Abstract
Levodopa is effective for the motor symptoms of Parkinson's disease (PD), but is associated with motor fluctuations and dyskinesia. Many patients require add-on therapy to improve motor fluctuations without exacerbating dyskinesia. The objective of this Phase III, multicenter, double-blind, placebo-controlled, parallel-group study was to evaluate the efficacy and safety of safinamide, an α-aminoamide with dopaminergic and nondopaminergic mechanisms, as add-on to l-dopa in the treatment of patients with PD and motor fluctuations. Patients were randomized to oral safinamide 100 mg/day (n = 224), 50 mg/day (n = 223), or placebo (n = 222) for 24 weeks. The primary endpoint was total on time with no or nontroublesome dyskinesia (assessed using the Hauser patient diaries). Secondary endpoints included off time, Unified Parkinson's Disease Rating Scale (UPDRS) Part III (motor) scores, and Clinical Global Impression-Change (CGI-C). At week 24, mean ± SD increases in total on time with no or nontroublesome dyskinesia were 1.36 ± 2.625 hours for safinamide 100 mg/day, 1.37 ± 2.745 hours for safinamide 50 mg/day, and 0.97 ± 2.375 hours for placebo. Least squares means differences in both safinamide groups were significantly higher versus placebo. Improvements in off time, UPDRS Part III, and CGI-C were significantly greater in both safinamide groups versus placebo. There were no significant between-group differences for incidences of treatment-emergent adverse events (TEAEs) or TEAEs leading to discontinuation. The addition of safinamide 50 mg/day or 100 mg/day to l-dopa in patients with PD and motor fluctuations significantly increased total on time with no or nontroublesome dyskinesia, decreased off time, and improved parkinsonism, indicating that safinamide improves motor symptoms and parkinsonism without worsening dyskinesia.
Keywords: MAO-B inhibitor, safinamide, dyskinesia
Although levodopa is very effective for the motor symptoms of Parkinson's disease (PD),1 its longer-term use is associated with motor fluctuations and dyskinesia.2 Patients with motor fluctuations often require add-on therapy, the aim of which is to improve motor fluctuations without exacerbating dyskinesia.3 Add-on dopaminergic agents improve motor fluctuations, but may do so at the expense of exacerbating dyskinesia.4 Also, as PD progresses, nondopaminergic pathways (eg, glutamate) become involved in the development of dyskinesia.5,6 Therefore, there is a need for new PD treatments with both dopaminergic and nondopaminergic effects.
Safinamide is a novel oral therapy in development for PD. It is an α-aminoamide that has both dopaminergic and nondopaminergic mechanisms of action, including inhibition of monoamine oxidase-B (MAO-B), sodium (Na+) channel blockade, and modulation of stimulated release of glutamate.7–10 Safinamide has been shown to reduce l-dopa-induced dyskinesias in animal models and humans.11
The aim of this study was to evaluate the efficacy and safety of safinamide, as an add-on therapy to stable l-dopa and other dopaminergic treatments in patients with PD and motor fluctuations.
Patients and Methods
Study Design
This Phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study comprised of 4 phases: a 10-day screening period; a 4-week l-dopa stabilization period; a 24-week treatment period; and an optional 1-week taper period. On completion of the 24-week treatment period patients continued on their randomized study medication in an 18-month double-blind, placebo-controlled extension study, except for those patients experiencing dose-limiting side effects, or clinically significant worsening.
Randomization was performed on a country-specific basis, using a computer-generated randomization schedule provided by the sponsor and administered via a central interactive voice-response system. Investigators, patients, and caregivers were blinded to treatment.
Patients and Treatment Setting
Investigators enrolled male and female patients aged 30 to 80 years with mid-to-late-stage PD, experiencing motor fluctuations while receiving l-dopa and other dopaminergic treatments at 52 centers in India (35), Romania (10), and Italy (7). Eligible patients had: idiopathic PD of ≥3 years' duration; Hoehn and Yahr stage I-IV during off; motor fluctuations (>1.5 hours' off time/day). Patients also had to be able to accurately maintain a diary.12
Patients with late-stage PD were excluded if they experienced severe, disabling peak-dose or biphasic dyskinesia, or unpredictable or widely swinging symptom fluctuations. Patients with evidence of dementia, major psychiatric illnesses, and/or severe and progressive medical illnesses were excluded.
Treatments
Patients were randomized to safinamide 100 mg/day, safinamide 50 mg/day, or placebo (1:1:1). Safinamide and placebo were identical in appearance. Doses were administered once-daily in the morning for 24 weeks. During screening, PD treatments were optimized and a 28-day fixed-dose period was required prior to randomization (“levodopa stabilization period”).
During the 24-week treatment period, doses of l-dopa and other PD therapies were to remain stable if possible. However, if a patient experienced deterioration in motor symptoms, the dose could be increased, or additional PD drugs (except MAO-B inhibitors) could be used as “rescue medication.” The l-dopa dose could also be decreased based upon the patient's condition or occurrence of adverse events (AEs). Patients in the safinamide 100 mg/day group could have their dose reduced to 50 mg/day if they did not tolerate the higher dose. The option to “reduce” the dose was also available in the other treatment groups, although the actual doses given were not changed.
Concomitant treatment with dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, amantadine, and/or anticholinergics (for PD) was permitted. Medications not permitted before or during the study included: MAO inhibitors, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.
Assessments
The primary efficacy variable was the change in mean daily total on time with no or nontroublesome dyskinesia (as used by Hauser et al.13) recorded in patient diaries.14 Secondary efficacy variables included: total daily off time; Unified Parkinson's Disease Rating Scale (UPDRS) Part III (motor) scores during on; Clinical Global Impression-Change (CGI-C) scores; off time following the first morning l-dopa dose; Dyskinesia Rating Scale (DRS) scores during on time;12 UPDRS Part II (activities of daily living) scores during on; Clinical Global Impression-Severity (CGI-S) scores; percentage change in l-dopa dose. Tertiary efficacy variables included the GRID Hamilton Rating Scale for Depression (GRID-HAM-D) total score and the Parkinson's Disease Questionnaire (PDQ-39) subscale scores.
Patients completed a diary at 30-minute intervals over 18 hours each day during the l-dopa stabilization period and in the 5 days preceding each study visit.13 After careful explanation and training by the investigator, the patient and/or caregiver completed the patient's diary in parallel with the investigator's assessment. Concordance between the patient's and investigator's ratings of whether the patient was currently: on with no dyskinesia, on with nontroublesome dyskinesia (dyskinesia does not interfere with function/cause meaningful discomfort), on with troublesome dyskinesia (interferes with function/causes meaningful discomfort), off, or asleep, was required prior to the start of the stabilization period. Patients' diaries were also evaluated remotely by an independent trained physician to ensure compliance with the diary completion and protocol selection criteria related to motor fluctuations. Patients unable to produce accurate diary recordings were retrained at baseline, and were dropped from the study if retraining was unsuccessful. Diary data from the 2 days preceding each study visit were used for analysis purposes, More than 20,000 diaries completed by patients during the l-dopa stabilization phase were evaluated for adequacy of diary completion. During the 6 months' treatment, out of a total of 18,682 completed diaries, only 13 from the placebo group, 9 from the safinamide 50 mg group, and 6 from the safinamide 100 mg group were considered unacceptable (ie, had >5 missing or erroneous entries). This is a reflection of the stringent requirements during screening; patients were required to demonstrate their ability to accurately maintain a diary. Safety assessments included treatment-emergent AEs (TEAEs), laboratory data, ophthalmological and dermatological examinations, electrocardiogram, and vital signs.
Statistical Analyses
It was calculated that a sample size of 568 patients completing the 24-week treatment period would achieve 87% power to detect a difference of 0.78 hours in mean daily on time, assuming an SD of 2.32 and a 2-tailed probability of type I error equal to 0.05. Treatment difference and SD estimates were derived from results of the PRESTO Study.15 Based on an estimated attrition rate of 14%, it was estimated that 660 patients should be recruited.
The intent-to-treat (ITT) population included all randomized patients and was used for all efficacy analyses. If a patient's dose of l-dopa or other PD therapy was increased by ≥20% or if rescue medication was used, data were censored at that point and week 24 evaluations were carried out before the intervention (“On Treatment” analysis). The safety population was defined as all patients who received at least one dose of study medication and had a subsequent safety assessment.
The change from baseline to week 24 for the primary efficacy variable was analyzed using a mixed linear model with baseline as a covariate. A sequence of comparisons approach was used: safinamide 100 mg/day versus placebo was tested first, and if significant, safinamide 50 mg/day was tested versus placebo. Mixed model repeated measures (MMRM) analysis was used for the primary efficacy variable. In this analysis, there are no imputations for missing data; instead, the analysis uses a likelihood-based method for analyzing incomplete data, and controls for Type 1 error rates at a nominal level. Sensitivity analyses were performed using the last observation carried forward (LOCF) analysis (in which the last available rating is used for all subsequent time-points) and an observed cases (OC) analysis (in which data are analyzed only up to the time they were recorded). All 3 analyses produced similar results.
Each secondary variable was analyzed sequentially as long as the difference between safinamide 100 mg/day and placebo was significant. Also, if the difference between safinamide 100 mg/day and placebo was significant, safinamide 50 mg/day and placebo were compared. If any of the tests were not significant (alpha = 0.05) subsequent tests were considered exploratory. For CGI-S the scores at week 24 were analyzed and for the remaining secondary endpoints, the change from baseline to week 24 was analyzed. DRS scores and changes in l-dopa dose were analyzed using Wilcoxon rank-sum test, CGI-C scores were analyzed using Cochran-Mantel-Haenszel test, and the remaining variables were analyzed using analysis of covariance with baseline values as covariates. TEAEs were compared across groups using Cochran-Mantel-Haenszel test stratified by center.
In order to correct changes in the dose of concomitant PD medications, and minor discrepancies in the date of onset and resolution of AEs, the Study 016 database was unlocked and relocked on 3 occasions without any effect on the overall safety/efficacy conclusions.
Classification of Level of Evidence
The primary research question was: does safinamide 50 mg and 100 mg as add-on to l-dopa and other dopaminergic treatments in patients with PD and motor fluctuations, improve on time (defined as on time without dyskinesia plus on time with nontroublesome dyskinesia). Based on the design and patient population, a positive result would provide Class I evidence of such an improvement.
Protocol Approval, Trial Registration, and Patient Consent
The protocol and all patient materials were approved by Independent Ethics Committees and Health Authorities in all 3 countries. All patients signed an informed consent form and the study was conducted according to the Declaration of Helsinki. The study is registered on Clinicaltrials.gov (NCT01187966).
Results
Patients
The first patient was screened on January 13, 2007 and the last patient completed on October 28, 2008. Patient disposition is summarized in Figure 1; all patients were analyzed in the treatment group to which they were assigned. Overall, 88.8% of enrolled patients completed the study. Baseline demographics and clinical characteristics were similar between groups (Table1). Overall, 80.6% of patients were from India, 15.8% were from Romania, and 3.6% were from Italy. Patient disposition by country was generally similar in the 3 groups.
fig. 1.
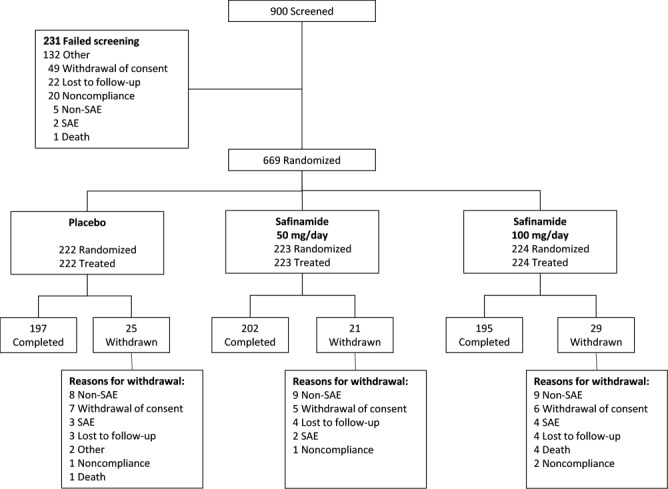
Patient disposition. None of the 5 deaths reported after randomization (1 in the placebo group and 4 in the safinamide 100 mg/day group) were considered to be related to the study drug. AE, adverse event; SAE, serious adverse event.
TABLE 1.
Baseline patient demographics and clinical characteristics
| Characteristic | Placebo (n = 222) | Safinamide 100 mg/day (n = 224) | Safinamide 50 mg/day (n = 223) |
|---|---|---|---|
| Gender, male, n (%) | 160 (72.1) | 163 (72.8) | 157 (70.4) |
| Race, n (%) | |||
| Asian | 180 (81.1) | 179 (79.9) | 180 (80.7) |
| White | 42 (18.9) | 45 (20.1) | 43 (19.3) |
| Age, years, mean (SD) | 59.4 (9.41) | 60.1 (9.19) | 60.1 (9.65) |
| H&Y stage, mean (SD) | 2.8 (0.7) | 2.8 (0.6) | 2.8 (0.6) |
| Disease duration, y, mean (SD) | 8.3 (3.8) | 8.2 (3.8) | 7.9 (4.0) |
| Mean daily total on time with no or nontroublesome dyskinesia, h, mean (SD) | 9.30 (2.155) | 9.52 (2.246) | 9.37 (2.259) |
| Off time, h, mean (SD) | 5.30 (2.06) | 5.2 (2.16) | 5.2 (2.08) |
| UPDRS-III, mean (SD) | 28.7 (12.02) | 28.3 (13.30) | 27.3 (12.66) |
| Concomitant PD medication, n (%) | |||
| Levodopaa | 222 (100) | 224 (100) | 223 (100) |
| Dopamine agonist | 137 (61.7) | 128 (57.1) | 142 (63.7) |
| Entacapone | 56 (25.2) | 55 (24.6) | 52 (23.3) |
| Anticholinergic | 87 (39.2) | 87 (38.8) | 74 (33.2) |
| Amantadine | 34 (15.3) | 30 (13.4) | 29 (13.0) |
Includes carbidopa/levodopa and entacapone tablets (Stalevo; Novartis).
H&Y, Hoehn and Yahr; on, on medication; off, off medication; UPDRS-III, Unified Parkinson's Disease Rating Scale Part III (motor) score; PD, Parkinson's disease.
Efficacy
The mean total on time with no or nontroublesome dyskinesia recorded in patient diaries increased over time in all 3 groups (Fig. 2). At week 24, there were significant differences in the least squares (LS) mean change versus placebo in both the safinamide 50 mg/day (0.51 hours; 95% CI, 0.07-0.94; P = 0.0223) and 100 mg/day (0.55 hours; 95% CI, 0.12-0.99; P = 0.0130) groups.
fig. 2.
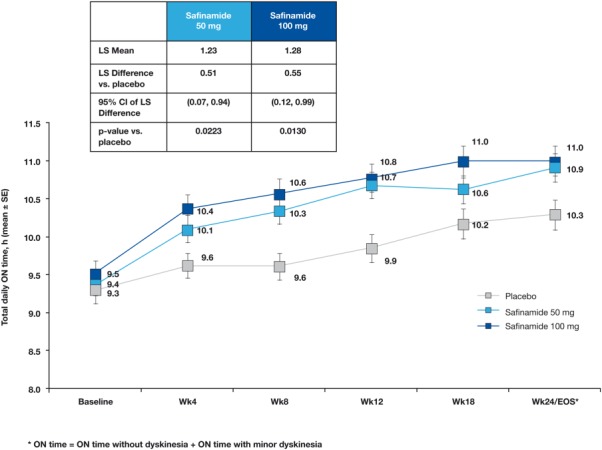
Primary endpoint. Mean change ± SE in on time with no or minor dyskinesia during the study (patient diary data). Using ANCOVA analysis (MMRM), all time points after baseline were statistically significant when compared with placebo, with the exception of safinamide 50 mg/day at week 18 (P = 0.0974). *P < 0.05 versus placebo. ANCOVA, analysis of covariance; MMRM, mixed model repeated measures; LS, least squares; CI, confidence interval; EOS, end of study; SE, standard error.
For off time, at week 24, LS mean differences versus placebo were significantly higher in both the safinamide 50 mg/day (−0.6; 95% CI, −0.9 to −0.2; P = 0.0043) and 100 mg/day (−0.6; 95% CI, −1.0 to −0.2; P = 0.0034) groups. Differences from placebo in on and off time were significant for both 50 and 100 mg/day doses from the first postbaseline evaluation (week 4) onward.
UPDRS-III (motor) scores were significantly improved in both 50 and 100 mg/day groups compared to placebo (LS mean changes: 50 mg/day: −1.8 [95% CI, −3.3 to −0.4; P = 0.0138]; and 100 mg/day: −2.6 in hours [95% CI, −4.1 to −1.1; P = 0.0006]).
There were also significant improvements in CGI-C, CGI-S, and off time following the morning l-dopa dose in both safinamide groups compared with placebo (Table2). There were no significant between-group differences in DRS scores during on time (Table2).
TABLE 2.
Changes in patient-recorded functional status, UPDRS scores, overall clinical status, PDQ-39, and depression scores at endpoint
| Placebo (n = 222) | Safinamide 100 mg/day (n = 224) | Safinamide 50 mg/day (n = 223) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | Change from baseline | Baseline | Change from baseline | P (vs placebo) | 95% CI | Baseline | Change from baseline | P (vs placebo) | 95% CI |
| Patient-recorded diary data | ||||||||||
| On time without dyskinesia, ha | 7.0 (3.33) | 0.6 (2.98) | 7.1 (3.50) | 1.1 (2.86) | 0.0103 | 0.2 to 1.1 | 7.1 (3.31) | 1.0 (3.24) | 0.0528 | −0.0 to 1.0 |
| On time with nontroublesome dyskinesia, ha | 2.3 (2.6) | 0.2 (1.92) | 2.4 (2.73) | 0.1 (2.07) | 0.4330 | −0.5 to 0.2 | 2.3 (2.52) | 0.2 (1.96) | 0.7897 | −0.4 to 0.3 |
| On time with troublesome dyskinesia, h | 1.0 (1.71) | −0.1 (1.48) | 0.7 (1.38) | 0.0 (1.78) | 0.6729 | −0.2 to 0.3 | 0.9 (1.73) | 0.0 (1.54) | 0.3259 | −0.1 to 0.4 |
| Off timeb, h | 5.30 (2.06) | −0.7 | 5.2 (2.16) | −1.3 | 0.0034 | −1.0 to −0.2 | 5.2 (2.08) | −1.3 | 0.0043 | −0.9 to −0.2 |
| Asleep, h | 2.4 (1.37) | 0.1 | 2.6 (1.38) | 0.0 | 0.6903 | −0.2 to 0.1 | 2.5 (1.25) | 0.0 | 0.6755 | −0.2 to 0.1 |
| Off time following first morning dose of levodopa, h | 4.8 (1.96) | −0.6 | 4.7 (2.07) | −1.2 | 0.0011 | −1.0 to −0.2 | 4.7 (2.0) | −1.1 | 0.0031 | −0.9 to −0.2 |
| Physician-related outcomes | ||||||||||
| UPDRS-III | 28.7 (12.02) | −4.3 | 28.3 (13.30) | −6.9 | 0.0006 | −4.1 to −1.1 | 27.3 (12.66) | −6.1 | 0.0138 | −3.3 to −0.4 |
| UPDRS-II | 12.3 (5.92) | −1.2 | 12.1 (5.82) | −2.2 | 0.0060 | −1.7 to −0.3 | 11.8 (5.66) | −1.7 | 0.1253 | −1.2 to 0.2 |
| CGI-C improvement, % patients | 55.4 | 64.3 | 0.0089 | 66.4 | 0.0010 | |||||
| CGI-S | 4.0 (0.66) | −0.2 | 4.0 (0.72) | −0.4 | 0.0448 | −0.2 to 0.0 | 4.0 (0.70) | −0.4 | 0.0060 | −0.3 to 0.0 |
| DRS score | 3.4 (3.93) | −0.2 (2.16) | 3.7 (4.07) | −0.3 (3.13) | 0.2431 | 3.9 (3.89) | −0.3 (2.52) | 0.1812 | ||
| Change in levodopa dose, % dose | −2.12 | −3.21 | 0.1092 | −1.41 | 0.1393 | |||||
| Patient-related outcomes | ||||||||||
| PDQ-39 | ||||||||||
| Total score | 230 (109.8) | −11.9 | 229 (124.1) | −28.4 | 0.0360 | −31.9 to −1.1 | 225 (110.5) | −16.4 | 0.5603 | −20.0 to 10.9 |
| Mobility | 41.8 (22.20) | −3.5 | 40.4 (25.81) | −5.5 | 0.2067 | −5.0 to 1.1 | 42.0 (23.24) | −5.9 | 0.1186 | −5.5 to 0.6 |
| Activities of daily living | 37.0 (21.85) | −1.5 | 36.5 (23.66) | −4.2 | 0.0940 | −5.7 to 0.5 | 37.0 (22.42) | −5.1 | 0.0256 | −6.6 to −0.4 |
| Emotional well-being | 30.4 (18.29) | −1.7 | 30.8 (18.86) | −5.1 | 0.0116 | −6.0 to −0.8 | 31.1 (19.70) | −2.4 | 0.6123 | −3.3 to 1.9 |
| Stigma | 31.4 (25.51) | −2.5 | 31.0 (26.16) | −2.9 | 0.8151 | −3.9 to 3.1 | 29.2 (25.66) | −3.9 | 0.4267 | −4.9 to 2.1 |
| Social support | 9.8 (16.70) | −0.2 | 11.2 (17.94) | 0.1 | 0.9684 | −2.8 to 2.7 | 9.5 (16.17) | 1.8 | 0.2498 | −1.1 to 4.3 |
| Cognition | 24.8 (17.58) | −0.5 | 23.7 (17.71) | −1.6 | 0.3775 | −3.6 to 1.3 | 22.6 (16.12) | 0.7 | 0.3081 | −1.2 to 3.7 |
| Communication | 25.9 (20.80) | −1.1 | 26.8 (22.33) | −4.4 | 0.0361 | −6.4 to −0.2 | 27.6 (20.90) | −2.6 | 0.3425 | −4.6 to 1.6 |
| Bodily discomfort | 28.8 (21.99) | 0.2 | 28.0 (21.43) | −3.5 | 0.0159 | −6.8 to −0.7 | 26.5 (20.06) | 1.3 | 0.4937 | −2.0 to 4.1 |
| GRID-HAM-D | ||||||||||
| Total score | 5.9 (3.70) | −0.3 | 6.0 (3.54) | −0.8 | 0.0731 | −1.0 to 0.0 | 6.0 (3.70) | −0.5 | 0.3922 | −0.8 to 0.3 |
Values are least square means (SD) unless otherwise stated.
The primary efficacy variable was the change in mean daily total on time with no dyskinesia plus mean daily total on time with nontroublesome dyskinesia (as used by Hauser et al.15).
Differences from placebo in off time (as for on time) were significant for both 50 and 100 mg/day doses from the first postbaseline evaluation (week 4) onward.
UPDRS, Unified Parkinson's Disease Rating Scale; PDQ-39, Parkinson's disease questionnaire; CI, confidence interval; on, on medication; off, off medication; UPDRS-III, UPDRS motor scale, UPDRS-II, UPDRS self-evaluation of the activities of daily life; CGI-C, Clinical Global Impression-Change; CGI-S, Clinical Global Impression-Severity; DRS, dyskinesia rating scale; GRID-HAM-D, GRID-Hamilton Rating Scale for Depression.
Safinamide 100 mg/day improved UPDRS-II (activities of daily living) scores compared with placebo (P = 0.006); however, this was not noted for the 50 mg/day group (Table2).
The dose of l-dopa remained stable in the majority of patients: 10% to 13% had their dose reduced during the study. There was a small percentage reduction in l-dopa dose in all groups and no statistically significant between-group differences.
There were also improvements in PDQ-39 total score (P = 0.0360) and subscale scores for emotional wellbeing (P = 0.0116), communication (P = 0.0361), and bodily discomfort (P = 0.0159) for safinamide 100 mg/day versus placebo (Table2). Changes in GRID-HAM-D scores from baseline to week 24 were numerically greater for both safinamide groups versus placebo (Table2).
Safety
Investigators chose the dose reduction option for 10 patients (4%) in the 100 mg/day group, 7 (3%) in the 50 mg/day group, and 9 (4%) in the placebo group. Completion rates were high (87%-90%), with no differences in discontinuation rates due to TEAEs.
In total, 446 (67%) patients experienced TEAEs (Table3); the majority (>90%) were rated mild or moderate. There were no significant differences among groups in the incidence of TEAEs (P = 0.5293), TEAEs related to treatment (P = 0.1395), or TEAEs leading to discontinuation (P = 0.8497) (Table3). The most common TEAEs by body system were nervous system disorders, followed by general disorders and gastrointestinal disorders. Worsening of PD and depression were reported more frequently in patients receiving placebo than patients on safinamide. Dyskinesia was reported more frequently in the safinamide groups and was generally mild or moderate in severity; only 1.8%, 0.9%, and 2.3% of patients in the 100 mg/day, 50 mg/day, and placebo groups, respectively, reported severe dyskinesia.
Table 3.
AE profile
| Placebo (n = 222) | Safinamide 100 mg/day (n = 224) | Safinamide 50 mg/day (n = 223) | |
|---|---|---|---|
| AE category | |||
| Patients with any TEAEs | 152 (68.5) | 147 (65.6) | 147 (65.9) |
| Patients with study drug-related TEAEs | 51 (23.0) | 67 (29.9) | 69 (30.9) |
| Patients with SAEs | 18 (8.1) | 22 (9.8) | 8 (3.6) |
| Patients discontinued due to AE | 12 (5.4) | 14 (6.3) | 11 (4.9) |
| Most common AEs (reported by ≥5% of patients in any group) | |||
| Dyskinesia | 28 (12.6) | 41 (18.3) | 47 (21.1) |
| Worsening PD | 18 (8.1) | 9 (4.0) | 12 (5.4) |
| Cataract | 13 (5.9) | 14 (6.3) | 11 (4.9) |
| Back pain | 13 (5.9) | 12 (5.4) | 10 (4.5) |
| Depression | 12 (5.4) | 4 (1.8) | 2 (0.9) |
| Headache | 10 (4.5) | 11 (4.9) | 13 (5.8) |
| Hypertension | 8 (3.6) | 10 (4.5) | 13 (5.8) |
Values are n (%).
AE, adverse event; TEAE, treatment-emergent adverse event; SAE, serious adverse event; PD, Parkinson's disease.
The incidence of serious TEAEs was higher in the placebo and safinamide 100 mg/day groups versus safinamide 50 mg/day (P = 0.0286) (Table3), but no specific pattern of serious TEAEs was observed. Seven deaths were reported: 2 in the placebo group and 5 in the safinamide 100 mg/day group. In the safinamide 100 mg/day group, 2 of the deaths were considered unrelated to the study drug; in 2 cases, the cause of death was unknown and 1 death occurred 49 days after discontinuation from Study 016, as a result of a posttraumatic subdural hematoma that was reported as a serious AE.
There were no significant findings for clinical laboratory tests, vital signs, or ophthalmological examination between treatment groups.
Discussion
In this study, safinamide as add-on therapy to l-dopa and other dopaminergic treatments in PD patients with motor fluctuations significantly improved on time with no/nontroublesome dyskinesia, and off time based on patient diary data. Patients consider on time with no or nontroublesome dyskinesia as “good” on time and it correlates with patients' perceived duration of a good response throughout the day.12,13 Importantly, both safinamide groups showed no increase in troublesome dyskinesia despite the significant increase in on time. Dyskinesia is the most disabling side effect of current PD medications and can have a significant impact on patients' quality of life.16 The study also showed improvements in motor function and patients' overall clinical status, activities of daily living, and some aspects of quality of life with safinamide. Importantly, the change in UPDRS-III from baseline with safinamide (Table2) represented a clinically important difference, according to criteria developed by Shulman et al.17 In general, the benefits of safinamide were more often observed with safinamide 100 mg/day, although the lower dose of 50 mg/day was significantly superior to placebo for most measures.
The MAO-B inhibitor rasagiline 1 mg/day significantly improved on time without increasing troublesome dyskinesia in the Lasting Effect in Adjunct therapy with Rasagiline Given Once Daily LARGO study,18 but in the PRESTO study, it significantly increased ON time with troublesome dyskinesia.15 The clinical data obtained with safinamide are interesting in the context of preclinical data in the methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) primate model of levodopa-induced dyskinesia, which show that safinamide potentiates l-dopa's antiparkinsonian effects while simultaneously improving dyskinesia.19 The antiparkinsonian effects at 50 mg/day may be related to MAO-B inhibition, although the superiority of the effects at 100 mg/day cannot be ascribed to this mechanism as MAO-B inhibition is virtually complete at a dose of 50 mg/day, suggesting the enhanced benefit at this dose and its antidyskinetic effects may be mostly due to nondopaminergic mechanisms.
Overall, incidences of TEAEs, drug-related TEAEs, and discontinuation due to TEAEs with safinamide were similar to placebo. Ophthalmological examination showed that safinamide was not associated with an increase in TEAEs compared with placebo, and therefore no further ocular monitoring was deemed necessary. Dyskinesia was the only drug-related TEAE reported more frequently with safinamide than placebo; the higher incidence in the 50-mg compared with the 100-mg group suggests no relationship with dose. Importantly, the data from other measures assessing dyskinesia (diaries, DRS score, UPDRS IV subitems, etc.) suggest no increase in the severity of dyskinesia with safinamide. Although the severity of dyskinesia reported as a TEAE was generally mild or moderate, the results appear to be inconsistent with those obtained for patient-recorded on time, which demonstrated that safinamide significantly improved on time with no or nontroublesome dyskinesia). This may reflect differences in the way the data were collected. On time was recorded by patients in their diaries every 30 minutes over an 18-hour period for 5 consecutive days preceding each visit, whereas AEs recorded at study visits may reflect whether a patient is experiencing any dyskinesia at that moment, or has experienced any dyskinesia at any time point from the start of the study irrespective of its impact on functioning.
Furthermore, DRS scores, recorded by clinicians at the end of the study for patients whose dose of l-dopa did not change during the 24-week treatment period, showed no worsening of dyskinesia between groups. It is not uncommon to see differences between patient-reported and physician-reported outcomes and correlations between these can be poor.20 Dyskinesia reported as an AE in this study is likely to be a class effect of dopaminergic drugs. Dyskinesia is mediated, at least in part, by changes in postsynaptic dopamine receptors secondary to nigral denervation and all dopaminergic drugs (and short-acting agents in particular) have the potential to cause dyskinesia TEAEs.21 However, safinamide may differ from other dopaminergic PD treatments in that it is has both dopaminergic and nondopaminergic mechanisms of action.
The proportion of patients taking anticholinergic medication at baseline was relatively high (37%). This reflects the high number of patients from India, where it is common clinical practice to administer anticholinergics for PD. However, as the percentage of patients taking this medication at baseline was similar between groups, we do not believe this will have affected the results. Although approximately 80% of patients were Asian, patient disposition was generally similar between countries and we have no reason to expect the clinical profile of safinamide to be different in non-Asian patients. Results from an ongoing Phase III study of safinamide as add-on to l-dopa (SETTLE), which will include a higher proportion of Caucasian patients, should confirm this.22
Our findings support previous clinical data on the efficacy and tolerability of safinamide as add-on therapy to l-dopa.23 The efficacy and tolerability of safinamide as add-on therapy to dopamine agonists has also been demonstrated in previous Phase II and Phase III studies.24–28 Additional Phase III studies will report the results on the use of safinamide as add-on to l-dopa (SETTLE) and dopamine agonist (safinaMide add-On To dopamine agonist in early Idiopathic ParkinsON's disease [MOTION]).22,29 The results of clinical studies with safinamide in early-stage PD also provide further evidence of its potential benefits in PD.30,31 In a short-term trial, safinamide 100 mg/day, when added to a stable dose of dopamine agonist in early PD, was associated with improvement from baseline to week 24 in UPDRS-III total score.30 In a recently reported long-term (18 months) placebo-controlled extension of the same study, efficacy benefits were seen to persist with safinamide 100 mg/day, resulting in a significantly lower rate of intervention compared with dopamine agonist monotherapy.31 Furthermore, the 100-mg dose significantly improved activities of daily living (UPDRS-II), motor function (UPDRS-III), and responder rate.31
The current study demonstrates the benefits of safinamide as add-on therapy to l-dopa and other dopaminergic treatments in mid-stage to late-stage PD patients with motor fluctuations. Based on patient diary data, safinamide 50 mg/day and 100 mg/day increased total daily on time with no or nontroublesome dyskinesia; ie, both doses increased good on time. Eighty-one percent of patients randomized to this study continued into an 18-month extension study, which has completed and will allow further evaluation of the long-term efficacy and safety of safinamide in this patient population.
Appendix
We thank the Study 016 Investigators: India: M. Illiyas Sahadulla, U. Kardan, B.S. Keshava, A. Kishore, S.S. Kothari, J.M. Krishna Murthy, S. Kumar, P. Kumar Pal, N. Mehta, C. Meshram, S. Prabhakar, S.Kr. Prabhakar, S. Pradhan, A.K. Roy, C. Sankhla, P.K. Sethi, A.B. Shah, N. Shankar, R. Shukla, A. Sowani, R. Srinivasa, M. Varma, D. Vasudevan, P. Vavilikolanu Sreenivas, C.U. Velmurugendran, K. Vijayan. Romania: O. Bajenaru, A. Bulboaca, A. Campeanu, D. Chirileanu, D. Muresanu, C. Panea, C. Popescu, M. Simu, J. Szasz, M. Ticmeanu. Italy: T. Avarello, U. Bonuccelli, R. Eleopra, M. Onofrj, R. Quatrale, P. Stanzione, F. Stocchi.
Acknowledgments
The Contract Research Organization was CliniRx Research Private Ltd. Native English editing and formatting was provided by Ray Hill on behalf of Health Publishing and Services Srl (HPS). This editorial assistance was funded by Newron and Merck Serono.
References
- 1.LeWitt PA. Levodopa therapeutics for Parkinson's disease: new developments. Parkinsonism Relat Disord. 2009;15(Suppl 1):S31–S34. doi: 10.1016/S1353-8020(09)70009-4. [DOI] [PubMed] [Google Scholar]
- 2.Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1–8. doi: 10.1159/000215875. [DOI] [PubMed] [Google Scholar]
- 3.Hinson VK. Parkinson's disease and motor fluctuations. Curr Treat Options Neurol. 2010;12:186–199. doi: 10.1007/s11940-010-0067-8. [DOI] [PubMed] [Google Scholar]
- 4.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. Eur J Neurol. 2006;13:1186–1202. doi: 10.1111/j.1468-1331.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 5.Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson's disease. Mol Neurobiol. 1996;12:73–94. doi: 10.1007/BF02740748. [DOI] [PubMed] [Google Scholar]
- 6.Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res. 2003;5:139–146. doi: 10.1007/BF03033378. [DOI] [PubMed] [Google Scholar]
- 7.Pevarello P, Bonsignori A, Dostert P, et al. Synthesis and anticonvulsant activity of a new class of 2-[(arylalky)amino]alkanamide derivatives. J Med Chem. 1998;41:579–590. doi: 10.1021/jm970599m. [DOI] [PubMed] [Google Scholar]
- 8.Caccia C, Maj R, Calabresi M, et al. Safinamide: from molecular targets to a new anti-Parkinson drug. Neurology. 2006;67(7 Suppl 2):S18–S23. doi: 10.1212/wnl.67.7_suppl_2.s18. [DOI] [PubMed] [Google Scholar]
- 9.Caccia C, Salvati P, Rossetti S, Anand R. Safinamide: beyond MAO-B inhibition. Parkinsonism Relat Disord. 2007;13(Suppl 2):S99. [Google Scholar]
- 10.Chazot PL. Safinamide for the treatment of Parkinson's disease, epilepsy and restless legs syndrome. Curr Opin Investig Drugs. 2007;8:570–579. [PubMed] [Google Scholar]
- 11.Grégoire L, Jourdain VA, Townsend M, Roach A, Di Paolo T. Safinamide reduces dyskinesias and prolongs l-DOPA antiparkinsonian effect in parkinsonian monkeys. Parkinsonism Relat Disord. 2013;19:508–514. doi: 10.1016/j.parkreldis.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, Stebbins GT, Shale HM, et al. Utility of an objective dyskinesia rating scale for Parkinson's disease: inter- and intrarater reliability assessment. Mov Disord. 1994;9:390–394. doi: 10.1002/mds.870090403. [DOI] [PubMed] [Google Scholar]
- 13.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23:75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hauser RA, Deckers F, Lehert P. Parkinson's disease home diary: further validation and implications for clinical trials. Mov Disord. 2004;19:1409–1413. doi: 10.1002/mds.20248. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol. 2005;62:241–248. doi: 10.1001/archneur.62.2.241. [DOI] [PubMed] [Google Scholar]
- 16.Chapuis S, Ouchchane L. Metz O, Gerbaud L. Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005;20:224–230. doi: 10.1002/mds.20279. [DOI] [PubMed] [Google Scholar]
- 17.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 18.Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double- blind, parallel-group trial. Lancet. 2005;365:947–954. doi: 10.1016/S0140-6736(05)71083-7. [DOI] [PubMed] [Google Scholar]
- 19.Gregoire L, Roach A, Di Paolo T. Safinamide reduces levodopa-induced dyskinesia in MPTP-lesioned primates while prolonging anti-parkinsonian efficacy. Mov Disord. 2010;25(Suppl 2):S411. [Google Scholar]
- 20.Arpinelli F, Bamfi F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual Life Outcomes. 2006;4:85. doi: 10.1186/1477-7525-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Encarnacion EV, Hauser RA. Levodopa-induced dyskinesias in Parkinson's disease: etiology, impact on quality of life, and treatments. Eur Neurol. 2008;60:57–66. doi: 10.1159/000131893. [DOI] [PubMed] [Google Scholar]
- 22.Schapira AH, Fox S, Hauser R, et al. SETTLE: a study design: 24-week, double- blind, placebo-controlled study of the efficacy and safety of safinamide as add-on therapy to levodopa in patients with Parkinson's Disease. Mov Disord. 2010;25(Suppl 2):S308. [Google Scholar]
- 23.Stocchi F, Vacca L, Grassini P, De Pandis MF, Battaglia G, Cattaneo C, Fariello RG. Symptom relief in Parkinson disease by safinamide: Biochemical and clinical evidence of efficacy beyond MAO-B inhibition. Neurology. 2006;67:S24–S29. doi: 10.1212/wnl.67.7_suppl_2.s24. [DOI] [PubMed] [Google Scholar]
- 24.Stocchi F, Arnold G, Onofrj M, et al. Improvement of motor function in early Parkinson disease by safinamide. Neurology. 2004;63:746–748. doi: 10.1212/01.wnl.0000134672.44217.f7. [DOI] [PubMed] [Google Scholar]
- 25.Borgohain R, Bhatt M, Rossetti S, Anand R. Safinamide potentiates the effects of dopamine (DA)-agonists in early stage Parkinson's disease patients. Parkinsonism Relat Disord. 2007;13(Suppl 2):S99. [Google Scholar]
- 26.Stocchi F, Borgohain R, Onofrj M, et al. Safinamide, a new anti-parkinson agent is effective and well-tolerated in early PD patients on a stable dose of a single DA- agonist: Results of a randomized, international, placebo-controlled Phase III trial. Neurology. 2007;68(12 Suppl 1):A109. [Google Scholar]
- 27.Anand R, Lucini V, Giuliani R, Rossetti S. Long-term double-blind, placebo-controlled evaluation of the safety and efficacy of safinamide as an add-on to stable dopamine agonist therapy in patients with Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl 2):S99–S100. [Google Scholar]
- 28.Schapira AH, Borgohain R, Stocchi F, et al. Study Investigators. Long-term safety and efficacy of safinamide added to dopamine agonists in early Parkinson's disease (PD) Neurology. 2008;70(11 Suppl 1):A424. [Google Scholar]
- 29.Barone P, Fernandez J, Ferreira JJ, et al. MOTION (safinaMide add-On To dopamine agonist in early Idiopathic ParkinsON's disease) study design: a 24 week, double-blind, placebo-controlled study of the efficacy and safety of safinamide. Mov Disord. 2010;25(Suppl 2):S289. [Google Scholar]
- 30.Stocchi F, Borgohain R, Onofrj M, et al. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson's disease patients. Mov Disord. 2012;27:106–112. doi: 10.1002/mds.23954. [DOI] [PubMed] [Google Scholar]
- 31.Schapira AH, Stocchi F, Borgohain R, et al. Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson's disease. Eur J Neurol. 2013;20:271–280. doi: 10.1111/j.1468-1331.2012.03840.x. [DOI] [PubMed] [Google Scholar]


