Abstract
The oxidation of l-proline to glutamate in Gram-negative bacteria is catalyzed by the proline utilization A (PutA) flavoenzyme, which contains proline dehydrogenase (PRODH) and Δ1-pyrroline-5-carboxylate (P5C) dehydrogenase domains in a single polypeptide. Previous studies have suggested that aside from providing energy, proline metabolism influences oxidative stress resistance in different organisms. To explore this potential role and the mechanism, we characterized the oxidative stress resistance of wild-type and putA mutant strains of Escherichia coli. Initial stress assays revealed that the putA mutant strain was significantly more sensitive to oxidative stress than the parental wild-type strain. Expression of PutA in the putA mutant strain restored oxidative stress resistance, confirming that depletion of PutA was responsible for the oxidative stress phenotype. Treatment of wild-type cells with proline significantly increased hydroperoxidase I (encoded by katG) expression and activity. Furthermore, the ΔkatG strain failed to respond to proline, indicating a critical role for hydroperoxidase I in the mechanism of proline protection. The global regulator OxyR activates the expression of katG along with several other genes involved in oxidative stress defense. In addition to katG, proline increased the expression of grxA (glutaredoxin 1) and trxC (thioredoxin 2) of the OxyR regulon, implicating OxyR in proline protection. Proline oxidative metabolism was shown to generate hydrogen peroxide, indicating that proline increases oxidative stress tolerance in E. coli via a preadaptive effect involving endogenous hydrogen peroxide production and enhanced catalase-peroxidase activity.
INTRODUCTION
The conversion of l-proline to glutamate is a four-electron oxidation process that is coordinated in two successive steps by the enzymes proline dehydrogenase (PRODH) and Δ1-pyrroline-5-carboxylate dehydrogenase (P5CDH) (Fig. 1) (1). In eukaryotes, PRODH and P5CDH are separately encoded enzymes localized in the mitochondrion. In Gram-negative bacteria, PRODH and P5CDH are combined into a bifunctional enzyme known as proline utilization A (PutA) (1, 2). The PRODH domain contains a noncovalently bound flavin adenine dinucleotide (FAD) cofactor and couples the two-electron oxidation of proline to the reduction of ubiquinone in the cytoplasmic membrane (3). The product of the PRODH reaction, Δ1-pyrroline-5-carboxylate (P5C), is subsequently hydrolyzed to glutamate-γ-semialdehyde (GSA), which is then oxidized to glutamate by the NAD+-dependent P5CDH domain (2). In certain Gram-negative bacteria such as Escherichia coli, PutA also has an N-terminal ribbon-helix-helix (RHH) DNA-binding domain (residues 1 to 47) (4). The RHH domain enables PutA to act as an autogenous transcriptional regulator of the putA and putP (high-affinity Na+-proline transporter) genes (4). PutA represses put gene expression by binding to five operator sites in the put regulatory region (5). Transcription of the put genes is activated by proline, which causes a reduction of the PutA flavin cofactor and subsequent localization of PutA on the membrane (5–9).
FIG 1.

Reactions catalyzed by the PRODH and P5CDH domains of PutA. Reduction of ubiquinone (CoQ) in the electron transport chain is coupled to proline oxidation.
Proline has been shown to be an important carbon and nitrogen source supporting growth under various nutrient conditions for Escherichia coli, Pseudomonas putida, Bradyrhizobium japonicum, and Helicobacter pylori (10–14). In H. pylori, l-proline is a preferred respiratory substrate in the gut, with proline levels 10-fold higher in the gastric juice of patients infected with H. pylori than in noninfected individuals (10, 15). An H. pylori putA mutant strain was shown to be less efficient in the colonization of mice than the wild-type strain (16). A putA mutant strain of the closely related mouse pathogen Helicobacter hepaticus also exhibited less pathogenicity in mice than the wild-type strain (17). Thus, in certain ecological niches, PutA and the proline catabolic pathway have a critical role in bacterial pathogenesis.
Besides being an important energy source, proline also provides protective benefits against abiotic and biotic stresses in a broad range of organisms (18–23). Proline is a well-known osmoprotectant (24, 25), and in E. coli, proline has been described as being a thermoprotectant by diminishing protein aggregation during heat stress (26). Proline has also been found to help combat oxidative stress, a property which has been explored in eukaryotes such as fungi, plants, and animals (19, 20, 27, 28). The mechanism by which proline protects against oxidative stress appears to involve protection of intracellular redox homeostasis and, in the fungal pathogen Colletotrichum trifolii, upregulation of catalase (27). In mammalian cells, proline protection was shown to require PRODH activity and activation of signaling cascades that promote cell survival (28).
To uncover fundamental mechanisms of proline-mediated stress protection, we explored whether proline metabolism has a role in oxidative stress resistance in E. coli. We provide evidence that proline enhances oxidative stress tolerance in E. coli and that protection is dependent on the catalytic activity of PutA. Evidence supporting the involvement of hydroperoxidase I (encoded by katG) and the OxyR regulon, which have critical roles in oxidative stress defense, is also shown. The results suggest that proline metabolism can promote oxidative stress adaption, a feature which may facilitate pathogenesis in certain biological environments.
MATERIALS AND METHODS
Reagents, bacterial strains, and culture conditions.
β-Mercaptoethanol, o-aminobenzaldehyde (o-AB), o-nitrophenyl-β-d-galactopyranoside, l-proline, and l-tetrahydro-2-furoic acid (l-THFA) were purchased from Sigma. All other chemicals were purchased from Thermo Fisher unless noted otherwise. The PutA-pUC18 plasmid was described previously (5). E. coli strains used in this study are listed in Table 1. The MG1655 ΔputA strain was generated in this work by P1 transduction of the MG1655 wild-type strain (29). E. coli cultures were grown in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, and 10 g NaCl per liter) or glucose minimal medium (0.5 g glucose, 0.1 g thiamine, 1 mM MgSO4, 0.5 g NaCl, 1 g NH4Cl, 3 g KH2PO4, and 6 g Na2HPO4·7H2O per liter). Ampicillin and kanamycin were used as needed at 50 μg/ml. Cultures were grown at 37°C with shaking at 225 rpm. To perform measurements upon exponentially growing cells, cultures grown overnight were diluted 1,000-fold with fresh medium and grown to mid-logarithmic phase, which corresponds to an optical density at 600 nm (OD600) of 0.3.
TABLE 1.
Strains used in this study
| E. coli K-12 strain | Relevant genotype | Reference |
|---|---|---|
| MG1655 | Wild type (F− Δλ− rph-1) | 65 |
| MG1655 ΔputA | MG1655 plus ΔputA758::kan | This work |
| AL441 | MG1655 plus Δ(lacZ1::cat)1 attλ::[pSJ501::katG′-lacZ+ cat+] | 66 |
| CSH4 | Wild type [F− lacZ1125 λ− trpA49(Am) relA1 rpsL150(Strr) spoT1] | 14 |
| JT31 | CSH4 plus putA1::Tn5 | 14 |
| JT34 | CSH4 plus putP3::Tn5 | 14 |
| BW25113 | Wild type [F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 hsdR514] | 42 |
| JW0999 | BW25113 plus ΔputA758::kan | 42 |
| JW1721 | BW25113 plus ΔkatE731::kan | 42 |
| JW3914 | BW25113 plus ΔkatG729::kan | 42 |
| JW3933 | BW25113 plus ΔoxyR749::kan | 42 |
| JW4024 | BW25113 plus ΔsoxR757::kan | 42 |
Disk assay for oxidative stress sensitivity.
Cells were grown in LB broth to an OD600 of 0.3. Aliquots (0.5 ml) from each culture were then mixed with 4.5 ml cooled-down soft agar (glucose minimal medium, 0.8% agar, and no antibiotics) and then poured immediately onto LB plates (no antibiotics). After the soft agar solidified, a round filter paper (0.8-cm diameter) saturated with 10 μl of 6.6 M H2O2 was placed in the center of the plate. Plates were then incubated at 37°C overnight. The inhibitory zone diameter was measured from three different directions to calculate a mean value for the diameter of the inhibition zone.
Cell counting assay.
Cells were grown in glucose minimal medium to an OD600 of 0.3 with (or without) 10 mM l-proline. Cells were then collected and diluted to an OD600 of 0.1 with fresh medium prior to a 30-min treatment with 5 mM H2O2. After serial dilution, cells were spread onto LB plates and allowed to grow overnight at 37°C. Cell survival rates were calculated as CFU of H2O2-treated cells divided by that of untreated cells.
β-Galactosidase activity.
To measure the effect of proline oxidation on katG expression, AL441 cells were grown in glucose minimal medium to an OD600 of 0.3 before treatment with 10 mM l-proline and l-THFA. Samples were collected at designated time points, and β-galactosidase activities were measured as described previously (30). To determine the effect of H2O2 on katG expression, cells were cultured as described above and then treated with different concentrations of H2O2 for 30 min, followed by measurement of β-galactosidase activity. β-Galactosidase activity assays were performed as previously described (30) and are reported in Miller units (29).
Catalase activity.
MG1655 wild-type and ΔputA cells were grown in glucose minimal medium to an OD600 of 0.3 with (or without) 10 mM l-proline. Cells were then collected, centrifuged, and lysed with bacterial extraction reagent (Pierce). Cell debris was removed by centrifugation, and catalase activity in the supernatant was measured with 20 μM H2O2 by an Amplex Red catalase assay kit (Life Technology), using a newly prepared H2O2 standard curve according to the manufacturer's protocols, and measuring the absorbance at 568 nm with a Powerwave XS microplate reader. Protein concentrations were determined with a 660-nm protein assay (Pierce). One unit of catalase activity is defined as the decomposition of 1.0 μmol H2O2 per min at pH 7.0 at 25°C.
H2O2 clearance assay.
To determine the effect of proline oxidation on H2O2 scavenging, MG1655 wild-type and ΔputA cells were grown in glucose minimal medium to an OD600 of 0.3 with (or without) 10 mM l-proline. Cells were then pelleted, washed, and resuspended in phosphate-buffered saline (PBS) to an OD600 of 0.1. After the addition of 5 μM H2O2, 0.45-ml aliquots were removed at different time intervals, and H2O2 was measured immediately by an Amplex Red hydrogen peroxide-peroxidase assay kit (Life Technology) at room temperature, as previously described (31). Fluorescence measurements were made with an Agilent (Varian) Cary Eclipse fluorescence spectrophotometer with excitation at 545 nm and fluorescence emission monitoring at 590 nm.
Real-time PCR.
MG1655 wild-type and ΔputA cells were grown in glucose minimal medium to an OD600 of 0.3. At time zero (0 min), 10 mM l-proline was added to the cultures. Immediately afterwards, a sample of 0.5 ml was taken and mixed with 1 ml of RNAprotect Bacteria reagent (Qiagen) for the time zero point. Additional samples were then withdrawn from the cultures at different time points. RNA was extracted by using an RNeasy minikit (Qiagen) according to the manufacturer's protocol. Genomic DNA was removed from the RNA preparations with RNase-free DNase I (Fermentas). The cDNA was synthesized by using the RevertAid First Strand cDNA synthesis kit (Fermentas) with 100 ng of template RNA and random hexamer primers. The following primers were used to analyze the expression of katG, grxA, trxC, and 16S rRNA: katG forward primer 5′-AATCCAGTTCGAAGCGGTAG-3′, katG reverse primer 5′-CACCAGCATTGT CGGTTTAC-3′, grxA forward primer 5′-GATCTGGCTGAGAAATTGAG-3′, grxA reverse primer 5′-GTTTACCTGCCTTTTGTTGT-3′, trxC forward primer 5′-AATACCGTTTGTACCCAT TG-3′, trxC reverse primer 5′-GCTTCGGTATTCACTTTCAC-3′, 16S rRNA forward primer 5′-GGATGATCAGCCACACTGGA-3′, and 16S rRNA reverse primer 5′-CCAAT ATTCCTCACTGCTGCC-3′. The 20-μl real-time PCR mixture contained 10 μl SsoFast EvaGreen Supermix (Bio-Rad), 300 nM primers, and 50 ng cDNA. Thermal cycling was performed by using an iCycler iQ instrument (Bio-Rad) for 40 cycles in 3 steps: 95°C for 15 s, 58°C for 30 s, and 65°C for 60 s. Relative mRNA levels were calculated by using the 2−ΔΔCT method and by using 16S rRNA as the internal control. PCR products were also analyzed by agarose gel electrophoresis to confirm product size and specificity.
PutA Western blotting.
The expression of PutA was confirmed by Western blot analysis, as described previously, using an antibody against a polypeptide containing PutA residues 1 to 47 (5).
H2O2 production assays.
H2O2 produced and accumulated within cells passes through membranes and equilibrates with the culture medium (32). To determine the effect of l-proline treatment on H2O2 production in vivo, BW25113 (Keio strain collection) wild-type and ΔkatG (strain JW3914) cells were grown to an OD600 of 0.3 in glucose minimal medium with (or without) 10 mM l-proline. Cells were then pelleted, and the H2O2 content in the supernatant was measured immediately by using the Amplex Red hydrogen peroxide-peroxidase assay kit with excitation at 545 nm and fluorescence emission monitoring at 590 nm.
The kinetics of H2O2 formation from proline were determined by using inverted membrane vesicles from the ΔkatG strain (JW3914). Inverted membrane vesicles were prepared, as described previously (33), from JW3914 (ΔkatG) cells grown in minimal A medium [33 mM KH2PO4, 51 mM K2HPO4, 8 mM (NH4)2SO4, 0.4 mM MgSO4, 0.5 mM tryptophan, 10 mM l-proline, 8% glycerol, and 0.05% glucose] to an OD600 of 0.7. Assays were performed at room temperature with 50 μg/ml of membrane vesicles (or membrane protein) and 10 mM l-proline to estimate the rate of H2O2 production. The assay buffer (pH 7.2) included 40 U/ml superoxide dismutase, 125 mM KCl, 4 mM KH2PO4, 14 mM NaCl, 20 mM HEPES-NaOH, 1 mM MgCl2, 0.2% bovine serum albumin (BSA), and 0.02 mM EDTA (34). H2O2 was quantified by using the Amplex Red hydrogen peroxide-peroxidase assay kit with excitation at 555 nm and fluorescence emission monitoring at 581 nm, as described previously (34), with the rate defined as pmol of H2O2 formed min−1 mg−1 of membrane protein. To determine the rate of intracellular H2O2 formation, we used the relationship that 1 ml of bacteria at 1.0 OD unit comprises ∼0.47 μl of cytosolic volume (35). For these assays, 20 mg of membrane protein vesicles was isolated from 500-ml cultures grown to 0.8 OD units, which corresponds to a total cytosolic volume of ∼188 μl. The rate of intracellular H2O2 formation (μM min−1) was calculated from the measured H2O2 formation rate: (pmol min−1 mg−1 of membrane protein) × [total membrane protein (20 mg)/total cytosolic volume (188 μl)]. The effect of l-THFA on H2O2 production was determined by using the same assay conditions as those described above, with the proline concentration fixed at 10 mM and various l-THFA concentrations (1 to 10 mM). Background formation of H2O2 was determined in control assays without l-proline. PutA activity in the membrane vesicles was confirmed by quantifying P5C production (nmol P5C min−1 mg−1 of membrane protein) using o-AB, as previously described (36).
Statistical analysis.
The reported mean values and standard deviations are from three to five experiments. Data were analyzed by Student's t tests, with statistical significance considered to be a P value of <0.05.
RESULTS
E. coli putA mutants have increased oxidative stress sensitivity.
Oxidative stress disk assays were performed with wild-type E. coli strain CSH4 and isogenic putA (JT31) and putP (JT34) mutant strains grown in LB broth to exponential phase (Fig. 2A). The inhibition zone found for the JT31 strain is almost twice the size of the zones observed for the CSH4 and JT34 strains (Fig. 2A and B). This finding indicates that JT31 cells have increased sensitivity to H2O2. The observed phenotype of the JT31 cells can be complemented by transformation with the pUC18 vector bearing wild-type PutA (Fig. 2), confirming that depletion of PutA contributes to the H2O2 sensitivity of JT31 cells. Expression levels of PutA in the different strains were confirmed by Western blot analysis, as shown in Fig. 2C.
FIG 2.

Depletion of PutA increases oxidative stress sensitivity. (A) Disk assays were performed with CSH4 (parental wild type), JT31 (putA1::Tn5), JT34 (putP3::Tn5), and JT31 transformed with the empty pUC18 vector or the pUC18 vector bearing wild-type PutA by using filter paper saturated with 10 μl of 6.6 M H2O2. (B) Inhibition zone diameters from five replicates of the disk assays shown in panel A (*, P < 0.05). (C) Western blot analysis of PutA expression in strains used for panels A and B.
The above-described experiments were performed in LB broth, which is abundant in l-proline (9.5 mM) and contains low glucose (<0.1 mM), necessitating E. coli to utilize amino acids for growth (37). It was previously reported that l-proline is significantly utilized by E. coli in LB broth (37). Thus, we hypothesized that proline catabolism may account for the differences in oxidative stress resistance observed between strains CSH4 and JT31. Although the CSH4 and JT31 strains are commonly used for proline studies (4, 5, 9), the CSH4 strain contains mutations in relA and spoT, which regulate (p)ppGpp levels and are important for bacterial survival under nutrient starvation and oxidative and osmotic stress conditions (38, 39). Thus, to further evaluate the effects of proline metabolism on oxidative stress sensitivity, a ΔputA strain of MG1655 was generated by P1 transduction (Fig. 3B). MG1655 wild-type and ΔputA strains exhibit similar growth profiles, and proline supplementation did not affect the growth of either strain (data not shown). Figure 3A shows that proline supplementation promotes cell survival of the wild-type MG1655 strain by 2-fold after exposure to H2O2, whereas no protection by proline was observed in ΔputA cells. To confirm that the lack of proline protection was due to the loss of PutA, MG1655 ΔputA cells were transformed with the PutA-pUC18 vector. PutA expression in the different strains was confirmed by Western blot analysis, as shown in Fig. 3B. MG1655 ΔputA cells transformed with the PutA-pUC18 vector showed increased survival with proline (Fig. 3A), while the empty pUC18 vector had no effect. Thus, PutA is required for the improved oxidative stress tolerance with proline. The PutA-pUC18 vector, however, did not completely restore the H2O2 resistance of ΔputA cells, as survival rates remained lower than those of wild-type cells. Figure 3A also shows that ΔputA cells were more sensitive to H2O2 in the absence of proline than wild-type cells. One explanation for this finding may be the inability of ΔputA cells to utilize endogenous proline, which would lower resistance to oxidative stress. Consistent with this possibility, the survival rate of MG1655 ΔputA cells increased nearly 2-fold with the PutA-pUC18 vector.
FIG 3.

Proline enhances cell survival and H2O2 clearance. (A) Cell survival rates of MG1655 wild-type (parental) and ΔputA strains and the ΔputA strain transformed with the empty vector or the PutA-pUC18 vector in the absence (Con) and presence (Pro) of 10 mM proline. Cells grown in minimal medium were treated with 5 mM H2O2 (30 min) prior to plating onto LB agar plates (*, P < 0.05). (B) Western blot analysis of PutA expression in strains used for panel A and the MG1655 ΔputA strain transformed with the PutA Lys9Met-pUC18 vector. (C) H2O2 clearance in MG1655 wild-type and ΔputA cells grown in minimal medium in the absence (Con) and presence (Pro) of 10 mM proline. After the addition of 5 μM H2O2 to cells suspended in PBS, H2O2 was measured at the indicated times by using Amplex Red (*, P < 0.05). The concentration of H2O2 remaining is inversely proportional to the amount of H2O2 scavenged.
Next, we questioned the mechanism by which proline enhances resistance to H2O2. Scavenging enzymes, like peroxidases and catalases, are a key defense mechanism against H2O2 (40). To test whether proline increased the scavenging of H2O2,which is membrane permeable, extracellular H2O2 levels (Fig. 3C) were measured in cultures of MG1655 wild-type and ΔputA cells grown to exponential phase in medium supplemented with and without proline. In wild-type cells, extracellular H2O2 (5 μM) was cleared at a significantly higher rate with proline than without proline (Fig. 3C). In the MG1655 ΔputA strain, proline had no effect on the H2O2 clearance rate, indicating that the faster clearance of H2O2 in wild-type cells with proline is dependent on PutA. Proline alone did not decrease H2O2 levels during 30 min of incubation of proline (10 mM) and H2O2 (5 μM) in medium without cells (data not shown).
Proline metabolism upregulates katG expression and activity.
The influence of proline on catalase activity was next evaluated as a possible means for the increased oxidative stress resistance and faster clearance of H2O2. Figure 4A shows that MG1655 wild-type cells exhibited 1.7-fold-higher catalase activity with proline than did cells without proline treatment. In MG1655 ΔputA cells, no significant change in catalase activity was observed with proline.
FIG 4.
Catalase expression and activity are upregulated by proline. (A) Catalase activity of MG1655 wild-type and ΔputA cells grown in minimal medium with (Pro) or without (Con) 10 mM proline (*, P < 0.05). (B) Time course of katG expression in MG1655 wild-type and ΔputA cells. After the addition of 10 mM proline to cells grown to exponential phase in minimal medium, cells were harvested at the times indicated, and katG expression was measured by real-time PCR (*, P < 0.05). 16S rRNA was used as the internal control. (C) Effect of proline on katG′ promoter activity was determined by monitoring katG′::lacZ reporter construct activity in AL441 cells grown to exponential phase in minimal medium. After reaching exponential phase, cells were treated with proline (10 mM) and l-THFA (10 mM), as indicated (0 to 60 min), and β-galactosidase activity was then measured (*, P < 0.05). (D) Same as panel C except that AL441 cells were treated with increasing concentrations of H2O2 (30 min) prior to measurement of β-galactosidase activity (*, P < 0.05). β-Galactosidase activity is reported as Miller units (U/OD600).
E. coli has two catalases, hydroperoxidase I and hydroperoxidase II, which are encoded by katG and katE, respectively (41). The expression of katE is regulated by RpoS and is upregulated during stationary phase, whereas the expression of katG is regulated by OxyR and is induced by H2O2 (41). Changes in expression levels of katG were quantified by real-time PCR in cells treated with proline for up to 60 min (Fig. 4B). A 3.5-fold increase in the katG expression level was observed for wild-type MG1655 cells at 20 min, and a >6-fold increase was observed by 40 min. In ΔputA cells, no increase in katG expression was observed in response to proline. Thus, proline induction of katG expression is dependent on PutA. Additional evidence for proline increasing katG expression levels was obtained by using a katG′::lacZ expression reporter construct in MG1655 cells (strain AL441), in which the expression of katG is monitored by changes in β-galactosidase activity. The expression level of katG was 1.5-fold higher in cells with proline than in control cells without proline at 60 min (Fig. 4C). Incubation of cells with proline and l-THFA, a competitive inhibitor of PutA/PRODH activity (Ki = 1.6 mM) (3), blocked the observed increase in β-galactosidase activity, suggesting that PutA catalytic activity is critical for the effects of proline on katG expression (Fig. 4C). The effect of proline metabolism on the katG′::lacZ reporter was then compared to the effect of the addition of H2O2 to the cell medium. Figure 4D shows that exposure of cells to 0.1 mM and 1 mM H2O2 for 30 min results in 1.7- and 2.5-fold increases in the katG expression level, respectively. Thus, the increased level of katG expression by proline is similar to that observed with 0.1 to 1 mM H2O2. Altogether, these results strongly suggest that proline metabolism promotes the expression of katG and catalase activity.
DNA-binding function of PutA is not required for increased oxidative stress resistance.
E. coli PutA contains an RHH DNA-binding domain (residues 1 to 47) that enables PutA to act as transcriptional repressor of the putA and putP genes (4). Because PutA is a DNA-binding protein, it is feasible that the effect of PutA on katG expression may be via direct PutA-DNA interactions. E. coli PutA-DNA binding involves a GTTGCA consensus motif (5), which is not found in the promoter region of katG. Nevertheless, to rule out the possibility that PutA regulates katG expression by DNA binding, we transformed the MG1655 ΔputA strain with the PutA Lys9Met (K9M) mutant (Fig. 3B). Previously, Lys9 was determined to be critical for PutA-DNA interactions, as the Lys9Met mutation abolished PutA-DNA binding (5). Figure 5 shows that ΔputA cells expressing the PutA K9M mutant responded to proline with a 2-fold increase in cell survival, similar to that observed with wild-type PutA (Fig. 3A). Expression of the PutA K9M mutant was confirmed by Western blot analysis (Fig. 3B). Therefore, the DNA-binding function of PutA is not required for the proline-dependent increase in oxidative stress resistance.
FIG 5.
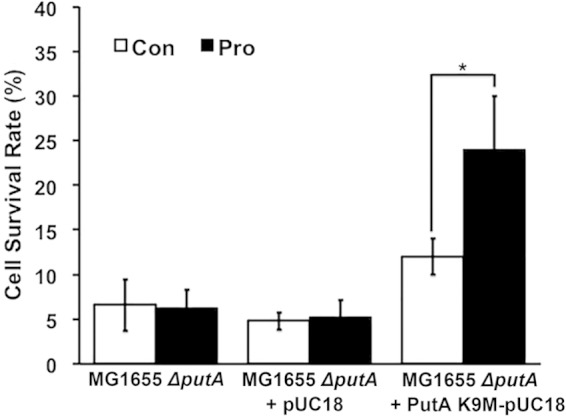
DNA binding is not required for proline-dependent oxidative stress resistance. Shown are survival rates of MG1655 ΔputA cells and ΔputA cells transformed with the empty vector or the PutA K9M-pUC18 vector. PutA K9M is a mutant that does not bind DNA (*, P < 0.05).
Proline metabolism activates the OxyR regulon.
The effect of proline on cell survival was next tested by using a panel of mutants from the E. coli Keio strain collection (42). The cell survival rates of BW25113 wild-type and mutant strains after H2O2 stress treatment were tested in the absence and presence of proline (Fig. 6A). As anticipated, proline increased the survival rate of BW25113 wild-type cells but not ΔputA cells. Similar to the ΔputA strain, the survival rates of the ΔkatG and ΔoxyR cells were not increased by proline, suggesting that OxyR, which regulates katG, is involved in the protective mechanism of proline. In contrast, proline increased the survival rates of ΔkatE and ΔsoxR cells, indicating that hydroperoxidase II and SoxR, which is a transcription factor activated in response to redox-active metabolites (43), are not essential for the mechanism of proline protection.
FIG 6.
Proline protection involves the OxyR regulon. (A) Cell survival rates of the BW25113 wild-type strain and the BW25113 ΔputA, ΔkatE, ΔkatG, ΔoxyR, and ΔsoxR mutants (*, P < 0.05). (B and C) Time course of grxA (B) and trxC (C) expression in MG1655 wild-type and ΔputA cells. Experiments were performed as described in the legend of Fig. 4B, using 16S rRNA as an internal control (*, P < 0.05).
Besides H2O2 scavenging, activation of OxyR initiates other oxidative stress systems, such as the sequestration of unincorporated iron by Dps and the repair of polypeptide cysteine oxidation by thioredoxins and glutaredoxins (40). Because OxyR appears to have a critical role in proline-promoted oxidative stress resistance, the transcription levels of other antioxidant genes in the OxyR regulon were evaluated. Similar to katG, the expression levels of grxA (glutaredoxin 1) and trxC (thioredoxin 2) in MG1655 wild-type cells increased (∼7-fold) in a time-dependent manner upon treatment with proline (Fig. 6B and C). In MG1655 ΔputA cells, no changes in grxA or trxC transcription levels were observed. These results are consistent with proline metabolism activating OxyR.
Proline catabolism generates reactive oxygen species.
Because of the above-described results, we suspected that proline respiration may generate sufficient H2O2 to activate OxyR. To test this, H2O2 levels in BW25113 wild-type and ΔkatG strains were measured with and without proline. Without proline, the estimated intracellular concentration of H2O2 in ΔkatG cells was nearly 3-fold higher (289 ± 46 nM) than that in wild-type cells (79 ± 7 nM). In both strains, H2O2 levels were significantly higher in the presence of proline, with a >2-fold increase being observed for ΔkatG cells (663 ± 9 nM). In wild-type cells, intracellular H2O2 levels increased to 145 ± 8 nM with proline.
To further evaluate reactive oxygen species (ROS) production by proline oxidative metabolism, we performed in vitro assays using membrane vesicles prepared from BW25113 ΔkatG mutant strain cells. The rate of H2O2 formation with 10 mM proline was 91 ± 9 pmol min−1 mg−1, which is equivalent to 9.7 ± 0.9 μM min−1 when converted into an intracellular endogenous rate (Fig. 7B). The activity of PutA in membrane vesicles was measured at 103 ± 7 nmol P5C min−1 mg−1, indicating that the H2O2 production rate is ∼0.1% of the PutA turnover rate. l-THFA was observed to inhibit H2O2 formation in a dose-dependent manner, with 5 mM l-THFA almost completely blocking H2O2 production (Fig. 7B). With membrane vesicles as the electron acceptor, PutA has a Km value of 1.5 mM proline (3). These results indicate that PutA/PRODH activity is required for H2O2 formation with proline.
FIG 7.
Proline metabolism generates H2O2. (A) BW25113 wild-type and ΔkatG (strain JW3914) cells were grown to exponential phase in minimal medium in the absence (Con) and presence (Pro) of 10 mM l-proline. The H2O2 content in the medium was measured by the Amplex Red assay and converted to an estimate of the intracellular H2O2 concentration (*, P < 0.05). (B) In vitro assays of H2O2 production using inverted membrane vesicles from ΔkatG (strain JW3914) cells. Assays were performed with 50 μg/ml of membrane vesicles and 10 mM proline in the presence of various concentrations of the PutA inhibitor l-THFA, as indicated. H2O2 formation was estimated by the Amplex Red assay (*, P < 0.05).
DISCUSSION
Proline is a multifaceted amino acid with important roles in carbon and nitrogen metabolism; protein synthesis; and protection against various environmental factors such as drought (44), metal toxicity (45, 46), osmotic stress (24, 25), ultraviolent irradiation (47), unfolded protein stress (26, 48), and oxidative stress (19, 23, 27, 28, 49). In this study, we explored the role of proline metabolism in oxidative stress protection by characterizing the oxidative stress response of wild-type and putA mutant E. coli strains. Wild-type E. coli strains exhibited significantly greater resistance to H2O2 stress than did the putA mutant strains in medium supplemented with proline. Complementation of the putA mutant strains with PutA restored oxidative stress protection to levels near those of the parent wild-type strain. These results indicate that stress protection afforded by proline is a general phenotype in E. coli and is dependent on PutA.
The addition of proline to the culture medium increased total catalase activity and led to significantly higher expression levels of katG in wild-type cells, whereas no significant increase in catalase activity was observed with proline in ΔputA cells. Proline did not protect ΔkatG cells, indicating that hydroperoxidase I is necessary for proline-enhanced protection against H2O2 stress in E. coli. The katG gene is regulated by the transcription factor OxyR (50), which is a critical regulator of the cellular response to H2O2 and thiol redox changes. The OxyR regulon regulates response genes, such as katG, grxA (glutaredoxin I), trxC (thioredoxin 2), and ahpCF (peroxiredoxin AhpCF), that provide protection against reactive oxygen species (51). Consistent with hydroperoxidase I having a critical role in proline protection, proline did not improve the oxidative stress survival of the oxyR mutant strain. In addition, proline increased the expression levels of other genes in the OxyR regulon, such as grxA and trxC. Whether proline broadly affects the OxyR regulon will require a more extensive profiling of gene expression changes. Altogether, our results indicate that proline catabolism activates OxyR, leading to increased expression of katG. In contrast to the oxyR mutant, proline enhanced the oxidative stress resistance of soxR mutant cells, indicating that SoxR is not essential for proline protection.
The finding that proline increases the transcription of genes in the OxyR regulon suggests that proline metabolism increases intracellular H2O2 levels. OxyR reacts with H2O2 to form a disulfide bond between Cys199 and Cys208, which results in transcriptional activation of the OxyR regulon (52, 53). Oxidation of OxyR and activation of the OxyR regulon have been reported to occur with 0.05 to 0.2 μM H2O2 (32, 54). We observed a significant increase in endogenous H2O2 levels in ΔkatG cells with proline, and in wild-type cells, H2O2 levels were found to be >0.1 μM with proline. Thus, it appears that proline oxidative metabolism can drive H2O2 concentrations to levels that are sufficient to induce OxyR. The rapid increase of katG transcription by proline treatment (20 min) is also consistent with the response time of the OxyR regulon to H2O2 stress (51).
Previous work has addressed metabolic sources of endogenous ROS in E. coli and indicated that the respiratory chain contributes to the majority of endogenous H2O2 production (55). It was found, however, that H2O2 can also be significantly generated in E. coli by enzymes not associated with the respiratory chain (56). The oxidation of proline by PutA provides reducing equivalents directly to the respiratory pathway via ubiquinone (3). The PRODH domain of PutA contains a FAD cofactor that couples the oxidation of proline (reductive half-reaction) to the reduction of ubiquinone in the membrane (oxidative half-reaction). The rate-limiting step in the proline:ubiquinone oxidoreductase reaction catalyzed by PutA is the oxidative step (reduced FAD [FADH2] oxidation by ubiquinone) (57). The production of H2O2 by proline oxidation would conceivably involve increased flux in the respiratory chain or aberrant electron transfer from FADH2 to molecular oxygen, generating superoxide anion radicals, a general feature of flavoenzymes. In a previous study, the reactivity of different PutA proteins with molecular oxygen was evaluated, and PutA from E. coli was shown to have a turnover rate of <0.3 min−1 with oxygen (58). Thus, we propose that endogenous H2O2 from proline metabolism is not generated directly by PutA but rather by PutA/PRODH mainly passing electrons into the ubiquinone pool and PutA/P5CDH producing NADH, both of which would lead to increased electron flux through the respiratory chain. Consistent with this, we observed that proline increases respiration in wild-type E. coli cells by 4-fold using the redox indicator 2,3,5-triphenyl tetrazolium chloride (data not shown). Superoxide that results from proline metabolism would be converted to H2O2 either nonenzymatically or enzymatically by superoxide dismutase. Our measurement of intracellular H2O2 in wild-type cells grown without proline is consistent with the physiological concentration of H2O2 (<0.1 μM) reported previously for E. coli in the exponential growth phase (59). Twofold increases in H2O2 production, which we observed with proline, have also been shown to significantly induce katG expression with intracellular H2O2 at 0.1 to 0.2 μM (55). Thus, increases in the endogenous levels of H2O2 as a by-product of proline metabolism are likely enough to activate OxyR and induce katG expression.
The observation that proline metabolism can influence hydroperoxidase I activity indicates that besides serving as an important growth substrate in nutritionally deplete microenvironments, proline may offer a competitive advantage to bacteria in harsh oxidative environments. Bacteria often encounter oxidative stress from the host immune system, such as the respiratory burst associated with phagocytic killing of microbes (60). Previously, pretreatment of E. coli cells with small amounts of H2O2 was shown to have a protective effect by a 10-fold induction of hydroperoxidase I (61, 62). Here, proline metabolism may also provide a preconditioning effect by generating H2O2 as a by-product and elevating hydroperoxidase I levels, thereby increasing the overall stress tolerance of the cell. Various studies of proline metabolism in eukaryotes have shown that proline oxidation, which in eukaryotes occurs in the mitochondrion, generates ROS (19, 23, 63, 64), which can mediate cell death (63), cell survival against oxidative stress (28), and life span (64). The results from this work further illustrate the fundamental importance of how H2O2, as a metabolic by-product, can enhance oxidative stress tolerance and appears to be an underlying feature of proline metabolism that is conserved between E. coli and mammals.
ACKNOWLEDGMENTS
We thank James Imlay for generously sharing E. coli strains MG1655 and AL441 and for insightful discussions. We also thank Melanie Simpson and Paul Black for sharing microplate reader instrumentation.
This research was supported in part by grants GM079393, GM061068, and P30GM103335 from the National Institutes of Health.
REFERENCES
- 1.Tanner JJ. 2008. Structural biology of proline catabolism. Amino Acids 35:719–730. doi: 10.1007/s00726-008-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menzel R, Roth J. 1981. Enzymatic properties of the purified putA protein from Salmonella typhimurium. J Biol Chem 256:9762–9766. [PubMed] [Google Scholar]
- 3.Moxley MA, Tanner JJ, Becker DF. 2011. Steady-state kinetic mechanism of the proline:ubiquinone oxidoreductase activity of proline utilization A (PutA) from Escherichia coli. Arch Biochem Biophys 516:113–120. doi: 10.1016/j.abb.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu D, Zhou Y, Kallhoff V, Baban B, Tanner JJ, Becker DF. 2004. Identification and characterization of the DNA-binding domain of the multifunctional PutA flavoenzyme. J Biol Chem 279:31171–31176. doi: 10.1074/jbc.M403701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Larson JD, Bottoms CA, Arturo EC, Henzl MT, Jenkins JL, Nix JC, Becker DF, Tanner JJ. 2008. Structural basis of the transcriptional regulation of the proline utilization regulon by multifunctional PutA. J Mol Biol 381:174–188. doi: 10.1016/j.jmb.2008.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Zhu W, Bellur PS, Rewinkel D, Becker DF. 2008. Direct linking of metabolism and gene expression in the proline utilization A protein from Escherichia coli. Amino Acids 35:711–718. doi: 10.1007/s00726-008-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Zhou Y, Becker DF. 2004. Regulation of PutA-membrane associations by flavin adenine dinucleotide reduction. Biochemistry 43:13165–13174. doi: 10.1021/bi048596g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrovsky De Spicer P, Maloy S. 1993. PutA protein, a membrane-associated flavin dehydrogenase, acts as a redox-dependent transcriptional regulator. Proc Natl Acad Sci U S A 90:4295–4298. doi: 10.1073/pnas.90.9.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood J. 1987. Membrane association of proline dehydrogenase in Escherichia coli is redox dependent. Proc Natl Acad Sci U S A 84:373–377. doi: 10.1073/pnas.84.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata K, Nagata Y, Sato T, Fujino MA, Nakajima K, Tamura T. 2003. L-Serine, D- and L-proline and alanine as respiratory substrates of Helicobacter pylori: correlation between in vitro and in vivo amino acid levels. Microbiology 149:2023–2030. doi: 10.1099/mic.0.26203-0. [DOI] [PubMed] [Google Scholar]
- 11.Curtis J, Shearer G, Kohl DH. 2004. Bacteriod proline catabolism affects N2 fixation rate of drought-stressed soybeans. Plant Physiol 136:3313–3318. doi: 10.1104/pp.104.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohl DH, Schubert KR, Carter MB, Hagedorn CH, Shearer G. 1988. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A 85:2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vílchez S, Manzanera M, Ramos J. 2000. Control of expression of divergent Pseudomonas putida put promoters for proline catabolism. Appl Environ Microbiol 66:5221–5225. doi: 10.1128/AEM.66.12.5221-5225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood JM. 1981. Genetics of L-proline utilization in Escherichia coli. J Bacteriol 146:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Amsterdam K, van der Ende A. 2004. Nutrients released by gastric epithelial cells enhance Helicobacter pylori growth. Helicobacter 9:614–621. doi: 10.1111/j.1083-4389.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima K, Natsu S, Mizote T, Nagata Y, Aoyania K, Fukuda Y, Nagata K. 2008. Possible involvement of putA gene in Helicobacter pylori colonization in the stomach and motility. Biomed Res 29:9–18. doi: 10.2220/biomedres.29.9. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan N, Doster AR, Duhamel GE, Becker DF. 2008. Characterization of a Helicobacter hepaticus putA mutant strain in host colonization and oxidative stress. Infect Immun 76:3037–3044. doi: 10.1128/IAI.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Villamor JG, Verslues PE. 2011. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabados L, Savoure A. 2010. Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Cecchini NM, Monteoliva MI, Alvarez ME. 2011. Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol 155:1947–1959. doi: 10.1104/pp.110.167163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita Y, Nakamori S, Takagi H. 2003. l-Proline accumulation and freeze tolerance of Saccharomyces cerevisiae are caused by a mutation in the PRO1 gene encoding gamma-glutamyl kinase. Appl Environ Microbiol 69:212–219. doi: 10.1128/AEM.69.1.212-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford JM, Kontnik R, Clardy J. 2010. Regulating alternative lifestyles in entomopathogenic bacteria. Curr Biol 20:69–74. doi: 10.1016/j.cub.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Zhang L, Natarajan SK, Becker DF. 2013. Proline mechanisms of stress survival. Antioxid Redox Signal 19:998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csonka LN. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet 182:82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- 25.Wood JM. 1988. Proline porters effect the utilization of proline as a nutrient of osmoprotectant for bacteria. J Membr Biol 106:183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- 26.Chattopadhyay MK, Kern R, Mistou MY, Dandekar AM, Uratsu SL, Richarme G. 2004. The chemical chaperone proline relieves the thermosensitivity of a dnaK deletion mutant at 42 degrees C. J Bacteriol 186:8149–8152. doi: 10.1128/JB.186.23.8149-8152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Dickman MB. 2005. Proline suppresses apoptosis in the fungal pathogen of Colletotrichum trifolii. Proc Natl Acad Sci U S A 102:3459–3464. doi: 10.1073/pnas.0407960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan SK, Zhu W, Liang X, Zhang L, Demers AJ, Zimmerman MC, Simpson MA, Becker DF. 2012. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic Biol Med 53:1181–1191. doi: 10.1016/j.freeradbiomed.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 30.Zhu W, Haile AM, Singh RK, Larson JD, Smithen D, Chan JY, Tanner JJ, Becker DF. 2013. Involvement of the beta3-alpha3 loop of the proline dehydrogenase domain in allosteric regulation of membrane association of proline utilization A. Biochemistry 52:4482–4491. doi: 10.1021/bi400396g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaver LC, Imlay JA. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Flecha B, Demple B. 1997. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol 179:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrahamson JL, Baker LG, Stephenson JT, Wood JM. 1983. Proline dehydrogenase from Escherichia coli K12. Properties of the membrane-associated enzyme. Eur J Biochem 134:77–82. [DOI] [PubMed] [Google Scholar]
- 34.Starkov AA. 2010. Measurement of mitochondrial ROS production. Methods Mol Biol 648:245–255. doi: 10.1007/978-1-60761-756-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imlay JA, Fridovich I. 1991. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem 266:6957–6965. [PubMed] [Google Scholar]
- 36.Srivastava D, Schuermann JP, White TA, Krishnan N, Sanyal N, Hura GL, Tan A, Henzl MT, Becker DF, Tanner JJ. 2010. Crystal structure of the bifunctional proline utilization A flavoenzyme from Bradyrhizobium japonicum. Proc Natl Acad Sci U S A 107:2878–2883. doi: 10.1073/pnas.0906101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sezonov G, Joseleau-Petit D, D'Ari R. 2007. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 189:8746–8749. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braeken K, Fauvart M, Vercruysse M, Beullens S, Lambrichts I, Michiels J. 2008. Pleiotropic effects of a rel mutation on stress survival of Rhizobium etli CNPAF512. BMC Microbiol 8:219. doi: 10.1186/1471-2180-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnusson LU, Farewell A, Nystrom T. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Switala J, O'Neil JO, Loewen PC. 1999. Catalase HPII from Escherichia coli exhibits enhanced resistance to denaturation. Biochemistry 38:3895–3901. doi: 10.1021/bi982863z. [DOI] [PubMed] [Google Scholar]
- 42.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh AK, Shin JH, Lee KL, Imlay JA, Roe JH. 2013. Comparative study of SoxR activation by redox-active compounds. Mol Microbiol 90:983–996. doi: 10.1111/mmi.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnett NM, Naylor AW. 1966. Amino acid and protein metabolism in Bermuda grass during water stress. Plant Physiol 41:1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CT, Chen L, Lin CC, Kao CH. 2001. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci 160:283–290. doi: 10.1016/S0168-9452(00)00393-9. [DOI] [PubMed] [Google Scholar]
- 46.Tripathi BN, Mehta SK, Amar A, Gaur JP. 2006. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 62:538–544. doi: 10.1016/j.chemosphere.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Saradhi PP, Alia, Arora S, Prasad KV. 1995. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209:1–5. doi: 10.1006/bbrc.1995.1461. [DOI] [PubMed] [Google Scholar]
- 48.Liang X, Dickman MB, Becker DF. 2014. Proline biosynthesis is required for endoplasmic reticulum stress tolerance in Saccharomyces cerevisiae. J Biol Chem 289:27794–27806. doi: 10.1074/jbc.M114.562827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan N, Dickman MB, Becker DF. 2008. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 44:671–681. doi: 10.1016/j.freeradbiomed.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visick JE, Clarke S. 1997. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J Bacteriol 179:4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. 2004. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol 11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 53.Zheng M, Aslund F, Storz G. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 54.Aslund F, Zheng M, Beckwith J, Storz G. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A 96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Flecha B, Demple B. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem 270:13681–13687. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 56.Seaver LC, Imlay JA. 2004. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem 279:48742–48750. doi: 10.1074/jbc.M408754200. [DOI] [PubMed] [Google Scholar]
- 57.Moxley MA, Becker DF. 2012. Rapid reaction kinetics of proline dehydrogenase in the multifunctional proline utilization A protein. Biochemistry 51:511–520. doi: 10.1021/bi201603f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnan N, Becker DF. 2006. Oxygen reactivity of PutA from Helicobacter species and proline-linked oxidative stress. J Bacteriol 188:1227–1235. doi: 10.1128/JB.188.4.1227-1235.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seaver LC, Imlay JA. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol 183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang F, Zhang Y, Dusting GJ. 2011. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 61.Imlay JA, Linn S. 1986. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol 166:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imlay JA, Linn S. 1987. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol 169:2967–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. 2012. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A 109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. 2012. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab 15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyer MS, Reed RR, Steitz JA, Low KB. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harbor Symp Quant Biol 45(Part 1):135–140. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Imlay JA. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]





