Abstract
What is known and objective
The pharmacokinetics (PK) and pharmacodynamics (PD) of levofloxacin were investigated following administration of levofloxacin injection in healthy Chinese volunteers for optimizing dosing regimen.
Methods
The PK study included single-dose (750 mg/150 mL) and multiple-dose (750 mg/150 mL once daily for 7 days) phases. The concentration of levofloxacin in blood and urine was determined using HPLC method. Both non-compartmental and compartmental analyses were performed to estimate PK parameters. Taking fCmax/MIC ≥5 and fAUC24 h/MIC ≥30 as a target, the cumulative fraction of response (CFR) of levofloxacin 750 mg for treatment of community-acquired pneumonia (CAP) was calculated using Monte Carlo simulation. The probability of target attainment (PTA) of levofloxacin at various minimal inhibitory concentrations (MICs) was also evaluated.
Results and discussion
The results of PK study showed that the Cmax and AUC0–∞ of levofloxacin were 14·94 μg/mL and 80·14 μg h/mL following single-dose infusion of levofloxacin. The half-life and average cumulative urine excretion ratio within 72 h post-dosing were 7·75 h and 86·95%, respectively. The mean Css,max, Css,min and AUC0–τ of levofloxacin at steady state following multiple doses were 13·31 μg/mL, 0·031 μg/mL and 103·7 μg h/mL, respectively. The accumulation coefficient was 1·22. PK/PD analysis revealed that the CFR value of levofloxacin 750-mg regimen against Streptococcus pneumoniae was 96·2% and 95·4%, respectively, in terms of fCmax/MIC and fAUC/MIC targets.
What is new and conclusion
The regimen of 750-mg levofloxacin once daily provides a satisfactory PK/PD profile against the main pathogenic bacteria of CAP, which implies promising clinical and bacteriological efficacy for patients with CAP. A large-scale clinical study is warranted to confirm these results.
Keywords: healthy volunteer, levofloxacin, Monte Carlo simulation, pharmacodynamics, pharmacokinetics
What is Known and Objective
Levofloxacin, a levoisomer of ofloxacin, belongs to fluoroquinolone antimicrobial agents with broad-spectrum antibacterial activity. It is highly active against Gram-negative aerobic bacteria including most of the Enterobacteriaceae species. Levofloxacin also provides good activity for Gram-positive aerobic bacteria. Moreover, some atypical pathogens are susceptible to levofloxacin.1 Levofloxacin is increasingly used in the treatment for community-acquired pneumonia (CAP) due to its potent bactericidal effect, higher blood drug concentration, extensive tissue distribution and higher bioavailability.2–5
The pharmacokinetic/pharmacodynamic (PK/PD) studies in recent years have proved that levofloxacin is a concentration-dependent antimicrobial agent. Its bactericidal activity increases with drug concentration over a certain range. It is proposed that the maximal bactericidal effect may be achieved by delivering fewer or single but high-level dose per day to maintain higher Cmax/MIC and AUC0–24/MIC ratios. Such a regimen may also be helpful to avoid the emergence of antibiotic resistance. For this reason, PK studies have been conducted for levofloxacin 750-mg injection (150 mL) in the United States and European countries.6,7 Intravenous administration of levofloxacin 750-mg injection (150 mL) once daily for 5 days achieved the same efficacy in severe CAP as the regimen of 500 mg once daily for 10 days. This new regimen has a shorter duration of treatment and lower exposure, less medical cost and may be associated with lower risk of developing resistance.8,9 However, no multiple-dose PK study of levofloxacin 750-mg IV infusion has been reported in Chinese subjects yet. It is necessary to clarify the PK/PD and safety profile of levofloxacin 750-mg IV infusion in Chinese volunteers for optimizing the dosing regimen.
This study was designed to explore the PK profiles of levofloxacin following single-dose and multiple-dose intravenous infusion of levofloxacin 750 mg for the first time in Chinese healthy volunteers. Meanwhile, considering the PD data of in vitro antimicrobial susceptibility testing for major CAP pathogens such as Streptococcus pneumoniae and Haemophilus influenzae,10 Monte Carlo simulation (MCS) method was used to perform PK/PD analysis. The results were compared with the PK/PD profile of levofloxacin 500-mg injection. An optimized dosing regimen, which is expected to achieve the maximal bactericidal effect in vivo, is recommended for patients with severe CAP.
Methods
Subjects
In the open-label single-dose study, nine enrolled healthy subjects received levofloxacin 750 mg/150 mL via intravenous infusion. In the randomized, double-blind, multiple-dose phase, 12 healthy subjects received levofloxacin 750 mg/150 mL or placebo (ratio of 3 : 1) via intravenous infusion over 90 min, once daily for seven consecutive days. The study protocol and informed consent were approved by the Institutional Ethics Committee. All subjects signed the written informed consent before participation in this study.
The nine male subjects in single-dose study were 23·51 ± 2·00 years of age and 65·67 ± 4·60 kg of body weight at average. Their creatinine clearance (CLcr) was 138·0 ± 13·28 mL/min before study administration. The nine male subjects receiving levofloxacin in multiple-dose study were 24·56 ± 2·41 years of age and 60·39 ± 4·61 kg of body weight at average. Their CLcr was 125·9 ± 16·5 mL/min before study initiation. The three male subjects receiving placebo were 26·24 ± 0·98 years of age and 62·00 ± 4·27 kg of body weight. Their CLcr was 116·6 ± 7·6 mL/min.
Antimicrobial agent
Levofloxacin 750-mg injection (Cravit, Lot: EXH0701, 150 mL/bottle containing levofloxacin 0·75 g and NaCl 1·35 g) was the product of Daiichi Pharmaceutical (Beijing, China). Placebo was 0·9% sodium chloride injection [Shanghai Huayuan Changfu Pharmaceutical (Group) Company, Lot: 07071104–1, 250 mL/bottle].
Single-dose study
Single dose of levofloxacin 750 mg was administered intravenously with an infusion pump at constant rate over 1·5 h. Blood sample (5 mL) was drawn before infusion, 0·75 h after infusion start and immediately, 0·25, 0·5, 1, 1·5, 2, 3, 4, 8, 12, 24, 36, 48, 60 and 72 h after the end of infusion. All urine was collected according to the following intervals: before infusion, 0–2, 2–4, 4–8, 8–12, 12–24, 24–48 and 48–72 h after the end of infusion.
Multiple-dose study
Levofloxacin 750 mg was administered intravenously to the volunteers once daily with an infusion pump at constant rate over 1·5 h for seven consecutive days. Blood sample (5 mL) was drawn before infusion, 0·75 h after infusion start and immediately, 0·25, 0·5, 1, 1·5, 2, 3, 4, 8 and 12 h after the end of infusion on Day 1, immediately before infusion (0 h) and immediately after the end of infusion from Day 2 to Day 6. The sampling time points on Day 7 were the same as that in single-dose study. All urine was collected on Day 1 and Day 7 according to the following intervals: before infusion, 0–2, 2–4, 4–8, 8–12 and 12–24 h. Urine volume was measured and recorded.
Sample processing and data collection
All the blood samples were put on ice bath and subjected to centrifugation within 30 min at 4 °C (1500 × g, 10 min) to separate plasma. The plasma was aliquoted to two tubes and stored at −40 ± 5 °C in a refrigerator for later analysis.
The volunteers were observed for any adverse event since the start of infusion, including clinical observation, vital signs (temperature, blood pressure and pulse rate), laboratory tests (haematology, urinalysis and serum biochemical tests), 12-lead electrocardiogram for safety evaluation of levofloxacin.
Levofloxacin assay
High-performance liquid chromatography (HPLC) (Waters 2695 HPLC System, Waters Company, Milford, MA, USA) was used to determine levofloxacin concentration in blood and urine samples. The solid phase was TSK-gel ODS-80TM gel column (4·6 mm × 150 mm, 5 μm). The mobile phase contained 50 mmol/L KH2PO4 (pH 2·0), THF and 1 m CH3COONH4 (92/7/1, V/V/V), respectively. The wavelength for fluorescence detection Ex/Em was 296 nm/504 nm, column temperature was 35 °C, flow rate was 1·0 mL/min, and injection volume for plasma and urine samples was 10 μL and 20 μL, respectively. The retention time was about 6·5 min for levofloxacin and 8·5 min for the internal standard (DL-8493, lot number 900131, provided by Daiichi Pharmaceutical). The range of standard curve was from 0·010 to 5·000 μg/mL for plasma and 0·100 to 100·0 μg/mL for urine. The curve linearity was 0·9989 and 0·9998, respectively. The assay method and its validation were previously described by Zhang J et al.11
Pharmacokinetic calculation
Phoenix WinNonlin 6.0 software (Pharsight Company, Sunnyvale, CA, USA) was used to calculate the non-compartmental PK parameters of levofloxacin following administration of levofloxacin. Peak concentration (Cmax) and C24 h were expressed as the observed values. AUC0–t was calculated based on the trapezoidal rule. Linear regression analysis was conducted using the terminal logarithmic concentration–time data to calculate the elimination rate constant (λ). The terminal elimination half-life (T1/2) was 0·693/λ. AUC0–∞ was calculated as AUC0–t + terminal concentration/λ. The total clearance (CLt) was calculated as the ratio of dose to AUC0–∞. Renal clearance (CLr) was the ratio of levofloxacin cumulative urinary excretion to AUC0–24 h. The apparent volume of distribution (Vd) is the ratio of CLt to λ. The mean residence time (MRT0–∞) was based on AUMC/AUC ratio.
Two-compartment model was employed to analyse the PK profile of levofloxacin in healthy volunteers using WinNonlin software. The removal of levofloxacin from central compartment to peripheral compartment and the transport between central compartment and peripheral compartment were all consistent with first-order kinetics. The elimination rate and clearance were represented by Ke and CL. The corresponding rate constant was expressed as K12 and K21, respectively. The clearance between compartments was expressed as Q. The distribution volume of central and peripheral compartments was represented by V1 and V2. The rate of distribution phase and elimination phase was represented by α and β, whereas the corresponding half-life was expressed as T1/2, α and T1/2, β. The weight of 1/C was used in the calculation.
Statistical analysis
The non-compartmental parameters of levofloxacin following multiple doses were compared between Day 1 and Day 7 to evaluate the PK behaviours over time following multiple doses, for example, the change in T1/2 and drug accumulation. The compartmental parameters of levofloxacin following single- or multiple-dose administration were compared to analyse the details of levofloxacin PK profile.
The correlation between compartmental parameters of levofloxacin and the relevant demographic covariates was also evaluated to preliminarily examine the potential factors influencing the disposition of levofloxacin in healthy adults. The data sets were examined by F-test for homogeneity of variance. The homogeneous data were compared by t-test between groups. Statistical significance was assumed at the level of P < 0·05. The above statistical analyses were completed with spss software package (Version 13.0, ibm spss Software, Armonk, NY, USA). The results were expressed as Mean ± SD.
Safety evaluation
The Criteria for Evaluation of Adverse Drug Reactions and Laboratory Abnormalities due to Antimicrobial Agents in Clinical Study issued by Japanese Society of Chemotherapy were referred to in this study to confirm the adverse events and the severity (mild, moderate and severe). The relationship between an adverse event and the study drug was judged as definitely, probably, possibly related, or possibly unrelated, unrelated or indeterminate. The events definitely, probably, possibly related to the study drug or indeterminate were classified as adverse drug reactions. In this study, the adverse events leading to study discontinuation, or requiring treatment, or resulting in QTc prolongation (≥60 msec longer or QTc interval ≥500 msec) were taken as significant adverse events.
PK/PD analysis and dosing regimen evaluation
PK/PD analysis was performed and evaluated for levofloxacin 750-mg dosing regimen based on the PK parameters of levofloxacin at steady state following dosing of 750 mg daily for seven consecutive days in this study, and the pharmacodynamic data from two clinical trials were as follows: levofloxacin tablet 500 mg in 881 patients with CAP or other lower respiratory tract infections during 2006–2007, and randomized controlled study of levofloxacin injection 750 mg vs. 500 mg in CAP during 2007–2008.10 Monte Carlo simulation was used to conduct the PK/PD analysis. The simulation was performed in 5000 patients with MATLAB software version 7.0.1 (Mathworks Company, Natick, MA, USA). It is expected to obtain a target PK/PD value indicating satisfactory clinical and bacteriological efficacy if fCmax/MIC ≥5 and/or fAUC24 h/MIC ≥30.12 The protein-binding rate of levofloxacin is 30%; hence, the fraction of free drug (f) is 0·7. The cumulative fraction of response (CFR) was calculated for 750-mg levofloxacin regimen in attaining the above target PK/PD value specific for major CAP pathogens. The susceptibility break point of levofloxacin against Streptococcus pneumoniae is ≤2 μg/mL.13 Therefore, this calculation is the pharmacodynamic probability of target attainment (PTA) in terms of fCmax/MIC ≥5 and fAUC24 h/MIC ≥30 when MIC = 0·25, 0·5, 1 or 2 μg/mL. In Monte Carlo simulation, the simulated steady-state AUC0–24 h and Cmax data were generated based on logarithmic normal distribution. The simulated MIC data were generated based on discrete distribution according to specified probability at each MIC level.
Results
Levofloxacin pharmacokinetics
The PK parameters in the nine subjects following single intravenous dose of levofloxacin 750 mg showed mean Cmax (14·94 ± 1·48) μg/mL, AUC0–72 h (79·97 ± 12·53) μg h/mL and AUC0–∞ (80·14 ± 12·55) μg h/mL. The terminal elimination half-life (t1/2) was (7·75 ± 0·59) h. Renal clearance (CLr) and CLt were (8·17 ± 1·00) L/h and (9·58 ± 1·62) L/h, respectively. The distribution volume (Vd) was (107·30 ± 20·73) L. The cumulative urinary excretion of levofloxacin was (86·95 ± 5·04)% of the administered dose 72 h post-dose (Table 1).
Table 1.
Non-compartmental and compartmental parameters of levofloxacin following intravenous infusion of levofloxacin 750 mg in healthy Chinese volunteers (n = 9, Mean ± SD)
| Non-compartmental | Compartmental | |||||
|---|---|---|---|---|---|---|
| Parameter | Single-dose PK | Multiple-dose PK | Parameter | Single-dose PK | Multiple-dose PK | |
| First Dose | Last Dose | |||||
| Cmax (μg/mL) | 14·9 ± 1·48 | 17·7 ± 4·22 | 13·3 ± 2·77c | T1/2,α (h) | 1·02 ± 0·323 | 0·305 ± 0·0473e |
| AUC0–24 h (μg·h/mL) | 73·3 ± 10·7 | 90·1 ± 11·9b | 94·1 ± 15·2b | T1/2,β (h) | 7·19 ± 0·503 | 7·22 ± 0·783 |
| AUC0–72 h (μg·h/mL) | 80·0 ± 12·5 | – | 103·7 ± 17·9 | K12 (1/h) | 0·220 ± 0·120 | 1·26 ± 0·336e |
| AUC0–∞ (μg·h/mL) | 80·1 ± 12·5 | 99·3 ± 13·7b | 104 ± 18·0b | K21 (1/h) | 0·465 ± 0·119 | 0·897 ± 0·237e |
| T1/2 (h) | 7·75 ± 0·591 | 7·52 ± 0·656 | 6·91 ± 0·806a,d | T1/2,Ke(h) | 4·62 ± 0·655 | 2·82 ± 0·854e |
| CLt (L/h) | 9·58 ± 1·62 | 8·46 ± 1·10 | 8·27 ± 1·31 | CL (L/h) | 10·2 ± 1·89 | 7·84 ± 1·10e |
| CLr (L/h) | 8·17 ± 1·28 | 6·40 ± 0·972b | 6·85 ± 1·06a | Q (L/h) | 13·8 ± 5·60 | 37·2 ± 6·05e |
| Vd (L) | 107·3 ± 20·7 | 91·3 ± 9·32 | 81·7 ± 10·5b, d | V1 (L) | 67·8 ± 13·2 | 31·6 ± 9·68e |
| MRT0–∞ (h) | 9·18 ± 0·786 | 9·72 ± 1·04 | 10·0 ± 0·97 | V2 (L) | 29·2 ± 7·61 | 43·4 ± 9·97e |
P < 0·05.
P < 0·01 vs. single-dose PK (non-compartmental parameter).
P < 0·05.
P < 0·01 vs. multiple-dose PK (first dose, non-compartmental parameter).
P < 0·01 vs. single-dose PK (compartmental parameter); the duration of levofloxacin infusion was 1·5 h.
The dosing regimen for the multiple-dose PK study was q24 h × 7 days.
In the nine subjects receiving multiple-dose intravenous infusion of levofloxacin 750 mg once daily for seven consecutive days, the Cmax was (17·69 ± 4·23) μg/mL on Day 1 and (13·31 ± 2·77) μg/mL on Day 7. The plasma concentration of levofloxacin at 24 h was (0·87 ± 0·22) μg/mL on Day 1 and (0·803 ± 0·258) μg/mL on Day 7. The corresponding AUC0–24 h was (90·05 ± 11·92) μg·h/mL and (94·1 ± 15·2) μg h/mL. The mean 24-hour cumulative urinary excretion rate was (75·69 ± 6·34)% and (84·16 ± 5·14)% of the administered dose, respectively.
When steady state was reached following consecutive dosing, the mean Css,max of levofloxacin was 13·31 μg/mL, mean Css,min 0·031 μg/mL, average plasma concentration (Cavg) (3·87 ± 0·62) μg/mL, mean AUC0–τ 94·12 μg h/mL (τ = 24 h) and accumulation factor (1·22 ± 0·14) (Table 1). Further analysis indicated that the ratio of geometric mean Cmax, C24 h, AUC0–24 h (Day 7/Day 1) and the corresponding 95% confidence interval were 0·76 (0·61–0·95), 0·91 (0·78–1·06) and 1·04 (0·97–1·11), suggesting no apparent accumulation in the body when steady state was reached following once-daily administration of levofloxacin for 7 days.
Compartmental model analysis
Two-compartment model well described the PK profile of levofloxacin in healthy volunteers following single- or multiple-dose administration (Fig. 1). The results showed that the distribution half-life after single dose of levofloxacin was (1·02 ± 0·32) h. The distribution volume of central and peripheral compartments was (67·8 ± 13·2) L and (29·2 ± 7·61) L, respectively. The clearance from central compartment was (10·2 ± 1·89) L/h and (13·8 ± 5·6) L/h between central and peripheral compartments.
Fig 1.
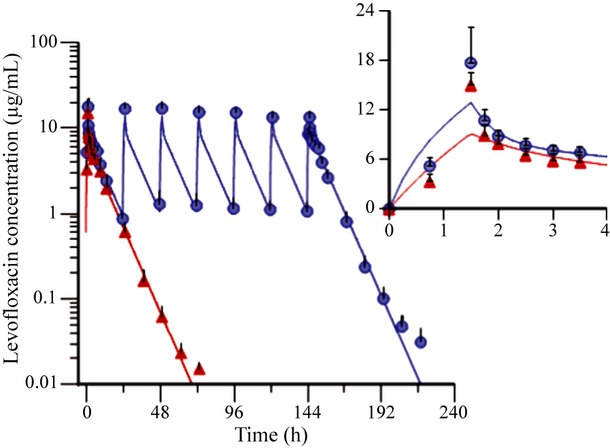
Concentration–time profiles of levofloxacin following single- or multiple-dose infusion of 750-mg levofloxacin in healthy Chinese volunteers (Mean ± SD, n = 9). The observed values and fittings obtained from two-compartment model were represented by dots and lines, whereas red and blue symbols represent single-dose PK group and multiple-dose PK group, respectively. The infusion time of single-dose levofloxacin was 1·5 h. The dosing regimen for multiple PK study was q24 h × 7 days.
Safety of levofloxacin
Six ADRs were reported in 6 of the 9 subjects receiving single dose of levofloxacin. The ADR was local adverse reaction in four subjects, that is, mild injection site hyperaemia, which started within 16–45 min since intravenous infusion, and resolved 30 min after the end of intravenous infusion. Both the increased lactate dehydrogenase in one subject and the conjugated bilirubin elevation in another subject developed 23 h after the end of intravenous infusion and normalized at Day 7 post-dose visit. All these ADRs were mild, transient and well tolerated.
Nine adverse events occurred in 8 of the 9 subjects receiving multiple doses of levofloxacin. Eight of these events in seven subjects were ADRs. Six ADRs were intermittent mild injection site hyperaemia during the consecutive 7-day intravenous infusion and resolved within 10–25 min after the end of intravenous infusion the same day. Two cases of laboratory abnormalities were transient creatine kinase elevation and increased total bilirubin. The increase was within two times the upper limit of normal in both cases. These ADRs were mild in severity and well tolerated. One of the three subjects receiving placebo developed two mild adverse events (decreased white blood cell count and increased uric acid), which were unrelated to the study drug.
Other laboratory tests, vital signs and 12-lead ECG parameters were all within the normal range in both single-dose and multiple-dose studies. No fatal or other significant adverse events were found. There was no significant adverse event leading to study discontinuation or abnormal QTc interval.
PK/PD analysis and dosing regimen evaluation
Figure 2 displays the CFR value of levofloxacin targeting 245 strains of Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, methicillin-susceptible Staphylococcus aureus (MSSA) or methicillin-resistant Staphylococcus aureus (MRSA). The CFR of levofloxacin 750-mg once-daily regimen was 96·2%, 98·5%, 95·3% and 100·0% for Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae and MSSA in terms of fCmax/MIC = 5, significantly better than that for MRSA (0·4%). The CFR was 95·4%, 98·0%, 95·2% and 100·0% in terms of fAUC24 h/MIC = 30, whereas for MRSA, the CFR was 0.
Fig 2.
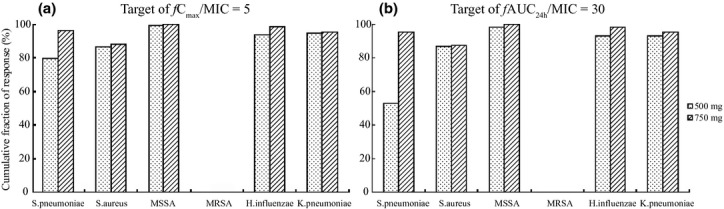
Cumulative fraction of response of fCmax/MIC (a) and fAUC24 h/MIC (b) for levofloxacin. The dosing regimen of levofloxacin was 500 mg or 750 mg (q.d.) for seven consecutive days, and f indicates the unbound fraction, the value of which was 0·7.
From Fig. 3, when MIC ≤1 μg/mL, the PTA of levofloxacin 750-mg dosing regimen was kept at 100% in terms of the target value of fCmax/MIC or fAUC24 h/MIC, suggesting that levofloxacin 750-mg dosing regimen can promise good clinical and microbiological efficacy targeting the bacteria for which levofloxacin MIC ≤1 μg/mL.
Fig 3.
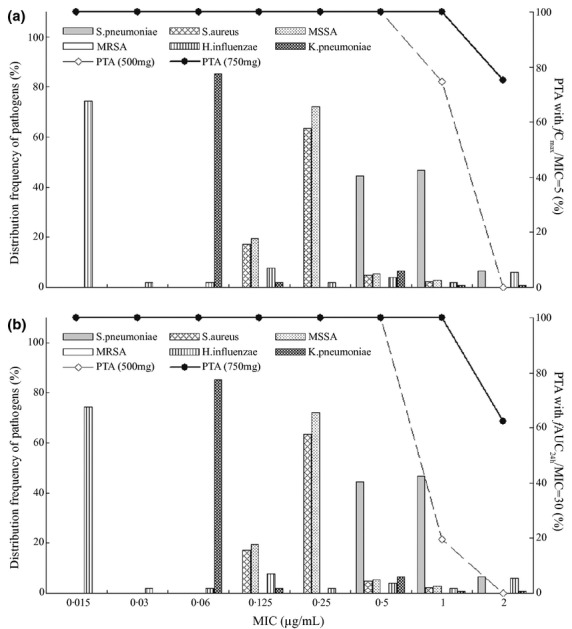
Distribution of CAP pathogens in terms of MIC level and the PTA of fCmax/MIC (a) and fAUC24 h/MIC (b) for levofloxacin following multiple dosing. The dosing regimen of levofloxacin was 500 mg or 750 mg (q.d.) for 7 consecutive days, and f indicates the unbound fraction, the value of which was 0·7. Histograms and lines represent the distribution frequency of MIC and PTA values, respectively. In the graph, the distribution data of MIC for CAP pathogens were obtained from the literature.10
Discussion
Following single- or multiple-dose intravenous infusion of levofloxacin 750 mg in healthy Chinese male volunteers, the Cmax of levofloxacin was 14·94 μg/mL and 13·31 μg/mL; AUC0–24 73·26 μg h/mL and 94·12 μg h/mL; T1/2 7·75 h and 6·91 h. These findings were comparable with the results reported by Chow et al.,6 which indicated that after single- or consecutive 10-day multiple-dose intravenous infusion of levofloxacin 750 mg, the Cmax was 11·3 mg/L and 12·4 mg/L, AUC0–24 90·9 mg h/L and 103 mg h/L, and T1/2 7·51 h.
The multiple-dose PK profile of levofloxacin showed more rapid terminal elimination and lower apparent volume of distribution compared with that after single-dose intravenous infusion (Table 1). This is reflected in the parameters of compartmental model, that is, both the clearance from central compartment and distribution volume decreased significantly (P < 0·01). Furthermore, the distribution rate of levofloxacin was significantly increased; meanwhile, the clearance between compartments and the distribution volume of peripheral compartment were also significantly higher following multiple-dose infusion. These results indicate that after levofloxacin multiple-dose infusion, the central-to-peripheral compartment distribution is faster. A fraction of levofloxacin was transported from central-to-peripheral compartment, reflecting the high tissue penetration and extensive, high-level distribution to peripheral tissues. This is helpful for levofloxacin to reach effective bactericidal concentration at the site of infection.
The distribution volume of levofloxacin is quite large, 1·64 L/kg on average, following single dose of 750 mg. This indicates that levofloxacin is distributed widely in tissues and body fluids. It is reported that levofloxacin can be extensively distributed to the skeletal muscle intercellular fluid, inflammatory blister fluid, subcutaneous soft tissue and pulmonary tissue; especially in lungs and bronchi, the concentration of levofloxacin is two times higher than that in blood. The subjects in this study were all healthy volunteers without infectious respiratory disease. Several studies reported that the levofloxacin concentration in the pulmonary tissue and alveolar fluid was higher than the corresponding concentration in blood in the patients with respiratory tract infection, and the levofloxacin concentration in the blood of the patients with infection was higher than that in healthy volunteers. Therefore, levofloxacin can reach higher concentration in the tissues and body fluids of the patients with infection compared with healthy volunteers.14–21
Correlation analysis showed that the endogenous creatinine clearance (CLcr) was well correlated with levofloxacin clearance (CL) in healthy subjects. Pearson's correlation coefficient (R) was 0·529 (P < 0·05). The body weight of volunteers was also correlated significantly with CL (R = 0·573, P < 0·01). The body weight of volunteers was positively correlated with the distribution volume of central compartment (R = 0·443). The distribution volume of peripheral compartment showed a trend of increase with age (R = 0·525, P < 0·01). This implies that age of volunteers may be an important factor influencing the distribution volume of levofloxacin in peripheral compartment. The endogenous creatinine clearance and body weight are covariates affecting the external clearance. This finding is consistent with the results of levofloxacin population PK study.11
In our previous study of levofloxacin 500 mg,22 after intravenous infusion of levofloxacin 500 mg, the mean Cmax and AUC0–∞ were (7·6 ± 1·1) μg/mL and (38·3 ± 4·9) μg h/mL, respectively. The average terminal elimination half-life (T1/2) was (6·5 ± 0·6) h. The CLr and CLt were (8·8 ± 1·4) L/h and (13·3 ± 1·7) L/h, respectively. Vd was (109·8 ± 10·8) L. At 24 h after dosing, the cumulative urinary excretion was (65 ± 4)% of the administered dose. According to the PK parameters of levofloxacin 750-mg and 500-mg studies, as well as the PD data (results of susceptibility testing of 245 strains of bacteria) from two clinical trials previously conducted in our institute,10 fCmax/MIC90 and fAUC24 h/MIC90 were calculated. The results demonstrated that except MRSA, both levofloxacin 750-mg and 500-mg dosing regimens can attain or exceed the target of fCmax/MIC ≥5 and fAUC24 h/MIC ≥30 for the common bacterial pathogens of CAP. Levofloxacin 750 mg provided higher PTA values than 500 mg (Table 2).
Table 2.
Susceptibility testing and PK/PD parameters of levofloxacin against 245 clinical isolates from community-acquired pneumonia
| Bacteria (No. of strains) | MIC90 | 500 mg | 750 mg | ||
|---|---|---|---|---|---|
| (μg/mL) | fCmax/MIC90 | fAUC24 h/MIC90 | fCmax/MIC90 | fAUC24 h/MIC90 | |
| Streptococcus pneumoniae (45) | 1 | 5·3 | 26·8 | 9·3 | 65·9 |
| Haemophilus influenzae (51) | 0·5 | 10·6 | 53·6 | 18·6 | 131·7 |
| Klebsiella pneumoniae (108) | 0·5 | 10·6 | 53·6 | 18·6 | 131·7 |
| Methicillin-susceptible Staphylococcus aureus (36) | 0·25 | 21·3 | 107·2 | 37·2 | 263·5 |
| Methicillin-resistant Staphylococcus aureus (5) | 32 | 0·2 | 0·8 | 0·3 | 2·06 |
The f means the unbound fraction of levofloxacin, the value of which was 0·7. The Cmax and AUC0–24 were the non-compartmental paramters following last dose of levofloxacin in the multiple PK study.
Monte Carlo simulation indicated that the CFR of levofloxacin 750-mg and 500-mg dosing regimens was 96·2% and 79·5% for Streptococcus pneumoniae, a common pathogen of CAP, in terms of fCmax/MIC ≥5. Similarly, the CFR of these two regimens was 95·4% and 52·9% in terms of fAUC24 h/MIC ≥30. When MIC ≤0·5 μg/mL, both dosing regimens offered best PTA (100%) in terms of fCmax/MIC ≥5 and fAUC24 h/MIC ≥30 for the common pathogens of CAP. When MIC = 1 μg/mL, the PTA for levofloxacin 750-mg dosing regimen was 99·9% vs. 73·7% for 500-mg dosing regimen in terms of fCmax/MIC ≥5, and 99·9% vs. 18·6% in terms of fAUC24 h/MIC ≥30. This suggests 750-mg dosing regimen of levofloxacin has higher probability in reaching the PD targets.
What is New and Conclusion
Considering the concentration-dependent PK/PD property of levofloxacin, which means higher blood concentration predicts better bactericidal activity, it is expected that the regimen of levofloxacin 750-mg q.d. will provide more potent bactericidal activity in human body and maintain effective bactericidal concentration at infection site. Such a dosing regimen can shorten treatment duration and decrease the exposure to sub-MIC level of levofloxacin, which can potentially reduce the emergence of resistant bacteria.
The results of this study provide positive evidence to support the regimen of levofloxacin 750-mg injection (150 mL) once daily via intravenous infusion for 5 days in the treatment for severe CAP. It is necessary to design a large-scale clinical trial in patients to confirm these results.
Acknowledgments
This study was supported by ‘The Major Research and Development Project of Innovative Drugs, Ministry of Science and Technology of China (2012ZX09303004-001)’. The work presented here was carried out in collaboration between all authors.
Conflict of Interest
None of the authors has any conflict of interest to declare.
References
- 1.Wang F, Zhang Y, editors. Practical antimicrobial therapeutics. Beijing: People's Medical Publishing House; 2004. [Google Scholar]
- 2.Friedman H, Song X, Crespi S, Navaratnam P. Comparative analysis of length of stay, total costs, and treatment success between intravenous moxifloxacin 400 mg and levofloxacin 750 mg among hospitalized patients with community-acquired pneumonia. Value Health. 2009;12:1135–1143. doi: 10.1111/j.1524-4733.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 3.Frei CR, Jaso TC, Mortensen EM, et al. Medical resource utilization among community-acquired pneumonia patients initially treated with levofloxacin 750 mg daily versus ceftriaxone 1000 mg plus azithromycin 500 mg daily: a US-based study. Curr Med Res Opin. 2009;25:859–868. doi: 10.1185/03007990902779749. [DOI] [PubMed] [Google Scholar]
- 4.Schein J, Janagap-Benson C, Grant R, Sikirica V, Doshi D, Olson W. A comparison of levofloxacin and moxifloxacin use in hospitalized community-acquired pneumonia (CAP) patients in the US: focus on length of stay. Curr Med Res Opin. 2008;24:895–906. doi: 10.1185/030079908X273408. [DOI] [PubMed] [Google Scholar]
- 5.Lynch JP, 3rd, File TM, Jr, Zhanel GG. Levofloxacin for the treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther. 2006;4:725–742. doi: 10.1586/14787210.4.5.725. [DOI] [PubMed] [Google Scholar]
- 6.Chow AT, Fowler C, Williams RR, Morgan N, Kaminski S, Natarajan J. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 2001;45:2122–2125. doi: 10.1128/AAC.45.7.2122-2125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noreddin AM, Elkhatib WF. Levofloxacin in the treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther. 2010;8:505–514. doi: 10.1586/eri.10.35. [DOI] [PubMed] [Google Scholar]
- 8.Anderson VR, Perry CM. Levofloxacin: a review of its use as a high-dose, short-course treatment for bacterial infection. Drugs. 2008;68:535–565. doi: 10.2165/00003495-200868040-00011. [DOI] [PubMed] [Google Scholar]
- 9.File TM, Jr, Milkovich G, Tennenberg AM, Xiang JX, Khashab MM, Zadeikis N. Clinical implications of 750 mg, 5-day levofloxacin for the treatment of community-acquired pneumonia. Curr Med Res Opin. 2004;20:1473–1481. doi: 10.1185/030079904X2556. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YY, Huang HH, Ren ZY, et al. Clinical evaluation of oral levofloxacin 500 mg once-daily dosage for treatment of lower respiratory tract infections and urinary tract infections: a prospective multicenter study in China. J Infect Chemother. 2009;15:301–311. doi: 10.1007/s10156-009-0713-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Xu JF, Liu YB, et al. Population pharmacokinetics of oral levofloxacin 500 mg once-daily dosage in community-acquired lower respiratory tract infections: results of a prospective multicenter study in China. J Infect Chemother. 2009;15:293–300. doi: 10.1007/s10156-009-0714-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones RN, Rubino CM, Bhavnani SM, Ambrose PG. Worldwide antimicrobial susceptibility patterns and pharmacodynamic comparisons of gatifloxacin and levofloxacin against Streptococcus pneumoniae: report from the Antimicrobial Resistance Rate Epidemiology Study Team. Antimicrob Agents Chemother. 2003;47:292–296. doi: 10.1128/AAC.47.1.292-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and laboratory standards institute (CLSI) Performance standards for antimicrobial susceptibility testing; twenty-first informational supplements. 2012;3(1) M100-S21, [Google Scholar]
- 14.Cook AM, Martin C, Adams VR, Morehead RS. Pharmacokinetics of intravenous levofloxacin administered at 750 milligrams in obese adults. Antimicrob Agents Chemother. 2011;55:3240–3243. doi: 10.1128/AAC.01680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Nandy P, Chien S, Noel GJ, Tornoe CW. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother. 2010;54:375–379. doi: 10.1128/AAC.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiser TH, Hoody DW, Obritsch MD, Wegzyn CO, Bauling PC, Fish DN. Levofloxacin pharmacokinetics and pharmacodynamics in patients with severe burn injury. Antimicrob Agents Chemother. 2006;50:1937–1945. doi: 10.1128/AAC.01466-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodvold KA, Danziger LH, Gotfried MH. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother. 2003;47:2450–2457. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odenholt I, Cars O. Pharmacodynamics of moxifloxacin and levofloxacin against Streptococcus pneumoniae Staphylococcus aureus Klebsiella pneumoniae and Escherichia coli: simulation of human plasma concentrations after intravenous dosage in an in vitro kinetic model. J Antimicrob Chemother. 2006;58:960–965. doi: 10.1093/jac/dkl356. [DOI] [PubMed] [Google Scholar]
- 19.Frei CR, Burgess DS. Pharmacodynamic analysis of ceftriaxone, gatifloxacin, and levofloxacin against Streptococcus pneumoniae with the use of Monte Carlo simulation. Pharmacotherapy. 2005;25:1161–1167. doi: 10.1592/phco.2005.25.9.1161. [DOI] [PubMed] [Google Scholar]
- 20.Sprandel KA, Schriever CA, Pendland SL, et al. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin at 750 milligrams and various doses of metronidazole in healthy adult subjects. Antimicrob Agents Chemother. 2004;48:4597–4605. doi: 10.1128/AAC.48.12.4597-4605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kays MB, Conklin M. Comparative in vitro activity and pharmacodynamics of five fluoroquinolones against clinical isolates of Streptococcus pneumoniae. Pharmacotherapy. 2000;20:1310–1317. doi: 10.1592/phco.20.17.1310.34899. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Yu JC, Shi YG, Zhou L, Ye XY, Zhu DM, Zhang YY. Study of pharmacokinetics/pharmacodynamics of levofloxacin. Zhonghua Yi Xue Za Zhi. 2005;85:1926–1932. [PubMed] [Google Scholar]


