97 (0.036) | 0.076 | |
| TT + CT vs. CC | Overall | 1.18b | 0.91–1.53 (0.205) | 0.000 |
| Han | 1.10b | 073–1.65 (0.648) | 0.021 | |
| Not stated | 1.35b | 0.92–1.98 (0.128) | 0.000 | |
| TT vs. CC + CT | Overall | 1.25b | 0.99–1.57 (0.060) | 0.014 |
| Han | 1.47b | 0.87–2.47 (0.148) | 0.005 | |
| Not stated | 1.22 | 1.03–1.44 (0.021) | 0.212 | |
| Source of controls | ||||
| Population-based | ||||
| T vs. C | – | 1.12b | 0.93–1.33 (0.225) | 0.001 |
| TT vs. CC | – | 1.37 | 1.14–1.65 (0.001) | 0.063 |
| TT + CT vs. CC | – | 1.14b | 0.86–1.52 (0.349) | 0.000 |
| TT vs. CC + CT | – | 1.25 | 1.07–1.45 (0.004) | 0.070 |
| Hospital-based | ||||
| T vs. C | – | 1.45b | 0.60–3.50 (0.414) | 0.000 |
| TT vs. CC | – | 0.49b | 0.09–2.66 (0.412) | 0.001 |
| TT + CT vs. CC | – | 1.53b | 0.48–4.86 (0.475) | 0.002 |
| TT vs. CC + CT | – | 1.65b | 0.53–5.09 (0.384) | 0.006 |
| Subgroup by area | ||||
| South China* | ||||
| T vs. C | – | 1.09b | 0.86–1.39 (0.481) | 0.000 |
| TT vs. CC | – | 1.20b | 0.77–1.89 (0.422) | 0.007 |
| TT + CT vs. CC | – | 1.13b | 0.81–1.57 (0.487) | 0.000 |
| TT vs. CC + CT | – | 1.06 | 0.87–1.30 (0.552) | 0.115 |
| North China** | ||||
| T vs. C | – | 1.28 | 1.14–1.44 (0.000) | 0.293 |
| TT vs. CC | – | 1.67 | 1.33–2.10 (0.000) | 0.615 |
| TT + CT vs. CC | – | 1.39 | 1.15–1.69 (0.001) | 0.178 |
| TT vs. CC + CT | – | 1.46b | 1.03–2.06 (0.034) | 0.031 |
P value for heterogeneity;
Estimates for random effects model.
South China including Taiwan, Yunnan, Guangzhou, Nanjing, Hubei and Jiangxi;
North China including Beijing, Shandong and Henan.
Table 3.
Summary ORs and 95% CI of MTHFR A1298C polymorphism and lung cancer risk.
| Analysis model | Ethnicity | OR | 95% CI (P value) | Pa |
|---|---|---|---|---|
| C vs. A | Overall | 1.05 | 0.92–1.19 (0.461) | 0.845 |
| Not stated | 1.01 | 0.87–1.17 (0.868) | 0.878 | |
| CC vs. AA | Overall | 1.33 | 0.87–2.02 (0.187) | 0.449 |
| Not stated | 1.17 | 0.70–1.95 (0.547) | 0.381 | |
| CC + AC vs. AA | Overall | 1.03 | 0.89–1.19 (0.682) | 0.913 |
| Not stated | 1.00 | 0.84–1.19 (0.998) | 0.904 | |
| CC vs. AA + AC | Overall | 1.31 | 0.86–1.99 (0.202) | 0.412 |
| Not stated | 1.16 | 0.70–1.92 (0.565) | 0.340 | |
| Source of controls | ||||
| Population-based | ||||
| C vs. A | – | 1.01 | 087–1.17 (0.868) | 0.878 |
| CC vs. AA | – | 1.17 | 0.70–1.95 (0.547) | 0.381 |
| CC + AC vs. AA | – | 1.07 | 0.90–1.27 (0.442) | 0.949 |
| CC vs. AA + AC | – | 1.16 | 0.70–1.92 (0.565) | 0.340 |
| Subgroup by area | ||||
| South China* | ||||
| C vs. A | – | 1.07 | 0.93–1.24 (0.336) | 0.805 |
| CC vs. AA | – | 1.26 | 0.80–2.00 (0.317) | 0.328 |
| CC + AC vs. AA | – | 1.07 | 0.90–1.27 (0.442) | 0.949 |
| CC vs. AA + AC | – | 1.24 | 0.79–1.95 (0.352) | 0.304 |
P value for heterogeneity.
South China including Taiwan, Yunnan, Guangzhou, Nanjing, Hubei and Jiangxi.
With respect to A1298C polymorphism, no significant association with lung cancer risk was demonstrated among overall analyses and subgroup analyses by ethnicity, geographical locations, and source of controls.
Sensitive analysis and bias diagnosis
To compare the difference and evaluate the sensitivity of the meta-analyses, we used both models (the fixed effects model and random effects model) to evaluate the stability of the meta-analysis. None of the results were materially altered (data not shown). Hence, results of the sensitivity analysis suggest that the data in this meta-analysis are relatively stable and credible.
The Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the literature. The shape of the funnel plots did not reveal obvious asymmetry (Figures not shown). Then, the Egger’s test was used to provide statistical evidence of funnel plot symmetry. The Egger’s test indicated that there was no obvious publication bias for MTHFR C677T in North China (T vs. C, t=0.12, p=0.925; TT vs. CC, t=−0.77, p=0.583; TT + CT vs. CC, t=−2.14, p=0.278; TT vs. CC + CT, t=1.21, p=0.441).
Discussion
Although many studies analyzing the research results about the MTHFR polymorphisms and their associations with lung cancer, definite conclusions cannot be drawn. Therefore, we did this updated meta-analysis to estimate the relationships between MTHFR polymorphisms and susceptibility to lung cancer among Chinese populations only, in order to lessen the impact of different genetic background. The meta-analysis involved 11 articles, of which 10 related to C677T polymorphism and 5 related to A1298C polymorphism. The results of this meta-analysis show that the variant genotypes of the MTHFRC677T polymorphisms were significantly associated with lung cancer risk in North China.
To the best of our knowledge, there are 6 published meta-analyses of MTHFR polymorphisms and lung cancer risk. Of these, 4 meta-analyses reported that there was no association between MTHFR polymorphisms (C677T and A1298C) and lung cancer risk [24–27]. Two found that MTHFR 677TT variant genotype was associated with an increased lung cancer risk [28,29], especially in Asians [28]. Furthermore, in the meta-analysis by et al. [29], stratified analysis by ethnicity indicated that there was no significant association observed in any genetic model of the MTHFR C677T polymorphism among Chinese populations, which is consistent with our results in overall analysis.
When we performed the subgroup analyses by ethnicity, source of controls, and geographical locations, significant association with susceptibility for the development of lung was found in North China and population-based studies, and this may be explained by several factors. First, the relationship between genes and lung cancer might vary by ethnicity. In addition, gene-environmental interaction might play an important role in susceptibility to lung cancer. Most importantly, according to the previous studies, there exist seasonal and sex differences in folate status among Chinese people [30,31]. People living in North China have a higher prevalence folate concentration deficiency [30]. Therefore, these results support the hypothesis that concomitant inadequate folate intake and impaired MTHFR activity might be important susceptibility factors for lung cancer.
Our meta-analysis has several strengths. First, we strictly followed the inclusion and exclusion criteria to reduce possible selection bias. Second, a funnel plot and Egger’s linear regression test were used to assess publication bias. Third, our inclusion of non-English-language reports was important in minimizing a major potential threat to the validity of any meta-analysis-publication bias and the related threat of a language bias. Fourth, the sensitivity analysis was performed to confirm the reliability and stability of this meta-analysis. Most importantly, the impact of different genetic backgrounds was minimized by including the studies performed in Chinese populations only, and the test of Hardy-Weinberg equilibrium for distribution of the genotypes in control groups suggested that there was no significantly different genetic background among the subjects. Therefore, the 11 studies would appear to be comparable in all respects relevant to our meta-analysis.
The limitations of this meta-analysis should be acknowledged. The key limitation of this study is the lack of an assessment of folate status. However, considering stratification by geographical locations, the folate status may have limited influence on the association between MTHFR polymorphisms and lung cancer susceptibility. Another potential limitation was that our results were based on unadjusted estimates. More precise analyses can be conducted if individual data were available, which would allow for the adjustment by other covariates, including age, sex, location, race, and other factors. Thirdly, the conclusions drawn from subgroup analyses might be limited because of the small sample size.
Conclusions
Our meta-analysis results support that MTHFR C677T polymorphism might contribute to individual susceptibility to lung cancer in North China. Concerning lung cancer with multifactorial etiology, to further evaluate gene–gene and gene–environment interactions on MTHFR polymorphisms and lung cancer, larger studies in selected populations with different environmental background or other risk factors are required. Such studies taking these factors into account may eventually lead to a better and more comprehensive understanding of the association between the MTHFR polymorphism and lung cancer risk.
Footnotes
Conflicts of interest
None.
Source of support: Self financing
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wang JJ, Zheng Y, Sun L, et al. CYP1A1 Ile462Val polymorphism and susceptibility to lung cancer: a meta-analysis based on 32 studies. Eur J Cancer Prev. 2011;20:445–52. doi: 10.1097/CEJ.0b013e328345f937. [DOI] [PubMed] [Google Scholar]
- 3.Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408. doi: 10.1016/S1470-2045(10)70126-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Wu J, Wu C, et al. A functional polymorphism (−1607 1G→2G) in the matrix metalloproteinase-1 promoter is associated with development and progression of lung cancer. Cancer. 2011;117:5172–81. doi: 10.1002/cncr.26154. [DOI] [PubMed] [Google Scholar]
- 5.Darby S, Whitley E, Doll R, et al. Diet, smoking and lung cancer: a case-control study of 1000 cases and 1500 controls in South-West England. Br J Cancer. 2001;84:728–35. doi: 10.1054/bjoc.2000.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldbohm RA, Voorrips LE. Epidemiology of nutrition and lung cancer. Nestle Nutr Workshop Ser Clin Perform Programme. 2000;4:23–37. doi: 10.1159/000061832. [DOI] [PubMed] [Google Scholar]
- 7.Mannisto S, Smith-Warner SA, Spiegelman D, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev. 2004;13:40–48. doi: 10.1158/1055-9965.epi-038-3. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, Wei Q, Pillow PC, et al. Dietary folate intake and lung cancer risk in former smokers: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2003;12:980–86. [PubMed] [Google Scholar]
- 9.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg I, Tran P, Christensen B, et al. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 11.Friedman G, Goldschmidt N, Friedlander Y, et al. A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr. 1999;129:1656–61. doi: 10.1093/jn/129.9.1656. [DOI] [PubMed] [Google Scholar]
- 12.Kim YI. Methylenetetrahydrofolate reductase polymorphisms,folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000;58:205–9. doi: 10.1111/j.1753-4887.2000.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeng YL, Wu MH, Huang HB, et al. The methylenetetrahydrofolate reductase 677C?T polymorphism and lung cancer risk in a Chinese population. Anticancer Res. 2003;23:5149–52. [PubMed] [Google Scholar]
- 14.Cui LH, Yu Z, Zhang TT, et al. Influence of polymorphisms in MTHFR 677 C→T, TYMS 3R→2R and MTR 2756 A→G on NSCLC risk and response to platinum-based chemotherapy in advanced NSCLC. Pharmacogenomics. 2011;12:797–808. doi: 10.2217/pgs.11.27. [DOI] [PubMed] [Google Scholar]
- 15.Liu CS, Tsai CW, Hsia TEC, et al. Interaction of methylenetetrahydrofolate reductase genotype and smoking habit in Taiwanese lung cancer patients. Cancer Genomics Proteomics. 2009;6:325–29. [PubMed] [Google Scholar]
- 16.Liu H, Jin G, Wang H, et al. Association of polymorphisms in one-carbon metabolizing genes and lung cancer risk: a case-control study in Chinese population. Lung Cancer. 2008;61:21–29. doi: 10.1016/j.lungcan.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Jin C, Zhang YH, Peng MF, et al. Study of the relationship between C677T, A1298C gene polymorphisms of methylenetetrahydrofolate reductase and lung cancer. Chin Clin Oncol (in Chinese) 2007;12:671–75. [Google Scholar]
- 18.Zhang XM, Miao XP, Tan W, et al. Association between genetic polymorphisms in methylenetetrahydrofolate reductase and risk of lung cancer. Acta Acad Med Sin (in Chinese) 2005;27:700–3. [PubMed] [Google Scholar]
- 19.Shen M, Rothman N, Berndt SI, et al. Polymorphisms in folate metabolic genes and lung cancer risk in Xuan Wei, China. Lung Cancer. 2005;49:299–309. doi: 10.1016/j.lungcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Yao QF, Chen X, Xue JR, et al. Relationship of polymorphisms of MTHFR gene and hypermethylation of tumor suppressor gene in lung cancers. Cancer Prev Treat (in Chinese) 2010;37:531–34. [Google Scholar]
- 21.Yang XX, Li FX, Yi JP, et al. Association between C677T genetic polymorphisms of methylenetetrahydrofolate reductase and risk of gastric cancer, colorectal cancer and lung cancer. Guangdong Med J (in Chinese) 2010;31:2375–78. [Google Scholar]
- 22.Cheng Z, Wang W, Song YN, et al. Association between C677T genetic polymorphisms of methylenetetrahydrofolate reductase and risk of lung cancer. Chin J Tuberc Respir Dis (in Chinese) 2011;34:57–58. [Google Scholar]
- 23.Ma QL, Li YF, Ji M, et al. Study of the association between C677T gene polymorphisms of methylenetetrahydrofolate reductase and susceptibility to lung cancer. Chin J Clinicians (in Chinese) 2012;6:213–15. [Google Scholar]
- 24.Mao R, Fan Y, Jin Y, et al. Methylenetetrahydrofolate reductase gene polymorphisms and lung cancer: a meta-analysis. J Hum Genet. 2008;53:340–48. doi: 10.1007/s10038-008-0262-6. [DOI] [PubMed] [Google Scholar]
- 25.Boccia S, Boffetta P, Brennan P, et al. Meta-analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and risk of head and neck and lung cancer. Cancer Lett. 2009;273:55–61. doi: 10.1016/j.canlet.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Chen GQ, Ji Y, et al. Quantitative assessment of the effect of MTHFR polymorphisms on the risk of lung carcinoma. Mol Biol Rep. 2012;39:6203–11. doi: 10.1007/s11033-011-1439-1. [DOI] [PubMed] [Google Scholar]
- 27.Hou XH, Huang YM, Mi YY. Methylenetetrahydrofolate reductase gene C677T polymorphism and lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev. 2012;13:2025–29. doi: 10.7314/apjcp.2012.13.5.2025. [DOI] [PubMed] [Google Scholar]
- 28.Liu ZB, Wang LP, Shu J, et al. Methylenetetrahydrofolate reductase 677TT genotype might be associated with an increased lung cancer risk in Asians. Gene. 2013;515:214–19. doi: 10.1016/j.gene.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Zhu N, Gong Y, He J, et al. Influence of methylenetetrahydrofolate reductase C677T polymorphism on the risk of lung cancer and the clinical response to platinum-based chemotherapy for advanced non-small cell lung cancer: an updated meta-analysis. Yonsei Med J. 2013;54:1384–93. doi: 10.3349/ymj.2013.54.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, Ma J, Stampfer MJ, et al. Geographical, seasonal and gender differences in folate status among Chinese adults. J Nutr. 2003;133:3630–35. doi: 10.1093/jn/133.11.3630. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Yao M, McCrory MA, et al. Dietary pattern is associated with homocysteine and B vitamin status in an urban Chinese population. J Nutr. 2003;133:3636–42. doi: 10.1093/jn/133.11.3636. [DOI] [PubMed] [Google Scholar]