Abstract
Background:
Physcion is an anthraquinone from rhubarb (rhizomes of Rheum tanguticum) and has been reported to have anti-inflammatory, hepatoprotective, antifungal, and anti-cancer activities. However, the growth inhibitory activity against human cancer cells and the underlying molecular mechanisms have been poorly determined. This study was designed to investigate the anti-proliferative activity of physcion by induction of cell cycle arrest and apoptosis in human MDA-MB-231 triple negative breast cancer cell line.
Methods:
MDA-MB-231 cells were treated with physcion, and the anti-proliferative activity was evaluated by the sulforhodamine B assay. The mechanisms of action for the growth inhibitory activity of physcion were evaluated by flow cytometry for cell cycle distribution, and by Western blot for the assessment of potential target proteins.
Results:
Physcion showed a significant anti-proliferative activity against MDA-MB-231 human breast cancer cells. Flow cytometric analysis indicated that physcion markedly induced the accumulation of cells in the G0/G1 phase and the increase of cell population in the sub-G1 phase. The G0/G1 cell cycle arrest by physcion was associated with the down-regulation of Cyclin D1, Cyclin A, CDK4, CDK2, c-Myc and phosphorylated Rb protein expressions. The increase of sub-G1 peak by physcion was closely correlated with the induction of apoptosis, which was confirmed by the induction of cleaved poly-(adenosine diphosphate ribose) polymerase, activation of Caspases, and suppression of Bid and Bcl-2 expression.
Conclusions:
The induction of G0/G1 cell cycle arrest and apoptosis might be one of the plausible mechanisms of actions for the anti-proliferative activity of physcion in human breast cancer cells.
Keywords: Physcion, Cell cycle arrest, Apoptosis, Rhubarb, Breast neoplasms
INTRODUCTION
Breast cancer is the second leading cause of cancer-related mortality in women worldwide.1,2 Five-year survival rate for breast cancer has increased over the last decade. The current clinical treatments for breast cancer include surgery, radiation, hormonal therapy and/or chemotherapy.3 However, a poor response to chemotherapy remains a major clinical obstacle to the successful treatment for breast cancer. Therefore, there is still a great need for developing effective therapeutic agents for breast cancer chemotherapy. Natural products are considered to be important sources in the development of potential chemotherapeutic agents against breast cancer cells.
Rhubarb is the rhizomes of Rheum species including Rheum tanguticum Maxim., R. officinale Baill., R. palmatum L., R. undulatum L., and R. coreanum Nakai (Polygonaceae), which is widely distributed in China, Korea and Japan.4 It has been used as a traditional medicine to exhibit anti-cancer, anti-oxidant, anti-inflammatory, anti-bacterial, and anti-allergy activities.5,6 Rhubarb contains anthraquinones, dianthrones, glycosides, and tannins. The main active compounds of rhubarb are thought to be anthraquinone derivatives, which include emodin, chrysophanol, aloe-emodin, rhein, and physcion.5 Among them, physcion is found in the R. tanguticum, R. emodii, and fungus Microsporum sp.,7–9 and has been demonstrated to have anti-inflammatory, hepatoprotective, and anti-fungal activities.8,10–13 It was also reported that physcion inhibited the growth of various human cancer cell lines, including HeLa (cervical cancer cells), A549 (lung cancer cells), HL-60 (leukemia cells), and SW680 (colon cancer cells).12 However, the anti-proliferative activity of physcion in human breast cancer cells and its underlying molecular mechanisms still remain to be elucidated yet.
In the present study, we investigated the anti-proliferative activities of physcion isolated from rhubarb (rhizomes of R. tanguticum) against MDA-MB-231 human breast cancer cells and explored its molecular mechanism of actions.
MATERIALS AND METHODS
1. Chemicals
Trichloroacetic acid (TCA), sulforhodamine B (SRB), bovine serum albumin, propidium iodide (PI), ribonuclease A (RNase A), dimethyl sulfoxide, and anti-β-actin antibody were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), RPMI1640 medium, fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid, and antibiotics-antimycotics solution were purchased from GIBCO-BRL (Grand Island, NY, USA). Antibodies against CDK2, CDK4, Cyclin A, PCNA, p21, c-Myc, Bcl-2, and all secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against Caspase-3, Caspase-8, Caspase-9, phospho-Rb (Ser807/811), Rb, and Poly adenosine diphosphate ribose polymerase (PARP) were obtained from Cell Signaling (Danvers, MA, USA). Antibodies against cyclin D, cyclin E, and PARP were purchased from BD Biosciences (San Diego, CA, USA).
2. Cell culture
Breast cancer cell line (MDA-MB-231) was provided by the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in DMEM medium supplemented with 10% heat-inactivated FBS and antibiotics-antimycotics (PSF; 100 units/mL penicillin G sodium, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B). The cells were incubated at 37°C and 5% CO2 in a humidified atmosphere.
3. Cell proliferation assay
Cells (5 × 104 cells/mL) were treated with various concentrations of compounds (total volume of 200 μL/well) in 96-well culture plates for 72 hours. After treatment, cells were fixed with 10% TCA solution, and cell viability was determined with a SRB assay.14 Results were expressed as percentages relative to solvent-treated control incubations, and IC50 values were calculated using non-linear regression analysis (percent survival versus concentration).
4. Cell cycle analysis
MDA-MB-231 cells were plated at a density of 1 × 106 cells per 100 mm culture dish and incubated for 24 hours. Fresh media containing various concentrations of test sample were added to culture dishes. Following a 24 hours incubation, the cells were harvested and fixed with 70% ethanol overnight at 4°C. Fixed cells were washed with phosphate buffered saline (PBS) and incubated with a staining solution containing RNase A (50 μg/mL) and PI (50 μg/mL) in PBS for 30 minutes at room temperature. The cellular DNA content was analyzed with a FACS Calibur flow cytometer (BD Biosciences). Approximately 10,000 cells were used for each analysis, and the distribution of cells in each phase of the cell cycle was displayed as histograms.
5. Western blot analysis
MDA-MB-231 cells were treated with various concentrations of physcion for 24 hours. After incubation, the cells were lysed and protein concentrations were determined by BCA method.14 Each protein (40 μg) was subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis. Proteins were transferred onto polyvinylidene fluoride membranes by electroblotting, and membranes were treated for 1 hour with blocking buffer (5% non-fat dry milk in phosphate-buffered saline-0.1% Tween 20 [PBST]). Membranes were then incubated with indicated antibodies (mouse anti-β-actin, diluted 1 : 2,000; other antibodies, diluted 1 : 1,000 in PBST) overnight at 4°C, washed three times for 5 minutes with PBST. After washing, membranes were incubated with horseradish peroxidas (HRP)-conjugated anti-mouse immunoglobulin G diluted 1 : 2,000 in PBST for 2 hours at room temperature, washed three times for 5 minutes with PBST, and visualized by HRP-chemiluminescent detection kit (Lab Frontier, Seoul, Korea) using LAS-3000 Imager (Fuji Film Corp., Tokyo, Japan).
6. Statistical analysis
Results were obtained from more than three independent experiments. Data were expressed as the mean ± standard deviation for the indicated number of independently performed experiments and analyzed by Student’s t-test (SigmaStat 3.1; Systat Software Inc. San Jose, CA, USA). Differences with the P-value less than 0.05 was considered statistically significant.
RESULTS
1. Anti-proliferative effects of physcion on MDA-MB-231 human breast cancer cells
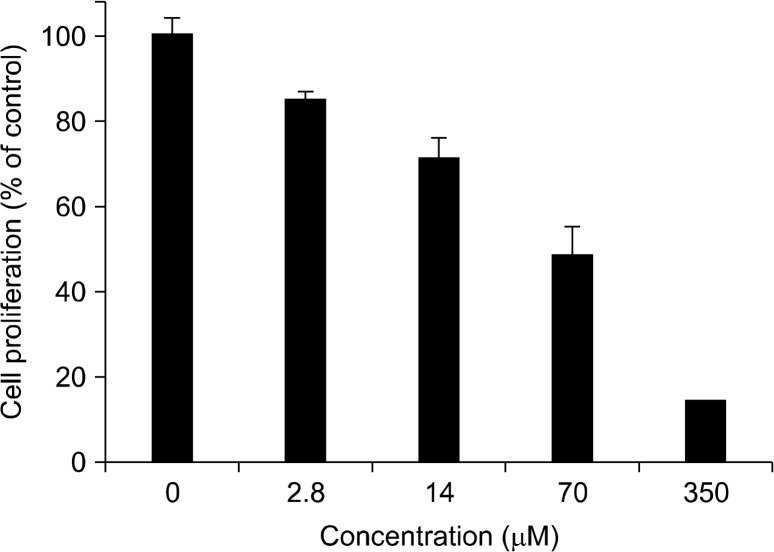
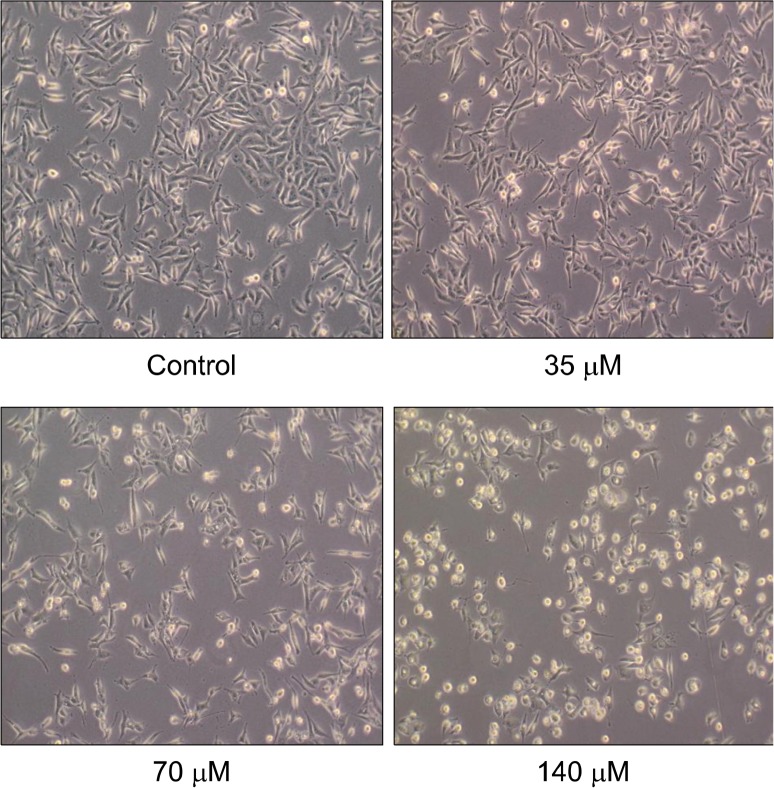
The anti-proliferative effect of physcion (Fig. 1) was determined in MDA-MB-231 human breast cancer cells by a colorimetric SRB protein dye staining method. As a result, the proliferation of MDA-MB-231 cells exposed to physcion was inhibited in a dose-dependent manner, with an IC50 value 45.4 μM at 72 hours incubation (Fig. 2). In addition, morphological changes were observed using a phase-contrast microscope. As shown in Figure 3, the cells treated with various concentrations (35, 70, and 140 μM) of physcion for 24 hours exhibited distinct cell shrinkage and rounded shapes in a concentration-dependent manner compared to control cells.
Figure 1.
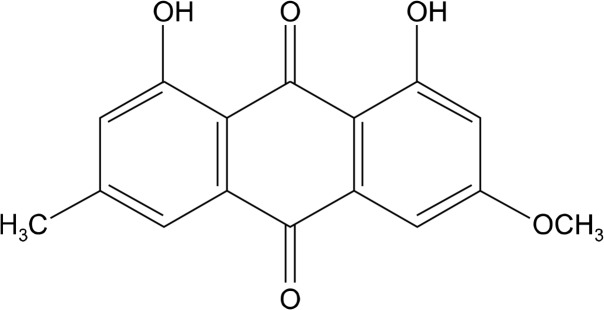
Chemical structure of physcion.
Figure 2.
Effect of physcion on the proliferation of human breast cancer MDA-MB-231 cells. MDA-MB-231 cells were plated at 10,000 cells in 96-well plate in RPMI supplemented with 10% fetal bovine serum, and incubated with the test compound as the indicated concentrations for 72 hours. Anti-proliferative activity was determined using the sulforhodamine B assay. The values (% of control) are calculated by the mean absorbance of samples/absorbance of vehicle- treated control. Data are represented as the mean ± standard deviation (n = 3).
Figure 3.
Morphological changes mediated by physcion in MDA-MB-231 cells. Cells were treated with various concentrations of physcion for 24 hours. Cells were photographed by inverted microscopy (×100).
2. Changes of cell cycle distribution by physcion
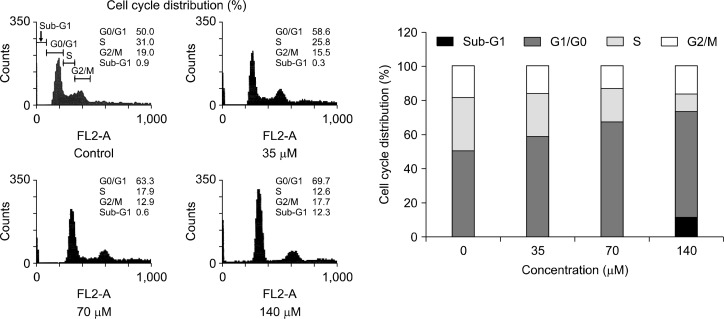
To determine the mechanism of action of anti-proliferative activity of physcion, the cell cycle distribution was evaluated by flow cytometric analysis. MDA-MB-231 cells were treated with the indicated concentrations of physcion for 24 hours, and the distribution of cells in various compartments of the cell cycle was analyzed by flow cytometry. As shown in Figure 4, the percentage of cells in the G0/G1 phase was markedly increased by physcion in a concentration-dependent manner. There was also a significant increase in the sub-G1 phase population in the highest concentration of physcion-treated cells.
Figure 4.
Effect of physcion on the cell cycle progression in the MDA-MB-231 cells. Cells were treated with vehicle or various concentration of physcion (35, 70, and 140 μM) for 24 hours. The cell cycle distribution was analyzed by flow cytometry as described in MATERIALS AND METHODS.
3. Effects of physcion on the expression of cell cycle regulatory proteins
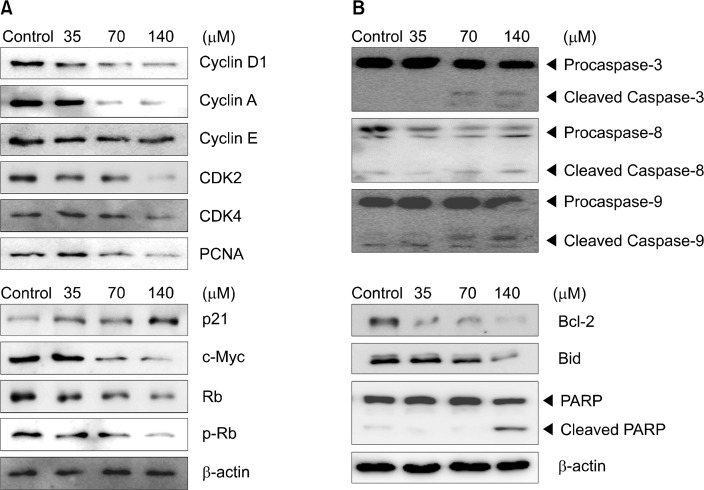
To further elucidate whether cell cycle arrest was related to the regulation of cell cycle checkpoint proteins, the expression of G0/G1 cell cycle regulatory proteins was determined by Western blot analysis. As shown in Figure 5A, treatment with physcion exhibited the down-regulation of the protein levels including Cyclin D, Cyclin A, Cyclin E, CDK2, CDK4, and PCNA in MDA-MB-231 cells. In addition, cyclin dependent kinase inhibitor p21 was increased and c-Myc, a specific protein that regulates cell cycle progression and cell proliferation, was suppressed by physcion. The active Cyclin-CDK complexes in G0/G1 phase mediate the inactivation of tumor suppressor protein Rb via phosphorylation at serine residues.15 Thus, we examined the regulation of Rb phosphrylation by physcion. As a result, both of total and phosphorylation of Rb were suppressed by treatment of physcion. Therefore, physcion-medicated G0/G1 cell cycle arrest was associated with the decrease of expression of the corresponding cyclins and CDKs in MDA-MB-231 cells.
Figure 5.
Effect of physcion on the expression of cell cycle regulatory proteins (A) and apoptosis related proteins (B) in the MDA-MB-231 cells. Cells were treated with the indicated concentrations of physcion for 24 hours. The expression level of proteins was analyzed by Western blot.
4. Effects of physcion on the expression of apoptosis-related proteins
In order to determine whether the anti-proliferative effect of physcion is related to the induction of apoptosis, MDA-MB-231 cells were exposed to physcion and the regulation of apoptosis-related proteins was evaluated by Western blot analysis. As shown in Figure 5B, physcion treatment significantly increased the expression of cleaved Caspases (-3, -8, -9) and cleaved-PARP. On the other hand, the expression of Bcl-2 and Bid was down-regulated. These results suggest that the increased protein expressions of cleaved PARP and cleaved Caspases (-3, -8, and -9), and decreased protein expressions of Bcl-2 and Bid by physcion were contributed to the induction of apoptosis in breast cancer cells.
DISCUSSION
Natural products have been evaluated as important source for developing the anti-cancer agents. Many plant-derived compounds have been shown to have potent anti-cancer activities and considered to be cancer therapeutic agents. As part of our on-going study on the identification of the potential anti-cancer agents from natural products, this study was designed to examine the anti-proliferative effects of physcion, an anthraquinone isolated from rhubarb (rhizome of R. tanguticum) and we further investigated the mechanisms of action in MDA-MB-231 human breast cancer cells.
The present study revealed that physcion inhibited the proliferation of MDA-MB-231 cells in a dose-dependent manner (Fig. 2). Based on the potential anti-proliferative activity of physcion, we further investigated the mechanisms of action related to cell cycle arrest and apoptosis. In physcion-treated cells, cell population in the S phase was decreased, whereas cell population in the G0/G1 phase was increased when compared with that of untreated cells. These data implied that physcion induced cell cycle progress in the G0/G1 phase arrest. A recent study reported that physcion 8-O-β-glucopyranoside exhibited an anti-proliferative activity of the A549 human lung cancer cells, and it was also associated with the G2/M phase cell cycle arrest and apoptosis.16 The different effects of physcion and physcion 8-O-β-glucopyranoside on the cell cycle progression might be in part related to the difference of chemical structures and cancer cell types.
The induction of G0/G1 phase cell cycle arrest by physcion was further confirmed by the analysis of expression of the cell cycle regulatory proteins. The progression of cell cycle involves the sequential activation of CDKs, whose association with corresponding regulatory cyclins is necessary for their activations.17 In particularly, cyclin D/CDK4 complex play an important role in the regulation of the G0/G1 phase, and cylicn E/CDK2 and cyclin A/CDK2 complexes are associated with the G1/S and initiation of S phase, respectively.18,19 Therefore, the suppression of cyclin D, cyclin E, cyclin A, CDK2, and CDK4 by physcion was involved in the G0/G1 and G1/S phase cell cycle arrest. The CDK inhibitor p21 can negatively regulate cell cycle progression by inhibiting the kinase activities of CDKs/cyclin complexes.20 In addition, cyclin- CDK complexes inactivated tumor suppressor Rb by phosphorylating Rb at serine site.15 As shown in Figure 5A, physcion induced the expression of p21 and suppression of pRb, suggesting the involvement of G0/G1 phase cell cycle arrest by physcion in MDA-MB-231 cells.
Apoptosis is one of the main types of programmed cell death, and many anti-cancer agents induce apoptosis to achieve therapeutic efficacy.21,22 There was a significant increase in the sub-G1 phase population in physcion (140 μM) treated cells (Fig. 5B). Therefore, the mechanistic activity of physcion was further analyzed by the expression of apoptosis-associated proteins. Apoptotic cell death involved two main molecular signaling pathways, the death-receptor extrinsic pathway and the mitochondrial intrinsic pathway.23 Caspase-8 and Bid are involved in the execution of apoptosis through the extrinsic pathway.23 On the other hand, caspase-9 is known to be involved in the activation of the intrinsic apoptosis pathway.24 Bcl-2 is traditionally known as a member of the BCL family and induces changes in the mitochondrial potential upon apoptotic stimuli, and this eventually leads to the activation of another proapoptotic proteins.25,26 As shown in Figure 5B, physcion induced an activation of Caspase-8 and Bid, which are critical in the extrinsic pathway, and also led to the down-regulation of Bcl-2 and activation of Caspase-9, which are essential in the intrinsic pathway. In addition, the two pathways converge at the point of Caspase-3 activation, and following the activation of Caspase-3, PARP is cleaved. The present results demonstrated that physcion increased the apoptotic cells and activation of Caspase-3, -8, -9, and PARP. Previous study in human cervical cancer cells showed that physcion inhibited the levels of Bcl-2 and activated the Caspase-3 pathway,9 suggesting that our findings are well correlated with the previous report.
In conclusion, the present study demonstrated that anti-proliferative activity of physcion is mediated by inducing G0/G1 phase arrest and apoptosis in MDA-MB-231 human breast cancer cells. These findings suggest that physcion might be a potential lead candidate for developing cancer chemotherapeutic agents from rhubarb (rhizome of R. tanguticum).
Acknowledgments
This research was supported by a grant (2009 and 12172MFDS 989) from Ministry of Food and Drug Safety, Korea in 2014.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–9. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He ZH, Zhou R, He MF, Lau CB, Yue GG, Ge W, et al. Anti-angiogenic effect and mechanism of rhein from Rhizoma Rhei. Phytomedicine. 2011;18:470–8. doi: 10.1016/j.phymed.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey JF, Kao GD. Aloe(-emodin) for cancer? More than just a comforting salve. Cancer Biol Ther. 2007;6:89–90. doi: 10.4161/cbt.6.1.3845. [DOI] [PubMed] [Google Scholar]
- 6.Cuéllar MJ, Giner RM, Recio MC, Máñez S, Ríos JL. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72:221–9. doi: 10.1016/S0367-326X(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang GY, Qi HY, Shi YP. Ultrasonic cell grinder extraction of anthraquinones from radix et rhizoma Rhei and determination by ultra- performance liquid chromatography. J Sep Sci. 2010;33:1730–8. doi: 10.1002/jssc.200900861. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–6. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 9.Wijesekara I, Zhang C, Van Ta Q, Vo TS, Li YX, Kim SK. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol Res. 2014;169:255–61. doi: 10.1016/j.micres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, Das Sarma M, Patra A, Hazra B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J Pharm Pharmacol. 2010;62:1158–66. doi: 10.1111/j.2042-7158.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YL, Wang JB, Zhou GD, Shan LM, Xiao XH. Investigations of free anthraquinones from rhubarb against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic Clin Pharmacol Toxicol. 2009;104:463–9. doi: 10.1111/j.1742-7843.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuete V, Wabo HK, Eyong KO, Feussi MT, Wiench B, Krusche B, et al. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One. 2011;6:e21762. doi: 10.1371/journal.pone.0021762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–30. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 14.Hong JY, Chung HJ, Lee HJ, Park HJ, Lee SK. Growth inhibition of human lung cancer cells via down-regulation of epidermal growth factor receptor signaling by yuanhuadine, a daphnane diterpene from Daphne genkwa. J Nat Prod. 2011;74:2102–8. doi: 10.1021/np2003512. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 16.Xie QC, Yang YP. Anti-proliferative of physcion 8-O-β-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complement Altern Med. 2014;14:377. doi: 10.1186/1472-6882-14-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–34. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 20.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 21.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4(Suppl 1):421–7. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153–9. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 26.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]