Abstract
Objective
This was a flexible-dosed study to evaluate the efficacy and safety of duloxetine 30–120 mg once daily in the treatment of generalized anxiety disorder (GAD) in older adult patients.
Methods
Patients with GAD, who were at least 65 years of age, were randomly assigned to double-blind treatment with either duloxetine (N = 151) or placebo (N = 140). The primary efficacy measure was the Hamilton Anxiety Rating Scale (HAM-A) total score, and the primary endpoint was at week 10. Global functioning was assessed by the Sheehan Disability Scale (SDS). Safety and tolerability was assessed by the occurrence of treatment-emergent adverse events, serious adverse events, laboratory analyses, and vital signs. Analyses were conducted on an intent-to-treat basis.
Results
The overall baseline mean HAM-A total score was 24, and SDS global score was 14. Completion rates were 75% for placebo and 76% for duloxetine. At week 10, duloxetine was superior to placebo on mean changes from baseline in HAM-A total scores (−15.9 vs. −11.7, p < 0.001) and in SDS global scores (−8.6 vs. −5.4, p < 0.001). Treatment-emergent adverse events occurred in ≥5% of duloxetine-treated patients and twice the rate than with placebo including constipation (9% vs. 4%, p = 0.06), dry mouth (7% vs. 1%, p = 0.02), and somnolence (6% vs. 2%, p = 0.14).
Conclusion
Duloxetine treatment was efficacious in the improvement of anxiety and functioning in older adult patients with GAD, and the safety profile was consistent with previous GAD studies. © 2014 The Authors. International Journal of Geriatric Psychiatry published by John Wiley & Sons, Ltd.
Key points
Treatment with duloxetine versus placebo can significantly reduce symptoms of generalized anxiety disorder and was associated with improved global function and increased enjoyment and satisfaction with life in patients 65 years and older.
The safety and tolerability profile for duloxetine in this older adult patient population was consistent with the established profile for treatment of generalized anxiety disorder in the broader mostly younger (≥18 years of age) population, and there were no new safety findings.
Keywords: anxiety, functioning, duloxetine
Introduction
Generalized anxiety disorder (GAD) is a chronic disabling condition characterized by persistent, excessive, and difficult-to-control worry (Wittchen et al., 2002). GAD typically onsets in young adulthood but is also known to onset later in life (Le Roux et al., 2005). In the National Comorbidity Replication Survey, GAD had a prevalence rate of nearly 12% in 2575 adults over the age of 55 years (Byers et al., 2010). A recent review of epidemiological data from European studies reported an estimated prevalence rate of 3.4% for GAD in persons >65 years of age (Wittchen et al., 2011).
Considering the neurobiology of GAD, pharmacological guidelines recommend treatment with selective serotonin reuptake inhibitors, selective serotonin–norepinephrine reuptake inhibitors, or pregablin (Montgomery et al., 2006; Hoffman and Mathew, 2008; Baldwin et al., 2012; Bandelow et al., 2012). Approved selective serotonin reuptake inhibitors for GAD include escitalopram (Davidson et al., 2004; Allgulander et al., 2006; Lenze et al., 2009) and paroxetine (Pollack et al., 2001; Rickels et al., 2003; Stocchi et al., 2003). Approved serotonin–norepinephrine reuptake inhibitors include duloxetine (Hartford et al., 2007; Koponen et al., 2007; Rynn et al., 2008; Nicolini et al., 2009) and venlafaxine (Davidson et al., 1999; Gelenberg et al., 2000; Rickels et al., 2000; Allgulander et al., 2001; Katz et al., 2002). A meta-analysis of 14 pharmacological treatment studies for GAD in older adults found an overall odds ratio (OR) of 0.32 favoring active treatment, with an OR of 0.19 for benzodiazepine treatments compared with OR of 0.46 for antidepressants (Gonçalves and Byrne, 2012).
Supportive data on the efficacy and safety of duloxetine in the treatment of older patients (≥65 years old) with GAD were provided from an analysis of 73 patients from four acute therapy, placebo-controlled trials (duloxetine n = 45, placebo n = 28) (Davidson et al., 2008). Given the relatively small sample size in that analysis, this clinical trial was undertaken to further evaluate the efficacy and safety of duloxetine in the treatment of patients with GAD, who were ≥65 years of age.
Methods
This phase IV study was conducted under protocol F1J-MC-HMGF (ClinicalTrials.gov identifier: NCT01118780) in 47 sites across nine countries: Argentina, Austria, Canada, Germany, Mexico, Poland, Spain, the UK, and the USA. Enrollment began in October 2010, and the study was completed in July 2012. Institutional review boards at each site approved the protocol, which was developed in accordance with the ethical guidelines of Good Clinical Practice and the Declaration of Helsinki. All patients provided written consent after the study was explained, their questions answered, and before any study procedures were initiated.
Male and female outpatients aged ≥65 years were eligible for study entry if they met criteria for GAD (DSM-IV TR) (APA, 1994) defined by the Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and had at least moderately severe symptoms as indicated by a Clinical Global Impressions (CGI) of Severity of Illness (Guy, 1976) score of ≥4, Covi Anxiety Scale (CAS) (Lipman, 1982) score of ≥9, no item score of >3 on the Raskin Depression Scale (RDS) (Raskin et al., 1969), the CAS total score had to be greater than the RDS total score, and a Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983) anxiety subscale score of ≥10. Stable medical comorbidities that did not pose a safety risk as determined by physical examination, electrocardiogram (ECG), and laboratory results were allowed. Patients were required to have a level of understanding that allowed them to communicate intelligibly and a Mini mental state examination (Folstein et al., 1975) score of ≥24.
Patients were excluded if they met criteria for any DSM-IV TR Axis I diagnosis other than GAD, with the exception of comorbid social/specific phobia, or any DSM-IV TR Axis II disorder that would impair study compliance, benzodiazepine use in the prior 14 days, judged clinically to be at serious risk of harm to self or others, history of alcohol or any psychoactive substance abuse or dependence (DSM-IV TR definition) within the past 6 months, positive urine screen for any substances of abuse, excessive use of caffeine, taken any excluded medication with central nervous system activity in the prior 7 days, treatment with monoamine oxidase inhibitor (MAOI) or fluoxetine within the prior 30 days or may need to use an MAOI during the study or within 5 days of discontinuation of study drug, and judged to be a poor medical or psychiatric risk for study compliance in the opinion of the investigator.
Study design
This was a randomized, double-blind, placebo-controlled, flexible-dose, parallel group trial that consisted of three study periods: screening/washout period (up to 30 days), 10-week therapy period, and a 2-week taper-off drug period. Study visits were conducted at weeks 2, 4, 7, and 10.
Eligible patients were randomly assigned (1 : 1) to treatment with oral duloxetine or placebo once daily (QD) via computer-generated random sequence using an interactive voice response system. Patients assigned to receive duloxetine initiated treatment with 30 mg QD for 2 weeks; then, the dose was increased to 60 mg QD based on investigator decision to maximize efficacy or if the CGI–Improvement (CGI-I) Scale (Guy, 1976) score was ≥3. Dose escalation was allowed only at scheduled visits if the patient was adequately tolerating the current dose. Dose increases were from 60 to 90 mg and from 90 to 120 mg QD. If the increased dose was not tolerated at any time, patients were returned to their previous dose, and patients were not allowed any additional dose changes for the remainder of the study. All patients were required to maintain a minimum dose of 30 mg to remain in the treatment phase of the study. Patients were required to undergo the double-blind drug-taper phase, unless the patient planned to start active treatment immediately upon completion of the study. During the drug-taper phase, patients on duloxetine 60 mg received 30 mg QD for 1 week followed by placebo for the second week. Patients taking duloxetine 90 or 120 mg received 60 mg QD for 1 week then 30 mg QD for the second week. Patients on duloxetine 30 mg QD or on placebo received placebo for both weeks.
Efficacy measures
The primary efficacy measure was Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959) total score. The HAM-A was chosen to assess anxiety because it is commonly used in pharmacological trials and was used in the four previous studies of duloxetine in GAD. HAM-A was administered at each visit and at the primary endpoint: week 10. The structured interview guide for HAM-A (Shear et al., 2001) was used to collect HAM-A data. Response was defined as a 50% reduction from baseline in the HAM-A total score (Allgulander et al., 2008), and remission was defined as a HAM-A total score of ≤7 (Doyle and Pollack, 2003).
The key secondary efficacy measure was the Sheehan Disability Scale (Sheehan et al., 1996) for global functioning that rates impairment in three life domains: work, social, and family/home management. Other secondary efficacy measures included the HAM-A psychic anxiety factor (sum of items assessing anxious mood, tension, fears, insomnia, concentration, depressed mood, and behavior at interview), HAM-A somatic anxiety factor (sum of items assessing somatic muscular, somatic sensory, cardiovascular, respiratory, gastrointestinal, genitourinary, and autonomic symptoms), HAM-A individual items for anxious mood and tension, and the HADS anxiety and depression subscales. Additional secondary outcomes included symptom improvement assessed by the CGI-I and by patients using the self-rating Patient Global Impression of Improvement Scale (Guy, 1976). Quality of life was assessed by the 16-item Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form (Endicott et al., 1993). In addition, patients were also assessed for the presence, severity, and interference of pain symptoms using the self-assessment Brief Pain Inventory (Cleeland and Ryan, 1994).
Safety and tolerability
Safety and tolerability was assessed through collection and monitoring discontinuation rates, treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), vital signs, laboratory analyses, and ECGs. In addition, a solicited assessment of the occurrence of any falls and the circumstances/consequences associated with any fall was made at every visit. Solicited questioning of suicide-related behavior and ideations was also completed at every visit using the Columbia Suicide Severity Rating Scale (Posner et al., 2011).
Statistical analysis
Approximately 288 patients were to be randomly assigned to receive either duloxetine or placebo treatment in a 1 : 1 ratio that would provide approximately 80% power to detect a 0.35 effect size relative to placebo in the baseline-to-endpoint mean change on the HAM-A total score. The effect size was based on HAM-A total score data collected from older patients, who participated in four placebo-controlled studies of duloxetine in GAD, and the pooled analysis effect size was 0.40. The sample size was determined using a two-sided test with p = 0.05 and assumed that 10% of the patients would discontinue by week 2 without providing postbaseline HAM-A data (Hartford et al., 2007; Koponen et al., 2007; Rynn et al., 2008; Nicolini et al., 2009).
All analyses were conducted on an intent-to-treat basis. Mean changes from baseline were analyzed using a restricted maximum likelihood-based repeated measures analysis using all postbaseline observations. The model included the fixed categorical effects of treatment, investigator, age, visit, and treatment-by-visit interaction, as well as the continuous, fixed covariates of baseline score and baseline-by-visit interaction. An unstructured covariance structure was used to model the within-patient errors. The Kenward–Rogers method was used to estimate denominator degrees of freedom. Type III sum of squares for the least squares mean was used. Analyses were implemented using sas (version 9.1). When analyzing efficacy variables using the repeated measures analysis, the treatment group contrast at the last visit of the double-blind acute therapy phase (week 10) was the primary comparison, and those at earlier postbaseline visits were secondary. In addition, treatment-by-investigator interaction was investigated by adding a treatment-by-investigator interaction term to the model described previously.
Treatment group differences were evaluated on the basis of a two-sided significance level of 0.05. No adjustments for multiple comparisons were made. Unless otherwise specified, the analysis of variance model used to analyze demographic, efficacy, or safety variables contained the main effects of treatment and investigator. The corresponding analysis of covariance models also included baseline as a continuous covariate and age group as a categorical covariate. Type III sum of squares for the least squares mean was used for the statistical comparison using analysis of variance or analysis of covariance. Unless otherwise specified, in all double-blind analyses, “baseline” refers to the last nonmissing observation at or before the randomization visit, and “endpoint” refers to the last nonmissing observation at or before week 10.
Categorical comparisons between treatment groups were performed using a Cochran–Mantel–Haenszel test controlling for pooled investigative site and Fisher's exact test, where appropriate, or Pearson's chi-square test.
Results
Patients
Figure 1 shows the number of patients across the stages of recruitment and study participation. Patient demographics and baseline illness severity are summarized in Table 1. The treatment groups did not differ significantly in any characteristic or severity of illness at baseline. Overall, 83.2% of patients had one or more preexisting stable medical condition, the most common of which was hypertension (44.0%), and 81.8% of patients used concomitant medications. One hundred fifteen (76.2%) duloxetine-treated patients and 105 (75.0%) placebo-treated patients completed the study (Figure 1). There were no significant differences between duloxetine and placebo treatment groups on rates of discontinuation because of adverse events (9.9% vs. 10.7%, respectively; p = 0.85).
Figure 1.
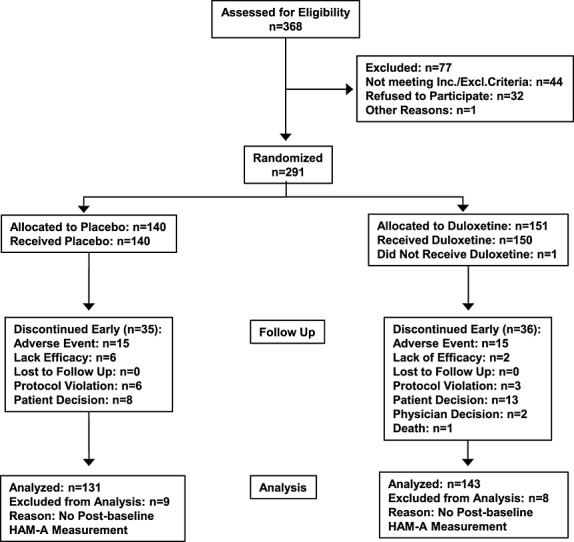
Consort diagram of recruitment and patient flow through the study.
Table 1.
Baseline characteristics and illness severity
| Variable | Placebo | Duloxetine |
|---|---|---|
| N = 140 | N = 151 | |
| Age, mean (SD) years | 71.7 (5.0) | 71.4 (5.4) |
| Female, n (%) | 112 (80.0) | 114 (75.5) |
| Ethnicitya | ||
| African descent, n (%) | 0 | 5 (3.3) |
| Caucasian, n (%) | 120 (85.7) | 129 (85.4) |
| Native American, n (%) | 18 (12.9) | 17 (11.3) |
| MMSE total score, mean (SD) | 28.5 (1.7) | 28.4 (1.7) |
| CGI-S, mean (SD) | 4.4 (0.6) | 4.4 (0.6) |
| HAM-A total score, mean (SD) | 24.4 (7.1) | 24.6 (6.4) |
| HAM-A Psychic Anxiety Factor score, mean (SD) | 13.4 (3.4) | 13.6 (3.2) |
| HAM-A Somatic Anxiety Factor score, mean (SD) | 10.9 (4.8) | 11.0 (4.3) |
| HADS Anxiety subscale score, mean (SD) | 13.6 (3.3) | 13.9 (3.1) |
| HADS Depression subscale, mean (SD) | 7.4 (4.3) | 7.4 (3.9) |
| SDS Global Functional Impairment score, mean (SD) | 14.2 (7.5) | 13.7 (7.6) |
| Q-LES-Q-SF, mean % of maximum score (SD) | 49.3 (14.6) | 49.0 (14.0) |
| BPI 24-h Average Pain Severity, mean (SD) | 3.2 (2.6) | 3.1 (2.6) |
| BPI Pain Interference, mean (SD) | 3.0 (2.4) | 2.8 (2.3) |
SD, standard deviation; BPI, Brief Pain Inventory; CGI-S, Clinical Global Impressions–Severity of Illness; HADS, Hospital Anxiety and Depression Scale; HAM-A, Hamilton Anxiety Rating Scale; MMSE, Mini mental state examination; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form; SDS, Sheehan Disability Scale.
There were no significance between treatment group differences in any of the baseline variables.
Two patients in the placebo group (1.4%) did not report ethnicity/race information.
Over the course of the study, 48 (31.8%) patients in the duloxetine arm did not have a dose escalation and remained on 30 mg, 52 (34.4%) had one escalation to 60 mg, 36 (23.8%) had two escalations to 90 mg, and 15 (9.9%) had three escalations to 120 mg. In the placebo arm, 31 (22.1%) of patients did not have a dose increase, 44 (31.4%) had one escalation, 28 (20.0%) had two escalations, and 37 (26.4%) had three escalations. Between-treatment differences in dose escalations were not significant.
Efficacy
Patients treated with duloxetine versus placebo had significantly greater baseline-to-endpoint improvement on the primary efficacy measure, the HAM-A total score (−15.9 vs. −11.7, p < 0.001) (Table 2). Significance between treatment group differences began as early as week 4 and continued to study end at week 10 (Figure 2). Treatment with duloxetine versus placebo was also associated with significantly greater improvement in other measures of anxiety, depression, quality of life, and overall functioning (Table 2). Significant improvement in global functioning, social/leisure activities, and in family/home management began at week 4 of treatment with duloxetine, which was sustained to study end. (Figure 3). Improvement in function for working separated from placebo at week 4 and at endpoint.
Table 2.
Least squares mean changes on efficacy measures and least squares mean endpoint improvement scores in patients treated with duloxetine or placebo
| Scale | Placebo | Duloxetine | p-value |
|---|---|---|---|
| N = 140 | N = 151 | ||
| Changes from baseline to endpoint, least squares mean (SE) | |||
| HAM-A total score | −11.7 (0.7) | −15.9 (0.6) | <0.001 |
| HAM-A Psychic Anxiety Factor score | −6.2 (0.4) | −8.6 (0.4) | <0.001 |
| HAM-A Somatic Anxiety Factor score | −5.6 (0.4) | −7.3 (0.3) | <0.001 |
| HADS Anxiety | −5.6 (0.4) | −7.8 (0.4) | <0.001 |
| HADS Depression | −1.6 (0.3) | −3.3 (0.3) | <0.001 |
| SDS Global Functional Impairment | −5.4 (0.6) | −8.6 (0.6) | <0.001 |
| Q-LES-Q-SF | 9.4 (1.5) | 15.1 (1.5) | 0.002 |
| BPI 24-h Average Pain Severity | −0.6 (0.2) | −1.1 (0.2) | 0.049 |
| BPI Pain Interference | −0.7 (0.2) | −1.2 (0.2) | 0.054 |
| Scores at endpoint, mean (SD) | |||
| CGI-I | 2.8 (1.3) | 2.3 (1.1) | <0.001 |
| PGI-I | 3.1 (1.4) | 2.5 (1.2) | <0.001 |
SE, standard error; BPI, Brief Pain Inventory; CGI-I, Clinical Global Impressions–Improvement; HADS, Hospital Anxiety and Depression Scale; HAM-A, Hamilton Anxiety Rating Scale; PGI-I, Patient Global Impression of Improvement; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form; SDS, Sheehan Disability Scale.
Figure 2.
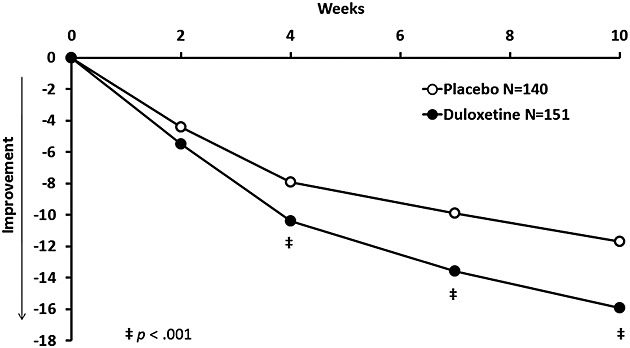
Least squares mean change from baseline to endpoint in HAM-A total score (MMRM analysis).
Figure 3.
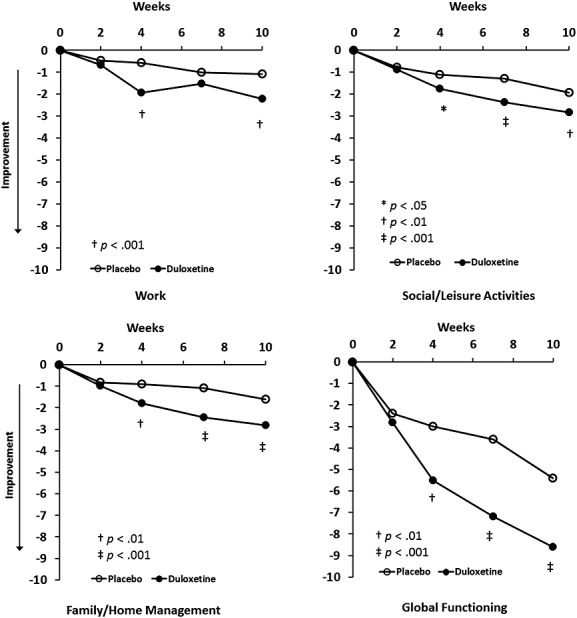
Least squares mean change from baseline to endpoint on the Sheehan Disability Scale items and Global Functioning Impairment scores (MMRM analysis).
Significantly, more patients treated with duloxetine versus placebo were perceived to be much improved with CGI-I scores of ≤2 (62.9% vs. 45.0%, p < 0.001). In addition, response rates were significantly higher in the duloxetine treatment group as compared with placebo (71.3% vs. 45.5%; p < 0.001) as were rates of remission (44.8% vs. 29.5%, p < 0.001). At study end, significantly more duloxetine-treated versus placebo-treated patients (74.6% vs. 55.6%, p = 0.001) had sustained improvement, defined as at least 30% reduction in HAM-A total scores at each visit from onset.
Safety and tolerability
Treatment-emergent adverse events that occurred in ≥5% of patients treated with duloxetine are summarized in Table 3. Dry mouth, constipation, and somnolence occurred at twice the rate as with placebo, and dry mouth frequency was significantly higher in the duloxetine group. On the basis of the solicited assessment of falls, four (3.5%) placebo-treated patients and five (4.4%) of duloxetine-treated patients (p = 0.75) reported a fall. Each fall incident was cross-checked with vital sign measurements, and no corresponding episodes of treatment-emergent orthostatic hypotension were identified. Treatment-emergent suicidal ideation assessed by the Columbia Suicide Severity Rating Scale was reported for nine patients, five (3.8%) treated with placebo and four (2.8%, p = 0.74) treated with duloxetine, and there were no attempted suicides.
Table 3.
Treatment-emergent adverse events with ≥5% incidence rate for patients treated with duloxetine
| Event, n (%) | Placebo | Duloxetine | p-value |
|---|---|---|---|
| N = 140 | N = 151 | ||
| Any event | 71 (50.7) | 91 (60.3) | 0.125 |
| Nausea | 9 (6.4) | 17 (11.3) | 0.157 |
| Headache | 9 (6.4) | 16 (10.6) | 0.218 |
| Constipation | 5 (3.6) | 14 (9.3) | 0.059 |
| Dizziness | 10 (7.1) | 12 (7.9) | 0.828 |
| Dry mouth | 2 (1.4) | 11 (7.3) | 0.021 |
| Somnolence | 3 (2.1) | 9 (6.0) | 0.141 |
Three SAEs occurred in the duloxetine group. One patient hospitalized approximately 2 months after initiating treatment was diagnosed with angina pectoris, recovered from the event, and completed the study. Another patient with preexisting cardiovascular disease was hospitalized after 6 days of treatment and diagnosed with hypertensive crisis, recovered, then was discontinued from the trial. A third patient hospitalized because of diarrhea following 68 days of treatment was diagnosed with a large intestinal obstruction and died of complications. These events were not considered by the study investigators to be associated with duloxetine treatment or because of any protocol procedure. There were no SAEs reported in the placebo group, and between-treatment differences were not significant (p = 0.25).
The incidence of abnormal laboratory parameters during the study was not statistically significantly different between treatment groups for any laboratory value. At study endpoint, six duloxetine-treated patients (4.4%) had elevated aspartate aminotransferase/serum glutamic oxaloacetic transaminase levels, which was statistically significantly greater than placebo (0%, p = 0.033). Overall, the mean level of aspartate aminotransferase/serum glutamic oxaloacetic transaminase elevation was less than two times the upper limit of normal, and there were no other related laboratory elevations. However, one duloxetine-treated patient, who was HBcAb IgM-positive, had mildly elevated alanine transaminase at baseline (54 IU/L), levels ≥3 × upper limit of normal at multiple time points, which subsequently declined while still on duloxetine treatment, and was largely normalized at poststudy follow-up.
The treatment groups did not significantly differ in mean baseline-to-endpoint changes in sitting systolic blood pressure, orthostatic vital signs, ECG parameters, or in weight, but there were statistically significant duloxetine/placebo treatment differences in sitting diastolic blood pressure (0.3 vs. −1.7 mmHg; p = 0.020) and sitting pulse rate (1.8 vs. −1.3 bpm; p = 0.003). These differences were not considered clinically significant.
Discussion
This was the first multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and tolerability of duloxetine in older patients with GAD. The results show that patients flexibly dosed with duloxetine 30–120 mg QD versus placebo had significantly reduced anxiety symptoms at 4 weeks, 7 weeks, and at the 10-week endpoint. The lack of separation from placebo at the week 2 visit may be associated with the fact that all duloxetine patients were on the 30 mg dose. It was only after the week 2 visit that duloxetine could be increased to 60 mg. Patients treated with duloxetine also demonstrated superior improvement over placebo-treated patients in each of the disease-specific secondary measures, as well as in global functioning, quality of life, and the patients' impression of feeling better. Duloxetine-treated patients were significantly more likely to meet treatment response and remission criteria and to experience sustained improvement during acute therapy.
The results presented here extend the findings of a post hoc age subgroup analysis of four placebo-controlled trials of duloxetine in patients with GAD. In that analysis, Davidson et al. (2008) reported significance between treatment group improvement in older patients (≥65 years of age) treated with duloxetine (n = 45) versus placebo (n = 28) on the HAM-A total, HAM-A psychic anxiety factor, and HADS anxiety but not the HAM-A somatic anxiety factor. They also reported no significant between-treatment differences in the CGI-I or the Patient Global Impression of Improvement scores, response, or remission rates, which they attributed to the small sample size.
The success of the current study may be attributed in part to initiating duloxetine treatment with the 30 mg dose for 2 weeks. Previous GAD studies started with 30 mg for 1 week (Hartford et al., 2007; Nicolini et al., 2009) or 60 mg with a dose reduction to 30 mg for 2 weeks if 60 mg was not well tolerated initially (Koponen et al., 2007; Rynn et al., 2008). The slower rate of duloxetine dose titration in the current study may have contributed to the higher completion rate and lower discontinuation rates because of adverse events. Completion rates for duloxetine-treated patients in previous GAD studies ranged from 54.3% (Hartford et al., 2007) to 69% (Nicolini et al., 2009), and discontinuation because of adverse events ranged from 11.3% (Koponen et al., 2007) to 20.2% (Rynn et al., 2008). The post hoc age subgroup analysis of these four studies reported a 66.7% completion rate and discontinuation because of an adverse event rate of 22.2% for the older patients treated with duloxetine (Davidson et al., 2008).
In the current study, 60.3% of duloxetine-treated patients experienced TEAE, which was less than that reported in the previous GAD studies that were as high as 83.3% (Rynn et al., 2008). TEAEs that occurred at 5% and twice the rate of placebo in the current study were fewer in number and with less frequency than in the previous GAD studies, and only one TEAE (dry mouth) occurred significantly more frequently in duloxetine-treated patients. Overall, the tolerability and safety findings in this older adult patient population were consistent with previous GAD studies in mostly young adults who were <65 years of age.
There are two other studies of duloxetine in older patients, both of which were for major depressive disorder. One study (Raskin et al., 2007) demonstrated significant improvement in cognition and depressive symptoms with duloxetine 60 mg/day as compared with placebo during 8 weeks of treatment. The other study (Robinson et al., 2014) did not meet the primary outcome of significant improvement in depression as assessed by the Maier subscale (Maier and Philipp, 1985) of the Hamilton Depression Rating Scale (Hamilton, 1960) even though 65.6% of patients treated with duloxetine achieved remission during the 12-week period. Both of these studies had high completion rates with duloxetine treatment (78.3% in Raskin et al., 2007 and 71.9% in Robinson et al., 2014), and rates of discontinuation because of adverse events in the duloxetine arms were relatively low (9.7% and 11.6%, respectively). TEAEs ≥5% and twice the rate of placebo occurring significantly more frequently for duloxetine included dry mouth, nausea, and diarrhea in one study and dry mouth, constipation, and diarrhea in the other study.
A major strength of this study was that patient demographics were comparable with epidemiological studies in elderly GAD, which also reported a greater preponderance of women and a high frequency of comorbid medical conditions (Mackenzie et al., 2011). An additional strength was that both clinician-rated and patient-rated measures of anxiety were used to assess disease severity, and there was good concordance between the outcomes. However, this study was limited by its short duration of 10 weeks, and GAD is a chronic illness, which limits the results regarding long-term efficacy and safety. Because common psychiatric comorbidities, such as major depression, were exclusions in this study, the results may not extend to all patients who are ≥65 years of age. In addition, the flexible-dose design did not allow definitive comparisons of duloxetine doses, because escalation was based on both efficacy and tolerability.
The results from this study demonstrated that duloxetine treatment was efficacious in the improvement of illness severity, functioning, and enjoyment of life for older adult patients with GAD. The safety profile for these older duloxetine-treated patients was consistent with previous GAD studies.
Acknowledgments
Our thanks to the patients who agreed to participate in this clinical trial and the investigators from the following countries: Argentina: S. M. Diamanti, C. M. Lupo, F. D. Medina, G. Noriega, and E. Serfaty; Austria: M. Schmitz, G. Schoenbeck, and A. Whitworth; Canada: L. Beauclair, R. Chandrasena, A. Fallu, P. Latimer, and A. Munshi; Germany: B. Dorn, H. J. Gertz, F. Kuhn, T. Lauter, J. Peltz, and H. P. Volz; Mexico: S. Aguilar, J. B. Corral Garcia, S. Lozano, G. Martinez, and S. Reyes; Poland: L. Bidzan, P. Bogacki, B. Janczewska, M. Perucki, J. Laczkowski, and J. Strzelec; Spain: J. R. Domenech Bisen, and A. Montejo Gonzalez; the UK: M. Blagden, B. Bodalia, and G. Chapman; and the USA: O. Caro, M. DiBuono, C. Ericksen, D. Gruener, A. Jonas, W. Julio, A. Khan, J. McDonough, A. Patel, J. Strawn, H. Thomas, and H. Hafez. This work was supported by Eli Lilly and Company, Indianapolis, IN, USA.
Conflict of interest
None declared.
References
- Allgulander C, Nutt D, Detke M. A non-inferiority comparison of duloxetine and venlafaxine in the treatment of adult patients with generalized anxiety disorder. J Psychopharmacol. 2008;22(4):417–425. doi: 10.1177/0269881108091588. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Hackett D, Salinas E. Venlafaxine extended release (ER) in the treatment of generalised anxiety disorder: twenty-four-week placebo-controlled dose-ranging study. Br J Psychiatry. 2001;179:15–22. doi: 10.1192/bjp.179.1.15. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Florea I, Huusom AK. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. Int J Neuropsychopharmacol. 2006;9(5):495–505. doi: 10.1017/S1461145705005973. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Baldwin DS, Allgulander C, Bandelow B, Ferre F, Pallanti S. An international survey of reported prescribing practice in the treatment of patients with generalised anxiety disorder. World J Biol Psychiatry. 2012;13(7):510–516. doi: 10.3109/15622975.2011.624548. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R. Guidelines for the pharmacological treatment of anxiety disorders, obsessive–compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67(5):489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Davidson JR, Dupont RK, Hedges D, Haskins JT. Efficacy, safety, and tolerability of venlafaxine extended release and buspirone in outpatients with generalized anxiety disorder. J Clin Psychiatry. 1999;60(8):528–535. doi: 10.4088/jcp.v60n0805. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress Anxiety. 2004;19(4):234–240. doi: 10.1002/da.10146. [DOI] [PubMed] [Google Scholar]
- Davidson J, Allgulander C, Pollack MH. Efficacy and tolerability of duloxetine in elderly patients with generalized anxiety disorder: a pooled analysis of four randomized, double-blind, placebo-controlled studies. Hum Psychopharmacol. 2008;23(6):519–526. doi: 10.1002/hup.949. [DOI] [PubMed] [Google Scholar]
- Doyle AC, Pollack MH. Establishment of remission criteria for anxiety disorders. J Clin Psychiatry. 2003;64:40–45. [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Lydiard RB, Rudolph R. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: a 6-month randomized controlled trial. JAMA. 2000;283(23):3082–3088. doi: 10.1001/jama.283.23.3082. [DOI] [PubMed] [Google Scholar]
- Gonçalves DC, Byrne GJ. Interventions for generalized anxiety disorder in older adults: systematic review and meta-analysis. J Anxiety Disord. 2012;26(1):1–11. doi: 10.1016/j.janxdis.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health (NIMH), Psychopharmacology Research Branch; 1976. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford J, Kornstein S, Liebowitz M. Duloxetine as an SNRI treatment for generalized anxiety disorder: results from a placebo and active-controlled trial. Int Clin Psychopharmacol. 2007;22(3):167–174. doi: 10.1097/YIC.0b013e32807fb1b2. [DOI] [PubMed] [Google Scholar]
- Hoffman EJ, Mathew SJ. Anxiety disorders: a comprehensive review of pharmacotherapies. Mt Sinai J Med. 2008;75(3):248–262. doi: 10.1002/msj.20041. [DOI] [PubMed] [Google Scholar]
- Katz IR, Reynolds CF, 3rd, Alexopoulos GS, Hackett D. Venlafaxine ER as a treatment for generalized anxiety disorder in older adults: pooled analysis of five randomized placebo-controlled clinical trials. J Am Geriatric Soc. 2002;50(1):18–25. doi: 10.1046/j.1532-5415.2002.50003.x. [DOI] [PubMed] [Google Scholar]
- Koponen H, Allgulander C, Erickson J. Efficacy of duloxetine for the treatment of generalized anxiety disorder: implications for primary care physicians. Prim Care Companion J Clin Psychiatry. 2007;9(2):100–107. doi: 10.4088/pcc.v09n0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13(1):23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rollman BL, Shear MK. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301(3):295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RS. Differentiating anxiety and depression in anxiety disorders: use of rating scales. Psychopharmacol Bull. 1982;18(4):69–77. [PubMed] [Google Scholar]
- Mackenzie CS, Reynolds K, Chou KL, Pagura J, Sareen J. Prevalence and correlates of generalized anxiety disorder in a national sample of older adults. Am J Geriatr Psychiatry. 2011;19(4):305–315. doi: 10.1097/JGP.0b013e318202bc62. [DOI] [PubMed] [Google Scholar]
- Maier W, Philipp M. Improving the assessment of severity of depressive states: a reduction of the Hamilton Depression Scale. Pharmacopsychiatry. 1985;18:114–115. [Google Scholar]
- Montgomery SA, Tobias K, Zornberg GL, Kasper S, Pande AC. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J Clin Psychiatry. 2006;67(5):771–782. doi: 10.4088/jcp.v67n0511. [DOI] [PubMed] [Google Scholar]
- Nicolini H, Bakish D, Duenas H. Improvement of psychic and somatic symptoms in adult patients with generalized anxiety disorder: examination from a duloxetine, venlafaxine extended-release and placebo-controlled trial. Psychol Med. 2009;39(2):267–276. doi: 10.1017/S0033291708003401. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Zaninelli R, Goddard A. Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62(5):350–357. doi: 10.4088/jcp.v62n0508. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin A, Schulterbrandt J, Reatig N, McKeon JJ. Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis. 1969;148(1):87–98. doi: 10.1097/00005053-196901000-00010. [DOI] [PubMed] [Google Scholar]
- Raskin J, Wiltse CG, Siegal A. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- Rickels K, Pollack MH, Sheehan DV, Haskins JT. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157(6):968–974. doi: 10.1176/appi.ajp.157.6.968. [DOI] [PubMed] [Google Scholar]
- Rickels K, Zaninelli R, McCafferty J. Paroxetine treatment of generalized anxiety disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2003;160(4):749–756. doi: 10.1176/appi.ajp.160.4.749. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Oakes TM, Raskin J. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22(1):34–45. doi: 10.1016/j.jagp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Rynn M, Russell J, Erickson J. Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial. Depress Anxiety. 2008;25(3):182–189. doi: 10.1002/da.20271. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13(4):166–178. [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Stocchi F, Nordera G, Jonkinen RH. Efficacy and tolerability of paroxetine for the long-term treatment of generalized anxiety disorder. J Clin Psychiatry. 2003;64(3):250–258. doi: 10.4088/jcp.v64n0305. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Kessler RC, Beesdo K. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(Suppl 8):24–34. [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


