Abstract
What is known and objective
Ponatinib is a potent oral tyrosine kinase inhibitor with activity against BCR-ABL, the primary driver of chronic myeloid leukaemia and Philadelphia chromosome–positive acute lymphoblastic leukaemia. This single-centre, single-dose, randomized, open-label, three-period crossover study evaluated the pharmacokinetics and bioavailability of a single oral dose of ponatinib (45-mg tablet) under fasting conditions and following consumption of high- and low-fat meals by healthy subjects.
Methods
Subjects were randomly assigned to one of the six possible treatment sequences, each evaluating three ponatinib 45-mg treatments: administered under fasting conditions; administered after a high-fat meal; or administered after a standardized low-fat meal. The high-fat meal derived approximately 50% of its total caloric content from fat, with approximately 150, 250 and 500–600 calories derived from protein, carbohydrates and fat, respectively (total of approximately 900–1000 calories). The standardized low-fat meal derived no more than 20% of total caloric content from fat, with approximately 56, 428 and 63 calories derived from protein, carbohydrates and fat, respectively (total of approximately 547 calories). During each of the three treatment periods, blood samples were collected predose and at 13 time points over the 96-h post-dose interval. Plasma concentrations of ponatinib were measured by liquid chromatography/tandem mass spectrometry. Mixed-model analyses of variance (anova) were performed on natural log-transformed PK parameters Cmax and AUC0-∞.
Results and discussion
Geometric mean maximum plasma concentration (Cmax) values for the fasted, low-fat and high-fat regimens were 54·7, 51·6 and 51·5 ng/mL, respectively. Geometric mean area under the concentration–time curve from time zero to infinity (AUC0-∞) values for the fasted, low-fat and high-fat regimens were 1273, 1244 and 1392 h × ng/mL, respectively. All limits of the 90% CIs of the estimated geometric mean ratios for Cmax and all AUC comparisons fell within the 80%–125% margins. These results indicate that consumption of a high- or low-fat meal within 30 min prior to administration of ponatinib had no effect on the single-dose pharmacokinetics of ponatinib.
What is new and conclusion
Food does not affect the single-dose pharmacokinetics of ponatinib. These data demonstrate that ponatinib may be administered with or without food.
Keywords: bioavailability, chemotherapy, haematology, oncology, therapeutics
Introduction
Clinical studies and current treatment guidelines acknowledge that the use of tyrosine kinase inhibitors (TKIs) is an appropriate treatment strategy for sustained disease control in chronic myeloid leukaemia (CML). Soon after the advent of the BCR-ABL TKI imatinib, it became evident that Philadelphia chromosome–positive cells could evolve to evade inhibition. Mutations in the kinase domain of BCR-ABL that impede effective inhibitor binding are the primary mechanisms of resistance to currently available agents including imatinib (Novartis, East Hanover, NJ, USA), nilotinib (Novartis), dasatinib (Bristol-Myers Squibb, Princeton, NJ, USA) and bosutinib (Pfizer, New York, NY, USA). In particular, the T315I gatekeeper mutant – the most commonly observed point mutation in clinical practice – confers universal resistance to these agents.1–3 At present, the only TKI that is effective in patients with the T315I mutation is ponatinib (ARIAD Pharmaceuticals, Inc., Cambridge, MA, USA).4
Ponatinib is a novel, synthetic, orally active, potent pan–BCR-ABL inhibitor and multi-targeted TKI that was discovered using a computational and structure-based drug design approach.5–7 Ponatinib was specifically designed to inhibit native BCR-ABL and mutant forms that can arise during treatment with other TKIs and cause resistance, including the T315I gatekeeper mutant. Based on results of phase 1 and 2 clinical trials in patients with CML and Philadelphia chromosome–positive acute lymphoblastic leukaemia (Ph+ ALL),4,8 ponatinib 45 mg once daily received accelerated approval in the United States for the treatment of patients with CML and Ph+ ALL who are resistant or intolerant to prior TKI therapy.9
Tyrosine kinase inhibitors have diverse characteristics regarding absorption from the gastrointestinal tract. In the phase 1 and 2 clinical studies, patients were instructed to take an oral dose of ponatinib with water approximately 2 h after a light meal and to not eat or drink anything other than water for 2 h after taking ponatinib. In the absence of data supporting a lack of food effect during the early clinical development of ponatinib, it was decided to dose patients under fasting conditions to ensure that food did not have an impact on the pharmacokinetic (PK) profile of the drug. However, the impact of co-administration with food requires more detailed evaluation. This study was conducted to evaluate the PK of the tablet formulation of ponatinib after consumption of high- and low-fat meals in healthy subjects.
Material and Methods
Subject selection and study design
This was a single-centre, single-dose, randomized, open-label, three-period, six-sequence crossover study conducted in healthy subjects in accordance with Food and Drug Administration guidelines for the assessment of food effects. The study was designed to enrol 24 male or female (of non-childbearing potential) subjects to ensure completion of at least 21 subjects. Subjects were included if they were between 18 and 55 years of age and in good health, as determined by medical history, physical examination, vital signs, electrocardiogram (ECG) results and laboratory tests conducted at screening. Each subject was required to have a body mass index (BMI) of 18·0–33·0 kg/m2 and a minimum weight of 50·0 kg at screening. Subjects were required to abstain from using prescription, investigational, over-the-counter or recreational drugs during the study until after the final follow-up visit. Subjects with a history or presence of clinically significant illnesses; surgical or medical conditions that could have interfered with the absorption, distribution, metabolism or excretion of the study drug; an acute disease state; or who reported use of over-the-counter drugs within 72 h before the first study drug administration or investigational or prescription drugs within 30 days of the first study drug administration were excluded. Also excluded were subjects with self-reported drug or alcohol abuse within 1 year of first drug administration; positive breath alcohol test at screening or at admission to any treatment period; clinically important drug allergies; positive serological findings for hepatitis B, hepatitis C or HIV antibodies; or who had recently donated or lost a clinically significant amount of blood.
All subjects provided signed informed consent. The protocol, the subject information and consent form, and other relevant study documentation were approved by Institutional Review Board Services Ontario prior to initiation of the study. The study was performed in accordance with the requirements of the World Medical Association Declaration of Helsinki in its revised edition, the International Conference on Harmonisation guidelines for current Good Clinical Practice and the demands of national drug and data protection laws and other applicable regulatory requirements.
Treatment administration
Ponatinib is a film-coated tablet with an immediate-release formulation. The effects of food on the PK of ponatinib were tested under three conditions: (A) a single dose of ponatinib 45 mg, administered under fasting conditions; (B) a single dose of ponatinib 45 mg, administered after completion of the Food and Drug Administration–recommended high-fat meal; and (C) a single dose of ponatinib 45 mg, administered after completion of a standardized low-fat meal. Subjects were randomly assigned to one of the six possible treatment sequences. In the fasted condition, subjects were administered the study drug with approximately 240 mL of water. No food was allowed for at least 10 h predose and at least 4 h post-dose; water was allowed as desired except for 1 h before and after drug administration. In the fed condition, subjects started the test meal 30 min before administration of the study drug. No additional food was allowed for at least 4 h post-dose; water was allowed as desired except for 1 h before and after drug administration. The high-fat meal derived 50% of its total caloric content from fat, with approximately 150, 250 and 500–600 calories derived from protein, carbohydrates and fat, respectively (total of approximately 900–1000 calories). The high-fat meal consisted of 240 mL of whole milk, two slices of toasted white bread, 3 × 6·5 g of butter (6·5 g for each toast and 6·5 g to fry two eggs), two fried eggs (scrambled), two hash browns (two ounces each) and two strips of bacon. The standardized low-fat meal derived no more than 20% of total caloric content of the meal from fat, with approximately 56, 428 and 63 calories derived from protein, carbohydrates and fat, respectively (total of approximately 547 calories). The low-fat meal consisted of 175 g of fat-free vanilla yogurt, one medium banana (18–20 cm), 125 mL of apple juice, one teaspoon of margarine, 15 mL of honey and two slices of toasted whole-wheat bread.
Sample collection and assessments
During each of the three treatment periods, 4-mL blood samples were collected prior to dosing and at 0·5, 1, 2, 4, 5, 6, 8, 12, 24, 36, 48, 72 and 96 h. Blood samples were collected by venepuncture or optional indwelling catheter into K2–ethylenediaminetetraacetic acid tubes and processed according to the clinical research site's study-specific procedures. All samples were stored at −20 ± 5°C.
Plasma ponatinib analyses
Concentrations of ponatinib were determined in plasma by liquid chromatography/tandem mass spectrometry (LC/MS/MS). Calibration standards (nine samples, covering the range 0·5–250 ng/mL) and quality control (QC) standards (four samples, covering the range 0·5–200 ng/mL) were prepared by spiking ponatinib solutions of known concentrations into blank human plasma. Aliquots (0·15 mL) of study plasma samples, calibrations standards, QC standards and control blanks were transferred to individual wells of a 96-well block. A 0·05-mL solution containing deuterated internal standards (IS, D3-ponatinib) was added to the plasma samples, followed by addition of 0·4 mL of 10 mm ammonium acetate buffer, pH 7·0. The samples were mixed thoroughly and centrifuged. The supernatants (0·6 mL) were passed through a preconditioned solid-phase extraction tube (Isolute, 100 mg; Biotage, Uppsala, Sweden). The tubes were washed with methanol, and the analytes were eluted into another 96-well block using 0·4 mL of 98/2 methanol/formic acid solution. The eluates were evaporated to dryness and reconstituted with 0·1 mL of a reconstitution solution (50 : 50 methanol/1000 : 10 water/1 m ammonium bicarbonate, pH 10). The final extracts were analysed by a validated LC/MS/MS method. Data were collected in positive-ion mode. Calculated correlations are based on peak area ratios of the analyte to its respective internal standard. The smoothing width was 3. The peak area for each analyte transition was divided by the peak area for the corresponding IS transition. The calibration curve for ponatinib was fit using a linear regression, weighted 1/x2. Intra- and interassay precision (% coefficient of variation [CV]) was ≤9·4% for ponatinib; the intra- and interassay accuracy (% relative error [RE]) was −10·4% to +10·5%. The lower limit of detection for ponatinib was 0·5 ng/mL.
Outcome measures
The primary objective of this study was to assess the effect of a high-fat meal compared with fasting on the relative bioavailability and PK of a single oral dose of ponatinib in healthy subjects. Secondary objectives included assessment of the effect of a low-fat meal compared with the fasting state on the relative bioavailability and PK of a single oral dose of ponatinib and to obtain additional safety and tolerability data of ponatinib in healthy subjects. Pharmacokinetic parameters assessed included maximum plasma concentration (Cmax); area under the plasma concentration–time curve from zero to the last measurable concentration (AUC0-t); area under the concentration–time curve from time zero to infinity (AUC0-∞); time to maximum concentration (tmax); terminal elimination half-life (t1/2); terminal phase rate constant (λZ); apparent total body clearance after oral administration (CL/F); and apparent distribution volume in the terminal elimination phase (V/F). Non-compartmental PK analyses were carried out to derive the PK parameters for ponatinib for all regimens using actual sampling times. Model 200 for extravascular administration from the WinNonlin library was applied.
Statistical methodology
Mixed-model analyses of variance (anova) were performed on natural log-transformed (Ln-transformed) PK parameters AUC0-∞, AUC0-t and Cmax. The anova model included sequence, treatment and period as fixed effects and subject nested within sequence as a random effect. Sequence was tested using subject nested within sequence as the error term, at a 10% level of significance; all other main effects were tested using the residual error (error mean square). Each anova included calculation of least squares mean (LSM), the difference between treatment LSM and the standard error associated with the difference. These were done using the SAS® Mixed procedure (SAS Institute Inc., Cary, NC, USA). Ratios of LSM were calculated using the exponential of the difference between treatment LSM from the analyses on the Ln-transformed AUC0-t, AUC0-∞ and Cmax. The comparison between (A) fasted and (B) high-fat conditions was considered as the primary analysis. The comparison between (A) fasted and (C) low-fat conditions was considered as a secondary analysis. Absence of food effect was to be concluded if the 90% confidence intervals (CIs) for the estimated mean ratios of ponatinib AUC0-∞, AUC0-t and Cmax for test (fed condition) to reference (fasted condition) were contained within the equivalence limits of 80%–125% for the primary comparisons. For each anova, only paired data sets were used; for each PK parameter, the subjects' data comprised a reliable value for the fasted regimen and one or both fed regimens. Differences between ponatinib tmax obtained for the fasted regimen and those for the fed regimens were evaluated by non-parametric testing of non-transformed data (Wilcoxon signed rank).
With the 90% CI for the geometric mean ratio (GMR) of fed versus fasted within the limits of 80%–125%, and the true fed/fasted GMR within 95%–105%, 21 subjects were required to have an 80% probability (power) of showing that the fed and fasted subject conditions would be population bioequivalent. This assumes that the within-subject variability of ponatinib PK would be approximately half that of the across-subject variability, corresponding to a correlation between time points of 0·875.9 If the true geometric mean ratio between fed and fasted is 1·00 (no food effect), then a study that enrolled 21 subjects would have a power of 97%. The false-positive rate (i.e. finding fed and fasted equivalent when they are not) would be <0·001. Twenty-four subjects were enrolled to ensure that at least 21 subjects completed all three periods of the study.
The number and percentage of subjects with specific adverse events (AEs) were tabulated by treatment at onset using MedDRA System Organ Classes and preferred terms and by maximum relationship and severity. Descriptive statistics and change from baseline (predose vital signs, laboratory values and ECG) were calculated for each parameter at each scheduled time point.
Results
A total of 24 subjects were randomized to treatment; of these, all but one subject completed all three treatments of the study. This subject was withdrawn from the study prior to period 3 because of elevated triacylglycerol lipase levels on the day before study drug administration; the levels subsequently returned to normal. One additional subject was excluded from the evaluable PK set because the subject's ponatinib plasma concentrations were all below the lower limit of quantification after administration of ponatinib during the high-fat regimen, suggesting that despite a visual mouth and hand check, the subject had not taken the dose as per protocol.
Demographics of all patients and the patients included in the PK set are shown in Table 1. With the exception of one female subject, all evaluable subjects were male (95·5%). Subjects were predominantly White (63·6%) or black/African American (22·7%); three subjects (13·6%) were Asian. The mean age of subjects in the PK set was 41·2 years (range, 24–53 years), and the mean BMI was 25·7 kg/m2 (range, 20·7–30·5 kg/mg2).
Table 1.
Demographic characteristics
| Characteristics | All subjects (N = 24) | PK set (N = 22) | |
|---|---|---|---|
| Age (years) | Mean (SD) | 41·3 (6·9) | 41·2 (7·2) |
| Median (range) | 41·0 (24–53) | 41·0 (24–53) | |
| Weight (kg) | Mean (SD) | 81·0 (12·9) | 80·5 (12·9) |
| Median (range) | 76·7 (63·7–110·6) | 76·7 (63·7–110·6) | |
| BMI (kg/m2) | Mean (SD) | 25·7 (2·9) | 25·7 (3·0) |
| Median (range) | 25·8 (20·7–30·5) | 25·8 (20·7–30·5) | |
| Gender | Male, n (%) | 23 (95·8) | 21 (95·5) |
| Female, n (%) | 1 (4·2) | 1 (4·5) | |
| Race | White, n (%) | 15 (62·5) | 14 (63·6) |
| Black or African American, n (%) | 6 (25·0) | 5 (22·7) | |
| Asian, n (%) | 3 (12·5) | 3 (13·6) | |
| Ethnicity | Non-Hispanic or Non-Latino, n (%) | 23 (95·8) | 21 (95·5) |
| Hispanic or Latino, n (%) | 1 (4·2) | 1 (4·5) | |
SD, standard deviation; BMI, body mass index.
All subjects were able to complete their entire meal within the required time period (30 min or less) for each of the two fed treatments of the study. Four subjects in each of the three treatment groups (12 subjects in total) did not receive the snack (one granola bar and 300 mL of grape juice) on the evening of the first day of period 1, which was part of the standardized protocol. Because this snack was to occur approximately 10–12 h after the dosing of ponatinib, it is unlikely to be relevant to the oral pharmacokinetics of ponatinib.
Maximum mean concentrations for the high-fat, low-fat and fasted regimens were reached approximately 5–6 h after oral administration of a single 45-mg ponatinib tablet and were between 51·7 and 54·5 ng/mL. Ponatinib in plasma was quantifiable through the 96-h sampling interval across all three treatment regimens. Notably, there were no differences between the respective absorption and distribution profiles for the three treatment regimens (Fig. 1). Ponatinib plasma concentrations declined in a multi-exponential manner; as expected, the elimination rate was not affected by the food regimen.
Fig 1.
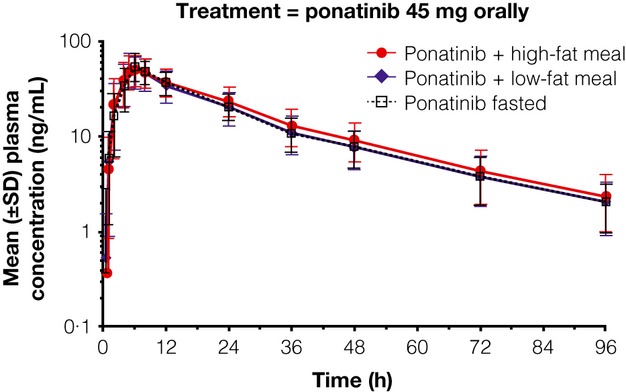
Mean ponatinib plasma concentrations vs. time for ponatinib + high- or low-fat meal, or fasting.
Descriptive statistics of the derived ponatinib PK parameters are summarized in Table 2. Median tmax values were similar overall in the fasting, high-fat and low-fat diets. Maximum plasma concentration was observed at ∼6 h post-dose (range, 5–8 h for the fasted and low-fat regimen and 4–12 h for the high-fat regimen). Geometric mean Cmax values for the fasted, low-fat and high-fat regimens were 54·7, 51·6 and 51·5 ng/mL, respectively. Geometric mean AUC0-∞ values for the fasted, low-fat and high-fat regimens were 1273, 1244 and 1392 h × ng/mL, respectively. There was no consistent difference between the three treatment regimens with regard to the mean (range, 23·9–24·7 h) and median (range, 23·3–24·2 h) ponatinib t1/2 values.
Table 2.
Ponatinib pharmacokinetic parameters in plasma under fasted and fed (high-fat and low-fat) conditionsa
| Tmax, hb | Cmax, ng/mL (%CV) | AUC0-t, h × ng/mL (%CV) | AUC0-∞, h × ng/mL (%CV) | T1/2, hc (%CV) | CL/F, L/h (%CV) | V/F, L (%CV) | |
|---|---|---|---|---|---|---|---|
| Fasted (n = 22) | 6·0 (5·0–8·0) | 54·7 (26·1) | 1203 (26·9) | 1273 (28·3) | 24·2 (14·7) | 35·4 (35·0) | 1242 (30·0) |
| High-fat meal (n = 22) | 6·0 (4·0–12·0) | 51·5 (26·3) | 1315 (27·5) | 1392 (29·1) | 23·5 (16·1) | 32·3 (34·2) | 1104 (28·8) |
| Low-fat meal (n = 22) | 5·0 (5·0–8·0) | 51·6 (27·2) | 1175 (28·7) | 1244 (29·7) | 23·3 (14·9) | 36·2 (38·7) | 1278 (37·6) |
Tmax, time to maximum concentration; Cmax, maximum plasma concentration; %CV, coefficient of variation; AUC0-t, area under the concentration–time curve from time zero to the last measurable time point; AUC0-∞, area under the concentration–time curve from zero to infinity; T1/2, terminal elimination half-life; CL/F, clearance after oral administration; V/F, volume in the terminal elimination phase.
Geometric mean values with %CV are shown.
Value for Tmax is median (range).
Value for T1/2 is median.
Table 3 summarizes the results of inferential analyses conducted to evaluate the effect of high-fat and low-fat meal intake on single-dose bioavailability of ponatinib. The relative bioavailability of a single dose of ponatinib 45 mg is not affected by the consumption of either a high-fat or a low-fat meal within 30 min prior to administration of ponatinib. All limits of the 90% CIs of the estimated geometric mean ratios for all of the comparisons fell within the 80%–125% margins required to conclude the absence of a clinically relevant effect of food intake on ponatinib single-dose bioavailability.
Table 3.
anova for impact of fasting/fed state on relevant ponatinib pharmacokinetic parameters
| 90% CI | |||||
|---|---|---|---|---|---|
| Reference treatment | Test treatment | Ln-transformed variable | Estimated mean ratio (T/R), % | Lower limit | Upper limit |
| Fasted | High-fat meal | AUC0-∞ | 109·55 | 105·85 | 113·38 |
| AUC0-t | 109·50 | 105·83 | 113·29 | ||
| Cmax | 94·22 | 89·70 | 98·97 | ||
| Fasted | Low-fat meal | AUC0-∞ | 97·85 | 94·54 | 101·27 |
| AUC0-t | 97·73 | 94·46 | 101·11 | ||
| Cmax | 94·29 | 89·77 | 99·05 |
CI, confidence interval; T/R, test/reference; AUC0-∞, area under the concentration–time curve from zero to infinity; AUC0-t, area under the concentration–time curve from time zero to the last measurable time point; Cmax, maximum plasma concentration.
In total, 20 AEs in 10 (41·7%) subjects were considered at least possibly related to treatment. Treatment-related AEs reported for more than one subject included headache (six subjects) and increased lipase levels (three subjects). Except for headache, which occurred more often during the fasting regimen, the incidence of treatment-related AEs was similar for the three regimens. No differences were observed for laboratory parameters among treatment regimens. A total of five clinically significant abnormalities were observed in a total of five subjects, including three subjects with high triacylglycerol lipase levels, one with high aspartate aminotransferase and one with a high prothrombin international normalized ratio. There were no severe AEs, one moderate AE (viral gastroenteritis, considered to be unrelated to treatment) and 29 mild AEs among 13 subjects. All AEs resolved by the end of the trial. No clinically significant abnormalities were observed for vital signs, ECG parameters or physical examinations.
Discussion
This study, which was conducted in healthy subjects who received a single dose of ponatinib 45 mg in tablet formulation (identical to the formulation used in clinical trials and the marketed product), demonstrates that the consumption of either a high- or a low-fat meal within 30 min of drug administration had no effect on the single-dose PK of ponatinib. Overall, ponatinib was generally well tolerated in these healthy subjects, regardless of the food intake regimen.
The other TKIs currently indicated for the treatment of CML have diverse characteristics regarding absorption from the gastrointestinal tract. Imatinib must be administered with a meal and a large glass of water,10 whereas food should be avoided for 2 h before and 1 h after administration of nilotinib.11 Bosutinib must be administered with food.12 In contrast, the PK of dasatinib is unaffected by food intake, and the drug may be taken with or without food.13
The flexibility afforded by the ability to administer a drug with or without food is a useful characteristic for any drug, particularly in disease states in which patients may be taking multiple medications with more stringent requirements regarding meal intake. Together, the data summarized here demonstrate that ponatinib may be administered without regard to meal intake.
Acknowledgments
This study was sponsored by ARIAD Pharmaceuticals, Inc. Professional medical writing assistance was provided by John Ferguson, PhD, Medicus International New York, and funded by ARIAD Pharmaceuticals, Inc.
References
- 1.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tokarski JS, Newitt JA, Chang CY, et al. The structure of dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg E, Manley P, Mestan J, Cowan-Jacob S, Ray A, Griffin JD. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94:1765–1769. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang WS, Metcalf CA, Sundaramoorthi R, et al. Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-y l)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J Med Chem. 2010;53:4701–4719. doi: 10.1021/jm100395q. [DOI] [PubMed] [Google Scholar]
- 6.O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou T, Commodore L, Huang WS, et al. Structural mechanism of the pan-BCR-ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance. Chem Biol Drug Des. 2011;77:1–11. doi: 10.1111/j.1747-0285.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 8.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A pivotal phase 2 trial of ponatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant or intolerant to dasatinib or nilotinib, or with the T315I BCR-ABL mutation: 12-month follow-up of the PACE trial. Blood. 2012;120 abstract 163. [Google Scholar]
- 9.ARIAD Pharmaceuticals, Inc. Ponatinib (Iclusig) prescribing information. Cambridge, MA: ARIAD Pharmaceuticals, Inc; 2012. [Google Scholar]
- 10.Novartis Pharmaceuticals Corporation. Imatinib (Gleevec) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011. [Google Scholar]
- 11.Novartis Pharmaceuticals Corporation. Nilotinib (Tasigna) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012. [Google Scholar]
- 12.Pfizer Inc. Bosutinib (Bosulif) prescribing information. New York, NY: Pfizer Inc; 2012. [Google Scholar]
- 13.Bristol-Myers Squibb. Dasatinib (Sprycel) prescribing information. Princeton, NJ: Bristol-Myers Squibb; 2011. [Google Scholar]


