Abstract
Bacteriophages and genetic elements, such as prophage-like elements, pathogenicity islands, and phage morons, make up a considerable amount of bacterial genomes. Their transfer and subsequent activity within the host's genetic circuitry have had a significant impact on bacterial evolution. In this review, we consider what underlying mechanisms might cause the spontaneous activity of lysogenic phages in single bacterial cells and how the spontaneous induction of prophages can lead to competitive advantages for and influence the lifestyle of bacterial populations or the virulence of pathogenic strains.
INTRODUCTION
The advancement of affordable and publicly available whole-genome sequencing platforms has given rise to sequence data on a wide range of bacterial species. These have revealed that DNA of viral origin represents a highly frequent element of bacterial genomes and can amount to a staggering 20% of the whole bacterial genome (1–3). While some of this DNA content can be accounted for by the presence of fully functional prophages, which are able to undergo a replicative, lytic life cycle, a considerable part of it is made up of prophage-like elements, phage remnants left after incomplete excision events, cryptic prophages, or genetic material acquired by horizontal gene transfer, such as genomic islands and plasmids (4) (Fig. 1). This genetic material can carry genes that influence the virulence of the bacterial host, e.g., cholera toxin (5) and Shiga toxin (6) genes, or the metabolic activities of the host. It is through this inclusion into the genetic circuitry of the microbial host that these elements may have a marked impact on host fitness (7). An important but often unnoted phenomenon is the spontaneous activation of these elements in single cells of bacterial populations even in the absence of an external trigger, a phenomenon dubbed “spontaneous prophage induction” (SPI).
FIG 1.
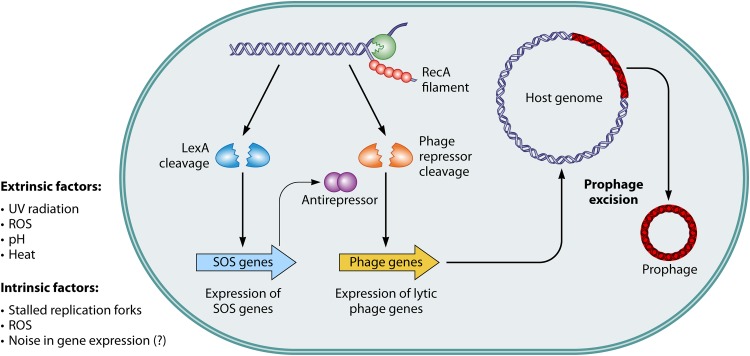
SOS-dependent SPI. A host of extrinsic and intrinsic factors have an influence on the host genome and can lead to spontaneous DNA lesions or stalled polymerases laying bare single-stranded DNA bound by polymerizing RecA proteins. The nucleoprotein filament in turn triggers the autocatalytic cleavage of LexA or CI-like phage repressors. Alleviation of LexA repression leads to the expression of SOS genes—initiating cell growth inhibition and DNA repair. An alternative route is the inactivation of the phage repressor by binding to antirepressor proteins. Inactivation of phage repressors leads to the derepression of the lytic promoters which facilitate the excision of the prophage from the host genome and its packaging into virions and release from the cell by holin- and lysin-mediated cell lysis.
As far back as the 1950s, it was observed that cultures of Bacillus megaterium lysogens exhibit free phages in the cultivation medium supernatant when grown under noninducing conditions (8). This spontaneous induction, often accompanied by lysis of the bacterial cell, was long seen as a potentially detrimental process for bacterial populations, as a small percentage of cells would be lost continuously. However, research in the fields of bacterial population dynamics, biofilm formation, and pathogenesis of human diseases has shed new light on the spontaneous induction of prophages.
While it is possible that SPI is merely the result of stochasticity in gene expression (genetic noise) (9) or results from a bona fide induction of the host SOS response (10), only a few studies have so far focused on the exact mechanisms underlying this phenomenon. The work performed in these studies was further supported by the advancement of single-cell analytics such as flow cytometry and time-lapse microscopy in microfluidic cultivation chambers, allowing the analysis of triggers for bacteriophages and mobile genetic elements working in a subset of bacterial populations (11–15). In this review, we set the focus on the triggering of spontaneous activity of prophages or phage remnants and the physiological consequences that this process can have for microbial populations. In the following, we use the term “spontaneous prophage induction” (SPI) not only to describe the intrinsic stability of lysogens (e.g., in a recA mutant background) but also for events specifically triggered in a subset of the population, e.g., during host infection or biofilm formation.
SOS-INDUCED SPI
Because an induced SOS response is responsible for the induction of many lambdoid lysogens, it was discussed by several researchers that spontaneous SOS induction in single cells might trigger the induction of prophages (10). During growth, ongoing (multifork) replication has been shown to cause sporadic DNA damage resulting in the derepression of the SOS genes (16). Recent single-cell studies revealed a small fraction of SOS-induced cells in clonal populations grown under standard conditions, and it is reasonable to infer that a prolonged induction will also lead to the activation of resident prophages (13, 17, 18).
Lwoff first described free phage appearing in the supernatant of noninduced cultures of lysogenic bacteria (8), and it was later shown that recombination-deficient recA mutant strains of Escherichia coli showed no discernible spontaneous induction of the prophage (19–21). Different parameters were shown to influence the spontaneous induction of phage λ expression in E. coli, such as overexpression of CI and deletion of recA, thus decreasing the growth rate, or exchanging glucose with glycerol, thus affecting cyclic AMP (cAMP) levels, which have an influence on the SOS response as well (22). In their recent study, Little and Michalowski found that the intrinsic switching rate of E. coli lambda lysogens is almost undetectably low (<10−8/generation) in a recA mutant background (21). Remarkably, the intrinsic stability very much depends on the cultivation conditions as well as on the occurrence of mutations which render the lysogen unstable. These findings emphasize that SPI has coevolved with its specific trigger (e.g., the SOS response) to optimize the switching frequency over a wide range of naturally occurring inducing conditions.
Recently, it was shown that single cells of Corynebacterium glutamicum undergo spontaneous SOS induction under standard cultivation conditions (13). This spontaneous induction of the SOS response is sufficient to induce key promoters within prophage CGP3, among them the genes encoding a putative integrase and lysin (13). Mutant strains lacking recA showed only a basal level of spontaneous CGP3 induction, which might be due to stochastic effects (see below) or mutations of the prophage. That stochasticity might play a rather minor role is also emphasized by a study by Livny and Friedman, who could show that SPI of the easily inducible H-19B prophage in almost all cases coincided with the induction of a second prophage in the same cells of Shiga toxin-producing E. coli (STEC) lysogens (23).
Another layer of control is added by RecA-independent and yet SOS-dependent SPI. In the case of coliphage 186, the viral Tum protein functions as an antirepressor which binds to the phage repressor, resulting in the induction of lytic growth (24, 25). Expression of tum itself is under the control of host LexA, and phage induction is thereby again linked to the SOS response system of the bacteria (Fig. 1). Similar mechanisms were also described for the N15 linear plasmid-prophage of E. coli and the Fels-2 Salmonella enterica phage (26, 27). Another interesting example is the induction of the CTX prophage of Vibrio cholerae, which encodes cholera toxin. Here, expression of the genes required for virion production is directly regulated by host LexA, which binds just upstream of the RstR phage-encoded repressor (28, 29). Repressor inactivation can also be achieved by the transcriptional regulator of the cps gene cluster RcsA or by the small RNA DsrA, which relieves repression of rcsA (30).
EXTRINSIC FACTORS
In addition to the intrinsic factors which affect genomic DNA or RecA and induce the SOS response, extrinsic factors, such as reactive oxygen species (ROS) generated within macrophages (31–33) and UV radiation, both of which induce DNA damage, or the effects of antibiotics such as mitomycin C (MmC) (34) and fluoroquinolones (Fq) (35), should be taken into account as well. Fqs and MmC are both able to induce pneumococcal prophages in multiple strains of Streptococcus pneumoniae (36). In this bacterium, induction acts via RecA and yet does so in an SOS-independent manner and represents a response to the topoisomerase IV-Fq complex formed or to transcriptional regulation of phage or bacterial genes by DNA supercoiling, which is a result of Fq treatment (36). Further factors which influence the lysogenic maintenance or induction of the prophage should be taken into account as well. The effects of pH, temperature, organic carbon, and the presence of chromium (VI) and the toxic compound potassium cyanide on prophage induction were studied in the ammonia-oxidizing Nitrospira multiformis bacterium. Whereas increasing levels of Cr (VI) and KCN led to an increase in prophage induction, a shift toward acidic pH had the most dramatic effect (37). The neutralophile Helicobacter pylori B45, which inhabits the human gastric mucosa, was shown to induce its phiHP33 bacteriophage after UV irradiation and at a low pH (38, 39). The opposite relationship was observed for prophage ϕLC3 of Lactococcus lactis, where a decrease in pH reduced the amount of spontaneously induced prophage (40). In their multifactorial experimental setup, the authors revealed that an increase in the levels of simultaneously acting stressors increased SPI. It is tempting to speculate that RecA and the SOS response are responsible for the prophage induction dynamics in this system, because the repressor of L. lactis prophage ϕLC3 belongs to the CI-like repressor family. Indeed, earlier studies showed that changes in intracellular pH influence the stability and binding properties of LexA or CI repressor and thus influence the transcription of target genes (41, 42).
IMPACT OF STOCHASTICITY IN GENE EXPRESSION
Gene expression can be surprisingly dynamic and heterogeneous. Cell-to-cell variation, even in clonal populations of cells grown under the same conditions, has been observed in a variety of different organisms, from mammalian stem cells to bacteria (9, 43). Hence, a plausible mechanism for SPI is that it could result from spontaneous fluctuations in the levels of repressor such that, below a threshold level, the lytic genes would be expressed, leading to switching to the lytic state. This model does not apply in the best-studied case, phage lambda, since SPI requires RecA; in addition, phage mutants with noncleavable repressors do not show SPI, despite the likelihood that expression levels of these mutant proteins would fluctuate in the same way as in the wild type. In contrast, the unstable mutant described by Little and Michalowski likely switches by this mechanism (21). Other examples of switching also appear to result from stochastic fluctuations in gene expression. Spontaneous excision of the mobile element ICEclc (integrative and conjugative element encoding clc genes) was, for example, shown from the genome of Pseudomonas knackmussii B13 (12, 44). Integrative and conjugative elements reside in the host genome and are able to excise and be transferred via conjugation. The excision of ICEclc depends on variations in the levels of the RpoS stationary-phase sigma factor among individual cells. RpoS levels reach a threshold at which ICEclc-encoded excision factors are expressed, and this state is locked in these single cells, thus maintaining ICEclc excision in the form of a bistable state (12). In their recent study on several mycobacteriophages, which are not SOS inducible, Broussard et al. described a novel class of simple switches relying on the site-specific recombination catalyzed by integrases as a key to decision (45). They also observed spontaneous switching from lysogeny to the lytic state and speculated that this might be due to a drop in the repressor level below the threshold or to sporadic expression of the integrase gene (45).
Noise in gene expression is ubiquitous. It can provide a selective advantage by increasing phenotypic heterogeneity within a clonal population of one species or even within microbial communities (e.g., biofilms). It is therefore conceivable that several mobile genetic elements, such as ICEclc, pathogenicity islands (PIs), and prophages, exploit noise as a function to modulate the frequency of their spontaneous activation and transfer (Fig. 1).
IMPACT OF LYSOGENIC PROPHAGES ON THE FITNESS OF BACTERIAL POPULATIONS
In their recent review, Bondy-Denomy and Davidson summarized the general positive impact that prophages can have on a population's fitness (46). For instance, this positive impact was impressively demonstrated by the work of Wang et al., who deleted nine cryptic prophages in E. coli K-12 (47). These cryptic prophages are unable to propagate into infectious virus particles, and it was assumed that functions which assist their host to propagate in adverse environments were retained. The study revealed not only a decreased growth rate in the prophage-cured strain but also that it was more sensitive to antibiotics, was impaired in adaptation to osmotic stress, and showed a decrease in biofilm formation (see the next section for more details on biofilm formation). Interestingly, seven of the nine deleted prophages were shown to excise spontaneously (47). As we go on to show, this spontaneous activity of prophage elements can have a high impact on the general fitness of bacterial populations under diverse conditions.
SPI can also promote the spread of phages and increased survival of lysogens when they are grown in mixed populations. Salmonella enterica serovar Typhimurium harbors four to five full-size prophages, with most being able to undergo lytic development (48–50). When clonal populations of strains carrying these prophages were cultivated, spontaneous prophage induction had no apparent consequences for the population due to its immunity to superinfection (51). However, when prophage-carrying strains and those cured of the prophages of the same origin (52) or of different origins (51) were cocultivated, a “selection regime” that forced maintenance and spread of viral DNA was set, with a portion of bacteria being killed due to lytic development of prophages and survivors undergoing lysogenic conversion. One may think of this process as lysogenic bacteria using spontaneous lytic development of their prophages as a weapon, giving them a competitive advantage against nonlysogenized cells. At the same time, the prophage uses this war to its own advantage, with the ultimate goal of spreading its DNA by lysogenic conversion of the nonlysogens (52).
The presence of inducible prophage elements can also be a selective disadvantage, as shown for Staphylococcus aureus in the nasopharynx. It is displaced by its relative Streptococcus pneumoniae, which generates H2O2 and is thus responsible for the formation of hyperoxides via the Fenton reaction. S. pneumoniae itself, however, is resistant to them (53). RecA-inducible prophages of S. aureus are induced, leading to cell death (54) and, ultimately, displacement of the strain. Thus, despite its positive effect, the presence of lysogenic bacteriophages may add a potential Achilles heel with respect to the fitness of the bacteria which is exploited by bacterial competitors and eventually might even be exploited by the human host.
PHAGES AFFECT MICROBIAL BIOFILM FORMATION
In nature, the vast majority of bacteria is thought to exist in surface-associated communities, commonly referred to as biofilms (55). In biofilms, the cells are encased in a matrix mainly consisting of various extracellular polymeric substances (EPS). The most prominent hallmark of cells growing in such communities is their increased tolerance of a wide range of environmental perturbations, including antibacterial and antibiotic treatments. Biofilm formation varies widely between different species but is often described as a developmental process (56). Phage-induced lysis within biofilms generally leads to an accumulation of extracellular DNA (eDNA) within the community. In concert with the high cell densities commonly occurring in biofilms, this provides a huge pool for horizontal gene transfer (reviewed in references 57 and 58).
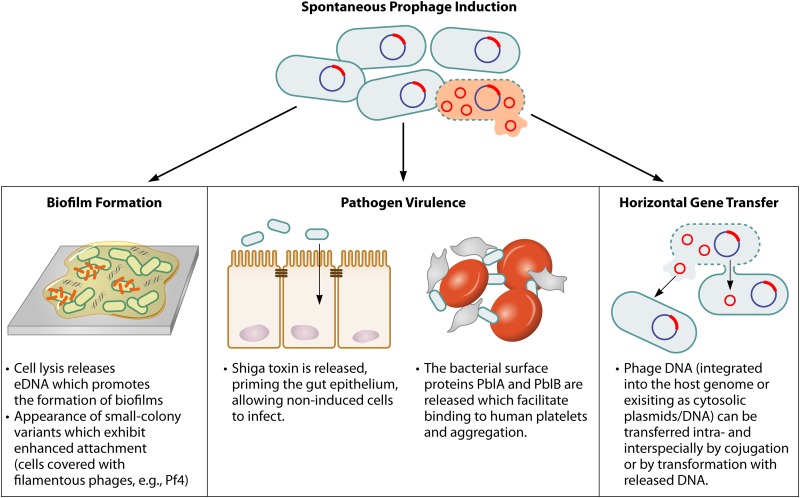
An increasing number of studies on several bacterial species have provided evidence that (pro)phages also directly affect the progression of biofilm formation through all stages (Fig. 2). The presence of the phages can lead to biofilm dispersal resulting from cell lysis while at the same time providing the enzymes needed to degrade the extracellular matrix. On the other hand, lysis of a cellular subpopulation may provide biofilm-promoting factors such as matrix components. And finally, phages drive the diversification of the biofilm community, a major factor responsible for the overall fitness of bacterial communities (59).
FIG 2.
Impact of SPI on host physiology. Spontaneous induction of lysogenic prophages can have manifold effects, such as enhancing biofilm formation, playing a vital role in bacterial virulence or leading to horizontal gene transfer of its own DNA (including virulence factors) and of host genes.
All three aspects of bacterium-phage interaction in biofilm formation could be identified in P. aeruginosa PAO1. When grown in flow cells (hydrodynamic growth conditions), this strain undergoes an intricate biofilm developmental cycle which is strongly affected by the Pf4 filamentous bacteriophage (60). During the initial steps of biofilm formation, Pf4 activity is strongly controlled by production of the PhdA “prevent-host-death” factor encoded by the host (61). However, activation of the Pf4 prophage in later stages of biofilm formation (62, 63), likely due to elevated ROS levels, results in formation of a hyperinfective form of Pf4 and extensive cell lysis within the three-dimensional structures, leading to dispersal of the biofilm (62, 63). In contrast, complete loss of the prophage or overproduction of the PhdA prevent-host-death factor leads to decreased cell lysis and, notably, less-stable biofilms, a result which was attributed to the lack of eDNA as an important structural component (60, 61). Thus, it was proposed that controlled Pf4-mediated cell lysis is required for the release of biofilm-promoting factors, such as eDNA, in P. aeruginosa biofilm formation (64, 65). Activity of Pf4 phages coincided with formation of small-colony variants of P. aeruginosa PAO1, which were characterized by increased biofilm formation capacities (66). In addition, loss of the phage significantly decreased the virulence of P. aeruginosa PAO1 in mice, which has been attributed to phage-induced strain variations (60). This example nicely illustrates how the components of phage-host interactions are intimately linked and have evolved to benefit microbial group behaviors.
This is further exemplified in E. coli, in which the CP4-57, DLP12, e14, and rac cryptic prophages affect the biofilm formation of their host (47, 67). Presumably, under conditions of nutrient limitation and the presence of uncharged tRNAs, the Hha regulator activates lytic prophage genes in CP4-57 and DLP12, resulting in cell death and dispersal (68). Notably, excision of CP4-57 and its subsequent loss from the chromosome predominantly occurred in biofilm cells. These cells exhibit enhanced flagellum-mediated motility and decreased nutrient metabolism, thereby broadening the diversity of the population (67).
The diverse roles phages might play during biofilm formation are reflected in several further studies. Propagation of biofilm formation through phage-mediated lysis and eDNA release has been proposed for two other bacterial species, Streptococcus pneumoniae and Shewanella oneidensis MR-1. Spontaneous SV1-mediated cell lysis was shown to occur in S. pneumoniae, and, accordingly, deletion of the SV1 phage lysin led to a decrease in cell lysis, eDNA release, and biofilm formation (69). In S. oneidensis, three prophages, MuSo1, MuSo2, and LambdaSo, jointly affect lysis and eDNA release, and consequently, a mutant devoid of all three phages is severely impaired in all stages of biofilm formation (70). Recent studies on this species have demonstrated that environmental iron levels are an important factor involved in inducing expression of LambdaSo in a RecA-dependent fashion during biofilm formation (71). Another highly interesting example for host-phage interactions is the lysogenic infection of P. aeruginosa PA14 with bacteriophage DMS3. Lysogeny results in decreased biofilm formation and bacterial swarming. Notably, this effect is dependent on the clustered regularly interspaced short palindromic repeat (CRISPR)-CAS system of the host (72).
These examples illustrate the complex interaction between (pro)phages and their hosts with respect to group behaviors such as biofilm formation. Given the fact that prophage genes are commonly among the highly regulated genes during biofilm formation and that many potential cell surface factors are encoded by prophages, it is likely that we have as yet seen only the tip of the iceberg.
SPONTANEOUS PROPHAGE ACTIVITY PROMOTES HOST VIRULENCE
A major reason we are interested in bacteria is their impact on humans. Early in the last century, d'Hérelle discovered that it is not only the relationship between humans and bacteria that is of importance but rather that bacteriophages need to be included as well (73). This seems plausible, especially when changing focus to the emergence of virulence in pathogenic bacterial strains. The formation of biofilms, which we have just covered, is in itself already an important factor for pathogen virulence. In many bacterial infections, the first step is attachment and adhesion to the right host cells (74).
Once they are established within the host, the release of extracellular toxins is a hallmark of several pathogens. For example, Shiga toxin-producing E. coli (STEC) bacteria release Shiga toxins in the gut. The life cycle of STEC bacteria and their Stx-encoding prophages exemplifies how the spontaneous induction of prophages in a small number of cells is important for pathogenic traits (Fig. 2). The colonization of the gastrointestinal tract of ruminants was assumed to rely on the spontaneous induction of a subset of STEC serovar O157:H7 cells (75). It was proposed that the presence of a small fraction of induced STEC cells would lead to a sufficient release of Shiga toxin and thus to diarrhea, enabling the spread of the remaining uninduced lysogenic cells (23). Indeed, a basal level of spontaneous induction could be shown for Stx-encoding prophage H-19B of STEC serovar O26:H19 (23). Remarkably, the level of SPI was significantly higher than the level of induction of λ in the same strain background, suggesting that this higher frequency of SPI coevolved to meet the requirements for toxin release of the pathogenic host strain.
In Shiga toxin-producing E. coli (STEC) bacteria, the spontaneous induction of prophages increases the fitness of the entire bacterial population within the host environment by priming the host's epithelial cells for infection (23, 75, 76). In the proposed model, a small fraction of cells induces the Stx-encoding prophages, leading to Shiga toxin release and, in consequence, priming the gut epithelial cells by the expression and relocation of nucleolin and further receptors to the epithelial cell surface. These receptors were previously shown to bind to the bacterial surface protein intimin, thus promoting STEC colonization (77–79). In these bacteria, expression of a type 3 secretion system (T3S), which is required for colonization, was shown to be influenced by the presence of Stx-encoding prophages. Reconstitution in an E. coli K-12 background provided evidence for a negative impact of lysogeny (in particular, that associated with cII) on expression of the T3S (reduction by about 2-fold). However, the effects described in this study were rather small and their actual impact on host-microbe interaction remains to be elucidated.
Evidence for the role of SPI in other human diseases is only beginning to emerge. One example is seen in infective endocarditis, which is an inflammation of the inner lining of the heart. It is often caused by Streptococcus mitis. In this organism, the surface expression of øSM1 prophage-encoded PblA and PblB is dependent on the presence of holin and lysin, which are expressed in the course of prophage induction (80). Upon permeabilization and lysis of the bacterial host cell, PblA and PblB are released into the culture medium, bind choline within the bacterial cell wall of unlysed cells, and mediate platelet binding and aggregation (81) (Fig. 2; deletion of pblA and pblB leads to a 40% reduction in binding). The bacterial lysin itself also plays a role in platelet binding. Through its interaction with choline, it becomes cell wall associated and can directly bind platelet fibrinogen (82). Enterococcus faecalis is a leading cause of hospital-acquired bacterial infections and may cause urinary tract infections, intra-abdominal infections, and infective endocarditis (83). E. faecalis strain V583 is polylysogenic for 7 prophage-like elements (termed V583-pp1 to V583-pp7) (Table 1). It was shown that three of these, pp1, pp4, and pp6, are important for adhesion to human platelets, which is possibly achieved by expression of the genes encoding PblA and PblB which are homologous to those of S. mitis (84). Thus, these examples nicely reveal that SPI triggered lysis of a small fraction of cells, possibly promoting the adhesion of the remaining noninduced part of the population.
TABLE 1.
Spontaneously induced genetic elements discussed in this review
| Species | Prophage(s) | Impact on physiology | Reference(s) | Classification | Induction |
|---|---|---|---|---|---|
| Corynebacterium glutamicum | CGP3 | The cryptic prophage CGP3 excises and replicates in a small fraction of wild-type populations; deletion of CGP3 leads to an increase in plasmid copy no. and heterologous protein production and increased transformation efficiency | 13, 99, 100 | Prophage | MmC, UV |
| Enterococcus faecalis | pp1, pp4, pp6 | Prophages promote platelet binding, encode PblA and PblB homologs from S. mitis | 84 | Prophage | pp1, pp4: MmC |
| Escherichia coli | Δ9 cryptic prophages | Deletion of all 9 cryptic prophages leads to a decreased growth rate, increased sensitivity to antibiotics, impaired adaptation to osmotic stress, and a decrease in biofilm formation; excision of CP4-57 in biofilms increases flagellum-mediated motility and decreases nutrient metabolism | 47, 67 | Cryptic prophages | e14 induced by MmC |
| Helicobacter pylori | phiHP33 | Bacteriophage phiHP33 expression is induced at a low level after exposure to UV treatment or acidic pH | 38 | Prophage | UV radiation and acidic environment |
| Lactococcus lactis | ϕLC3 | Nutrient availability, temp, pH, and osmolarity influence the induction of the prophage, either stabilizing the lysogenic state or inducing spontaneous induction dramatically | 40 | Prophage | Excised by different environmental conditions |
| Shiga toxin-producing Escherichia coli | H19-B, 933W | Induction of Stx-producing prophages leads to expression of stx genes and release of Shiga toxin into the surrounding area; Shiga toxin primes the gut epithelium for infection of the noninduced by expression of a type 3 secretion system | 23 | Prophage | UV, MmC, pressure |
| Pseudomonas aeruginosa PAO1 | Pf4 | The presence and activation of Pf4 at later stages are required for proper biofilm formation and survival in host systems; conversion to a hyperinfectious form enables biofilm dispersal and leads to small-colony variants with increased biofilm formation ability | 60–63, 66 | Prophage | |
| Pseudomonas aeruginosa PA14 | DMS3 | The prophage decreases biofilm formation and swarming in concert with the host's CRISPR-CAS system | 72 | Prophage | |
| Salmonella enterica | P22, SE1, Gifsy-1, Gifsy-2, Gifsy-3, Fels-1 | The prophages of S. enterica influence one another; infection with P22/SE1 leads to induction of the SOS response, which in turn activates antirepressors of the Gifsy-1 and Gifsy-3 repressors; the Gifsy-2 repressor, in turn, is inactivated by the antirepressor of Fels-1 prophage, a CI-like repressor | 95 | Prophage | Gifsy-1, Gifsy-2, Gifsy-3, Fels-1, P22: MmC |
| Shewanella oneidensis MR-1 | MuSo1, MuSo2, LamdaSO | The prophages jointly enhance biofilm formation through release of biofilm-promoting factors such as eDNA | 70 | Prophage | UV radiation |
| Staphylococcus aureus | 80α, ϕ11 | Induction of prophages by induction of the SOS response leads to cell lysis | 54 | Prophage | MmC, ciprofloxacin |
| Staphylococcus aureus | SaPIbov1, SaPIbov2 | Virulence factors encoded on pathogenicity islands SaPIbov1 and SaPIbov2 are transferred by horizontal gene transfer, playing a role in acquisition of novel virulence factors | 89, 90 | Pathogenicity island | Spontaneous induction by Sip integrase; horizontal dissemination stimulated by SOS-induced prophages 80α, ϕ11, and ϕ147 |
| Streptococcus mitis | øSM1 | Proteins PblA and PblB are released by cell lysis; they bind to choline in the bacterial cell wall and promote binding to human platelets and aggregation | 81 | Prophage | NDa |
| Streptococcus pneumoniae | SV1 | The prophage enhances biofilm formation through release of biofilm-promoting factors such as eDNA | 69 | Prophage | MmC |
ND, not determined.
Besides having a direct effect on bacterial virulence, the role that spontaneous prophage activity plays in horizontal gene transfer of virulence-associated factors is of great concern (85, 86). S. aureus pathogenicity islands encoding toxins (SaPI) are highly mobile and packaged into small infectious particles (87, 88). SaPIbov1 (89) and SaPIbov2 (90) are two SaPIs which contain the tst gene encoding the toxic shock syndrome toxin (TSST) and the bap (biofilm-associated protein) gene (91), which is needed for persistence in mammary tissue (90), respectively. Once excised, both PIs are transferred due to the action of resident prophages. It was shown for both PIs that they spontaneously excise from S. aureus genomes. Consequently, it was postulated that, due to the spontaneous induction of resident prophages in combination with the low-level activation of SaPIs, packaging into transducible phage particles is ensured and represents a serious case of horizontal gene transfer in habitats colonized by staphylococci (92).
PHAGE-PHAGE INTERACTION
Besides the effect that phages have on their bacterial host or on human physiology, phages can also influence one another. For STEC cells, it was shown that repressor proteins of different Stx-encoding prophages mutually influence their spontaneous induction, in turn influencing the stability of the lysogenic state and, ultimately, the virulence due to Stx production (93). Another example is found in Salmonella species. Infection of Salmonella enterica with P22 or SE1 leads to a kil-dependent induction of the SOS response (94). This in turn activates the LexA-controlled antirepressors which bind the Gifsy phage repressors (Fig. 1) (95). The phage repressor of Gifsy-2, however, is insensitive to the antirepressors of Gifsy-1 and Gifsy-3. Rather, it is inactivated by the antirepressor of the Fels-1 prophage. This Fels-1 antirepressor is neither regulated by LexA nor involved in Fels-1 induction at all (Fels-1 induction relies on a λ CI-like repressor cleavage mechanism). Thus, the ability of prophages to influence the stability of other prophages and the properties of antirepressors with respect to recognizing noncognate substrates allow multilayered induction regulation in polylysogenic strains.
CONCLUSION
More and more studies are indicating that the spontaneous activity of prophages or prophage-like elements has a marked impact on their bacterial host and even on the host the bacteria live in. Looking back on the advances that have been made since the turn of the century, it is fascinating to think about the developments that are going to be achieved in the upcoming years. Key to research questions centering on the phenotypic heterogeneity of populations is the advancement of the single-cell analytic platforms which have become common in modern microbiology and which will see an even broader use in the upcoming years (11, 14, 96, 97). Recent studies have shown the power of flow cytometry coupled with the use of fluorescent reporters and live-cell imaging enabled by microfluidic devices in elucidating mechanisms governing the activity of foreign DNA within bacterial genomes (12, 13). Combined with classical molecular biology approaches, these recent advances in single-cell analytics shed new light on the dynamics of microbial populations and host-microbe interaction.
Spontaneous prophage activity affects the formation of biofilms and the virulence of human pathogens. It will be through studies on the evolution of prophages and bacteria and the biology underlying distinct activities, such as spontaneous excision or induced cell death, that we gain the understanding to utilize the weapons used in the ongoing war between phages and bacteria to our advantage in the form of phage therapy (98).
ACKNOWLEDGMENTS
Our work is supported by the Deutsche Forschungsgemeinschaft (SPP 1617 grant FR 2759/2-1) and by the Helholtz Association (Young Investigator grant VH-NG-716).
Especially, we thank reviewer 3 of the Journal of Bacteriology for critical reading of the manuscript and for many helpful suggestions.
Biographies

Arun M. Nanda (Dipl. Biol.) studied biology at the Heinrich Heine University in Düsseldorf, Germany. After electrophysiological characterization of a plant MCF protein, he joined the group of Prof. Andreas Weber and performed genetical and biochemical work in Arabidopsis thaliana and studied the C4 syndrome in Cleome sp. He performed his diploma thesis work at the Department of Plant Biochemistry under the supervision of Andreas P. M. Weber. After his foray into botany, he joined the research group of Juniorprof. Dr. Julia Frunzke at the Forschungszentrum Jülich and started his work on the spontaneous SOS response and prophage excision in Corynebacterium glutamicum. Currently, he is working for a pharmaceutical company and is writing his Ph.D. thesis.

Kai Thormann studied biology in Göttingen, Germany, and received his Ph.D. in microbiology from the University of Ulm, Ulm, Germany. After postdoctoral research at Stanford University at the Department of Civil and Environmental Engineering, he went back to Germany, where he became group leader at the University of Bochum in the Department of Microbiology. He then moved as a Research Group Leader to the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany. Since 2013, he has held the position of a full professor at the Justus Liebig University of Giessen, Giessen, Germany. Since 2005, his group has been interested mainly in adaptation processes in bacterial motility and biofilm formation, using species of the genus Shewanella as model organisms. His interest in prophage induction started when it became apparent that prophages are a major factor in S. oneidensis biofilm formation through eDNA release.

Julia Frunzke studied biology at the Philipps University of Marburg and performed her diploma thesis at the Max Planck Institute of Terrestrial Microbiology. In 2004, she joined the group of Prof. M. Bott at the Forschungszentrum Jülich for her Ph.D. work. After her postdoctoral work in the laboratory of Prof. Julia Vorholt at the ETH Zurich, she came back to Jülich as a junior group leader. Since 2011, she has been head of the Helmholtz Young Investigator group “Population heterogeneity of industrial microorganisms” and has been associated with the University of Düsseldorf (since 2013) as a Juniorprofessor (W1). During her Ph.D. research, she observed the spontaneous activation of prophage elements in Corynebacterium glutamicum and became interested in the impact of SPI on the physiology of bacterial populations.
REFERENCES
- 1.Canchaya C, Fournous G, Brüssow H. 2004. The impact of prophages on bacterial chromosomes. Mol Microbiol 53:9–18. doi: 10.1111/j.1365-2958.2004.04113.x. [DOI] [PubMed] [Google Scholar]
- 2.Casjens S. 2003. Prophages and bacterial genomics: What have we learned so far? Mol Microbiol 49:277–300. doi: 10.1046/j.1365-2958.2003.03580.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatfull GF, Hendrix RW. 2011. Bacteriophages and their genomes. Curr Opin Virol 1:298–303. doi: 10.1016/j.coviro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrindt U, Hochhut B, Hentschel U, Hacker J. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol 2:414–424. doi: 10.1038/nrmicro884. [DOI] [PubMed] [Google Scholar]
- 5.Waldor MK, Mekalanos JJ. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 6.Neely MN, Friedman DI. 1998. Arrangement and functional identification of genes in the regulatory region of lambdoid phage H-19B, a carrier of a Shiga-like toxin. Gene 223:105–113. doi: 10.1016/S0378-1119(98)00236-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown NF, Wickham ME, Coombes BK, Finlay BB. 2006. Crossing the line: selection and evolution of virulence traits. PLoS Pathog 2:e42. doi: 10.1371/journal.ppat.0020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lwoff A. 1953. Lysogeny. Bacteriol Rev 17:269–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elowitz MB, Levine AJ, Siggia ED, Swain PS. 2002. Stochastic gene expression in a single cell. Science 297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 10.Little JW. 1990. Chance phenotypic variation. Trends Biochem Sci 15:138. [DOI] [PubMed] [Google Scholar]
- 11.Locke JCW, Elowitz MB. 2009. Using movies to analyse gene circuit dynamics in single cells. Nat Rev Microbiol 7:383–392. doi: 10.1038/nrmicro2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki R, Minoia M, Pradervand N, Sulser S, Reinhard F, van der Meer JR. 2012. Cellular variability of RpoS expression underlies subpopulation activation of an integrative and conjugative element. PLoS Genet 8:e1002818. doi: 10.1371/journal.pgen.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanda AM, Heyer A, Krämer C, Grünberger A, Kohlheyer D, Frunzke J. 25 October 2013. Analysis of SOS-induced spontaneous prophage induction in Corynebacterium glutamicum at the single-cell level. J Bacteriol doi: 10.1128/JB.01018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nebe-von-Caron G, Stephens PJ, Hewitt CJ, Powell JR, Badley RA. 2000. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J Microbiol Methods 42:97–114. doi: 10.1016/S0167-7012(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro HM. 2000. Microbial analysis at the single-cell level: tasks and techniques. J Microbiol Methods 42:3–16. doi: 10.1016/S0167-7012(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 16.Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. 2000. The importance of repairing stalled replication forks. Nature 404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 17.McCool JD, Long E, Petrosino JF, Sandler HA, Rosenberg SM, Sandler SJ. 2004. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol Microbiol 53:1343–1357. doi: 10.1111/j.1365-2958.2004.04225.x. [DOI] [PubMed] [Google Scholar]
- 18.Pennington JM, Rosenberg SM. 2007. Spontaneous DNA breakage in single living Escherichia coli cells. Nat Genet 39:797–802. doi: 10.1038/ng2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks K, Clark AJ. 1967. Behavior of λ bacteriophage in a recombination deficient strain of Escherichia coli. J Virol 1:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertman I, Luria SE. 1967. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol 23:117–133. doi: 10.1016/S0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- 21.Little JW, Michalowski CB. 2010. Stability and instability in the lysogenic state of phage lambda. J Bacteriol 192:6064–6076. doi: 10.1128/JB.00726-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czyz A, Los M, Wrobel B, Wegrzyn G. 2001. Inhibition of spontaneous induction of lambdoid prophages in Escherichia coli cultures: simple procedures with possible biotechnological applications. BMC Biotechnol 1:1. doi: 10.1186/1472-6750-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livny J, Friedman DI. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol Microbiol 51:1691–1704. doi: 10.1111/j.1365-2958.2003.03934.x. [DOI] [PubMed] [Google Scholar]
- 24.Lamont I, Brumby AM, Egan JB. 1989. UV induction of coliphage 186: prophage induction as an SOS function. Proc Natl Acad Sci U S A 86:5492–5496. doi: 10.1073/pnas.86.14.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shearwin KE, Brumby AM, Egan JB. 1998. The Tum protein of coliphage 186 is an antirepressor. J Biol Chem 273:5708–5715. doi: 10.1074/jbc.273.10.5708. [DOI] [PubMed] [Google Scholar]
- 26.Bunny K, Liu J, Roth J. 2002. Phenotypes of lexA mutations in Salmonella enterica: evidence for a lethal lexA null phenotype due to the Fels-2 prophage. J Bacteriol 184:6235–6249. doi: 10.1128/JB.184.22.6235-6249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mardanov AV, Ravin NV. 2007. The antirepressor needed for induction of linear plasmid-prophage N15 belongs to the SOS regulon. J Bacteriol 189:6333–6338. doi: 10.1128/JB.00599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinones M, Kimsey HH, Waldor MK. 2005. LexA cleavage is required for CTX prophage induction. Mol Cell 17:291–300. doi: 10.1016/j.molcel.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Waldor MK, Friedman DI. 2005. Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol 8:459–465. doi: 10.1016/j.mib.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Rozanov DV, D'Ari R, Sineoky SP. 1998. RecA-independent pathways of lambdoid prophage induction in Escherichia coli. J Bacteriol 180:6306–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueroa-Bossi N, Bossi L. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol Microbiol 33:167–176. doi: 10.1046/j.1365-2958.1999.01461.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee K-M, Park Y, Bari W, Yoon MY, Go J, Kim SC, Lee H-I, Yoon SS. 2012. Activation of cholera toxin production by anaerobic respiration of trimethylamine N-oxide in Vibrio cholerae. J Biol Chem 287:39742–29752. doi: 10.1074/jbc.M112.394932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner PL, Neely MN, Zhang X, Acheson DWK, Waldor MK, Friedman DI. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J Bacteriol 183:2081–2085. doi: 10.1128/JB.183.6.2081-2085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasz M. 1995. Mitomycin C: small, fast and deadly (but very selective). Chem Biol 2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 35.Piddock LJV, Wise R. 1987. Induction of the SOS response in Escherichia coli by 4-quinolone antimicrobial agents. FEMS Microbiol Lett 41:289–294. doi: 10.1111/j.1574-6968.1987.tb02213.x. [DOI] [Google Scholar]
- 36.López E, Domenech A, Ferrándiz MJ, Frias MJ, Ardanuy C, Ramirez M, García E, Liñares J, de la Campa AG. 2014. Induction of prophages by fluoroquinolones in Streptococcus pneumoniae: implications for emergence of resistance in genetically-related clones. PLoS One 9:e94358. doi: 10.1371/journal.pone.0094358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, Kotay SM, Goel R. 2010. Various physico-chemical stress factors cause prophage induction in Nitrosospira multiformis 25196–an ammonia oxidizing bacteria. Water Res 44:4550–4558. doi: 10.1016/j.watres.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 38.Alves de Matos AP, Lehours P, Timóteo A, Roxo-Rosa M, Vale FF. 2013. Comparison of induction of B45 Helicobacter pylori prophage by acid and UV radiation. Microsc Microanal 19:27–28. doi: 10.1017/S1431927613000755. [DOI] [Google Scholar]
- 39.Lehours P, Vale FF, Bjursell MK, Melefors O, Advani R, Glavas S, Guegueniat J, Gontier E, Lacomme S, Alves Matos A, Menard A, Megraud F, Engstrand L, Andersson AF. 2011. Genome sequencing reveals a phage in Helicobacter pylori. mBio 2:e00239-11. doi: 10.1128/mBio.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunde M, Aastveit AH, Blatny JM, Nes IF. 2005. Effects of diverse environmental conditions on {phi}LC3 prophage stability in Lactococcus lactis. Appl Environ Microbiol 71:721–727. doi: 10.1128/AEM.71.2.721-727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dri AM, Moreau PL. 1994. Control of the LexA regulon by pH: evidence for a reversible inactivation of the LexA repressor during the growth cycle of Escherichia coli. Mol Microbiol 12:621–629. doi: 10.1111/j.1365-2958.1994.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuldiner S, Agmon V, Brandsma J, Cohen A, Friedman E, Padan E. 1986. Induction of SOS functions by alkaline intracellular pH in Escherichia coli. J Bacteriol 168:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine JH, Lin Y, Elowitz MB. 2013. Functional roles of pulsing in genetic circuits. Science 342:1193–1200. doi: 10.1126/science.1239999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazaki R, van der Meer JR. 2011. A dual functional origin of transfer in the ICEclc genomic island of Pseudomonas knackmussii B13. Mol Microbiol 79:743–758. doi: 10.1111/j.1365-2958.2010.07484.x. [DOI] [PubMed] [Google Scholar]
- 45.Broussard GW, Oldfield LM, Villanueva VM, Lunt BL, Shine EE, Hatfull GF. 2013. Integration-dependent bacteriophage immunity provides insights into the evolution of genetic switches. Mol Cell 49:237–248. doi: 10.1016/j.molcel.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bondy-Denomy J, Davidson AR. 2014. When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness. J Microbiol 52:235–242. doi: 10.1007/s12275-014-4083-3. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. 2010. Cryptic prophages help bacteria cope with adverse environments. Nat Commun 1:147–147. doi: 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figueroa-Bossi N, Uzzau S, Maloriol D, Bossi L. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol Microbiol 39:260–271. doi: 10.1046/j.1365-2958.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- 49.Hooton SPT, Timms AR, Moreton J, Wilson R, Connerton IF. 2013. Complete genome sequence of Salmonella enterica serovar Typhimurium U288. Genome Announc 1:e00467-13. doi: 10.1128/genomeA.00467-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porwollik S, Wong RM-Y, McClelland M. 2002. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc Natl Acad Sci U S A 99:8956–8961. doi: 10.1073/pnas.122153699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bossi L, Fuentes JA, Mora G, Figueroa-Bossi N. 2003. Prophage contribution to bacterial population dynamics. J Bacteriol 185:6467–6471. doi: 10.1128/JB.185.21.6467-6471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gama JA, Reis AM, Domingues I, Mendes-Soares H, Matos AM, Dionisio F. 2013. Temperate bacterial viruses as double-edged swords in bacterial warfare. PLoS One 8:e59043. doi: 10.1371/journal.pone.0059043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol 185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa I, Novick RP, Penadés JR. 2009. Killing niche competitors by remote-control bacteriophage induction. Proc Natl Acad Sci U S A 106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costerton JW, Lewandowski Z. 1995. Microbial biofilms. Annu Rev Microbiol 49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 56.Monds RD, O'Toole GA. 2009. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol 17:73–87. doi: 10.1016/j.tim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Madsen JS, Burmolle M, Hansen LH, Sorensen SJ. 2012. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65:183–195. doi: 10.1111/j.1574-695X.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 58.Molin S, Tolker-Nielsen T. 2003. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol 14:255–261. doi: 10.1016/S0958-1669(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 59.Veening J-W, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 60.Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Way M, Hauser A, McDougald D, Webb JS, Kjelleberg S. 2009. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J 3:271–282. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrova OE, Schurr JR, Schurr MJ, Sauer K. 2011. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Mol Microbiol 81:767–783. doi: 10.1111/j.1365-2958.2011.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirov SM, Webb JS, O'May CY, Reid DW, Woo JKK, Rice SA, Kjelleberg S. 2007. Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 153(Pt 10):3264–3274. doi: 10.1099/mic.0.2007/009092-0. [DOI] [PubMed] [Google Scholar]
- 63.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, Kjelleberg S. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol 185:4585–4592. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 65.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 66.Webb JS, Lau M, Kjelleberg S. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J Bacteriol 186:8066–8073. doi: 10.1128/JB.186.23.8066-8073.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Kim Y, Wood TK. 2009. Control and benefits of CP4-57 prophage excision in Escherichia coli biofilms. ISME J 3:1164–1179. doi: 10.1038/ismej.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.García-Contreras R, Zhang X-S, Kim Y, Wood TK. 2008. Protein translation and cell death: the role of rare tRNAs in biofilm formation and in activating dormant phage killer genes. PLoS One 3:e2394. doi: 10.1371/journal.pone.0002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrolo M, Frias MJ, Pinto FR, Melo-Cristino J, Ramirez M. 2010. Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS One 5:e15678. doi: 10.1371/journal.pone.0015678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gödeke J, Paul K, Lassak J, Thormann KM. 2011. Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. ISME J 5:613–626. doi: 10.1038/ismej.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Binnenkade L, Teichmann L, Thormann KM. 2014. Iron triggers λSo prophage induction and release of extracellular DNA in Shewanella oneidensis MR-1 biofilms. Appl Environ Microbiol 80:5304–5316. doi: 10.1128/AEM.01480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zegans ME, Wagner JC, Cady KC, Murphy DM, Hammond JH, O'Toole GA. 2009. Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J Bacteriol 191:210–219. doi: 10.1128/JB.00797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.d'Hérelle F. 1917. Sur un microbe invisible antagoniste des bacilles dysentérique. Acad Sci Paris 165:373–375. [Google Scholar]
- 74.Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 75.Xu X, McAteer SP, Tree JJ, Shaw DJ, Wolfson EBK, Beatson SA, Roe AJ, Allison LJ, Chase-Topping ME, Mahajan A, Tozzoli R, Woolhouse MEJ, Morabito S, Gally DL. 2012. Lysogeny with Shiga toxin 2-encoding bacteriophages represses type III secretion in enterohemorrhagic Escherichia coli. PLoS Pathog 8:e1002672. doi: 10.1371/journal.ppat.1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Łoœ JM, MŁoœ Wêgrzyn A, Wêgrzyn G. 4 January 2013, posting date Altruism of Shiga toxin-producing Escherichia coli: recent hypothesis versus experimental results. Front Cell Infect Microbiol doi: 10.3389/fcimb.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson CM, Sinclair JF, Smith MJ, O'Brien AD. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc Natl Acad Sci U S A 103:9667–9672. doi: 10.1073/pnas.0602359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimizu T, Ohta Y, Noda M. 2009. Shiga toxin 2 is specifically released from bacterial cells by two different mechanisms. Infect Immun 77:2813–2823. doi: 10.1128/IAI.00060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tyler JS, Beeri K, Reynolds JL, Alteri CJ, Skinner KG, Friedman JH, Eaton KA, Friedman DI. 2013. Prophage induction is enhanced and required for renal disease and lethality in an EHEC mouse model. PLoS Pathog 9:e1003236. doi: 10.1371/journal.ppat.1003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siboo IR, Bensing BA, Sullam PM. 2003. Genomic organization and molecular characterization of SM1, a temperate bacteriophage of Streptococcus mitis. J Bacteriol 185:6968–6975. doi: 10.1128/JB.185.23.6968-6975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell J, Siboo IR, Takamatsu D, Chambers HF, Sullam PM. 2007. Mechanism of cell surface expression of the Streptococcus mitis platelet binding proteins PblA and PblB. Mol Microbiol 64:844–857. doi: 10.1111/j.1365-2958.2007.05703.x. [DOI] [PubMed] [Google Scholar]
- 82.Seo HS, Xiong YQ, Mitchell J, Seepersaud R, Bayer AS, Sullam PM. 2010. Bacteriophage lysin mediates the binding of Streptococcus mitis to human platelets through interaction with fibrinogen. PLoS Pathog 6:e1001047. doi: 10.1371/journal.ppat.1001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matos RC, Lapaque N, Rigottier-Gois L, Debarbieux L, Meylheuc T, Gonzalez-Zorn B, Repoila F, Lopes MDF, Serror P. 2013. Enterococcus faecalis prophage dynamics and contributions to pathogenic traits. PLoS Genet 9:e1003539. doi: 10.1371/journal.pgen.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheetham BF, Katz ME. 1995. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol Microbiol 18:201–208. doi: 10.1111/j.1365-2958.1995.mmi_18020201.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen J, Novick RP. 2009. Phage-mediated intergeneric transfer of toxin genes. Science 323:139–141. doi: 10.1126/science.1164783. [DOI] [PubMed] [Google Scholar]
- 87.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol 29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 88.Ruzin A, Lindsay J, Novick RP. 2001. Molecular genetics of SaPI1 - a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald JR, Monday SR, Foster TJ, Bohach GA, Hartigan PJ, Meaney WJ, Smyth CJ. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J Bacteriol 183:63–70. doi: 10.1128/JB.183.1.63-70.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ubeda C, Tormo MA, Cucarella C, Trotonda P, Foster TJ, Lasa I, Penadés JR. 2003. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol 49:193–210. doi: 10.1046/j.1365-2958.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 91.Cucarella C, Solano C, Valle J, Amorena B, Lasa Í, Penadés JR. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penadés JR. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 93.Serra-Moreno R, Jofre J, Muniesa M. 2008. The CI repressors of Shiga toxin-converting prophages are involved in coinfection of Escherichia coli strains, which causes a down regulation in the production of Shiga toxin 2. J Bacteriol 190:4722–4735. doi: 10.1128/JB.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campoy S, Hervàs A, Busquets N, Erill I, Teixidó L, Barbé J. 2006. Induction of the SOS response by bacteriophage lytic development in Salmonella enterica. Virology 351:360–367. doi: 10.1016/j.virol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Lemire S, Figueroa-Bossi N, Bossi L. 2011. Bacteriophage crosstalk: coordination of prophage induction by trans-acting antirepressors. PLoS Genet 7:e1002149. doi: 10.1371/journal.pgen.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grünberger A, Paczia N, Probst C, Schendzielorz G, Eggeling L, Noack S, Wiechert W, Kohlheyer D. 2012. A disposable picolitre bioreactor for cultivation and investigation of industrially relevant bacteria on the single cell level. Lab Chip 12:2060–2068. doi: 10.1039/c2lc40156h. [DOI] [PubMed] [Google Scholar]
- 97.Wang P, Robert L, Pelletier J, Dang WL, Taddei F, Wright A, Jun S. 2010. Robust growth of Escherichia coli. Curr Biol 20:1099–1103. doi: 10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu TK, Koeris MS. 2011. The next generation of bacteriophage therapy. Curr Opin Microbiol 14:524–531. doi: 10.1016/j.mib.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 99.Baumgart M, Unthan S, Rückert C, Sivalingam J, Grünberger A, Kalinowski J, Bott M, Noack S, Frunzke J. 2013. Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl Environ Microbiol 79:6006–6015. doi: 10.1128/AEM.01634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frunzke J, Bramkamp M, Schweitzer JE, Bott M. 2008. Population heterogeneity in Corynebacterium glutamicum ATCC 13032 caused by prophage CGP3. J Bacteriol 190:5111–5119. doi: 10.1128/JB.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]