Abstract
Hantaviruses are the causative agents of haemorrhagic fever with renal syndrome (HFRS) in Eurasia and of hantavirus cardiopulmonary syndrome (HCPS) in the Americas. The case fatality rate varies between different hantaviruses and can be up to 40%. At present, there is no specific treatment available. The hantavirus pathogenesis is not well understood, but most likely, both virus-mediated and host-mediated mechanisms are involved. The aim of the present study was to investigate the association among Puumala hantavirus (PUUV) viral RNA load, humoral immune response and disease severity in patients with HFRS. We performed a study of 105 PUUV-infected patients that were followed during the acute phase of disease and for up to 1–3 months later. Fifteen of the 105 patients (14%) were classified as having moderate/severe disease. A low PUUV-specific IgG response (p <0.05) and also a higher white blood cell count (p <0.001) were significantly associated with more severe disease. The PUUV RNA was detected in a majority of patient plasma samples up to 9 days after disease onset; however, PUUV RNA load or longevity of viraemia were not significantly associated with disease severity. We conclude that a low specific IgG response was associated with disease severity in patients with HFRS, whereas PUUV RNA load did not seem to affect the severity of HFRS. Our results raise the possibility of passive immunotherapy as a useful treatment for hantavirus-infected patients.
Keywords: Age, disease severity, hantavirus, haemorrhagic fever with renal syndrome, humoral immune response, immunoglobulins, neutrophil, Puumala virus, viral load, white blood cell count
Introduction
Hantaviruses can cause two febrile diseases in humans: haemorrhagic fever with renal syndrome (HFRS) in Europe and Asia, and hantavirus cardiopulmonary syndrome (HCPS) in the Americas. Humans are infected by inhalation of aerosolized rodent excreta containing virus. Puumala virus (PUUV) is endemic in Central and Northern Europe and causes a relatively mild form of HFRS, also known as Nephropathia Epidemica. The most common symptoms of PUUV infection are fever, headache, nausea, vomiting, myalgia, abdominal/back pain and visual disturbances 1. The majority of patients also have signs of renal failure and other important clinical features are mild haemorrhagic manifestations and respiratory symptoms 2. Predictors of severe renal impairment in hantavirus-infected patients include high leucocyte counts at the time of admission 3,4.
To date, there is no approved specific treatment for hantavirus-infected patients, instead supportive care is applied 5,6. Hantaviruses are believed to be non-cytopathic and the mechanisms behind the capillary leakage and haemorrhage are poorly understood. Studies imply that the pathogenesis is multifactorial and includes contributions from immune responses, platelet dysfunction, dysregulation of the endothelial cell barrier and genetic host factors 1,7,8. Among immune parameters, over-activation of CD8 T cells and natural killer cells, and induction of cytokines are thought to play important roles 9–12. Previous studies of viraemia in HCPS and HFRS suggest an association between initial high viral load of Dobrava, Sin Nombre and Hantaan viruses, respectively, and a more severe clinical outcome 13–16. This association implicates a potential direct role for the virus in pathogenesis and interventions with antivirals as a possible therapy for hantavirus-infected patients. However, contradictory results have been reported from studies regarding antiviral treatment of HFRS and HCPS patients and one theory is that Ribavirin may have been given too late in the course of infection 6,17,18. More data regarding the duration of viraemia are needed to determine the window of opportunity for antiviral therapy. Another approach for specific treatment of hantavirus infection may be administration of human neutralizing antibodies. However, there are no published controlled clinical trials of immunotherapy for hantavirus-infection. Studies on humoral immune responses to hantavirus infection have shown an association between low specific IgG antibody titres and disease severity for HCPS patients, suggesting that a strong IgG response to infection might be protective 19,20. The results on specific IgA and IgM responses are contradictory regarding an association with disease severity in HCPS 19,20. In HFRS caused by PUUV not only IgG but also IgA is known to neutralize the virus 19–21.
In this study on 105 PUUV-infected patients we analysed the duration of viraemia and the association between disease severity and viral load, humoral immune response and different laboratory parameters. Our results address the potential use of specific treatment, i.e. antivirals and immunotherapy in hantavirus-infected patients.
Methods
Study design and patient material
Patients with acute HFRS were included at the Clinic of Infectious Diseases, Umeå University Hospital. All patients were serologically verified by immunofluorescence assay for PUUV-specific IgM and IgG. If only IgM was present in the first serum sample the patient diagnosis had to be confirmed by seroconversion of IgG in follow-up samples. In three patients only IgG was present and the acute infection was verified by RNA detection with PCR or through avidity testing of the IgG antibodies 22. Both hospitalized patients and outpatients were followed with regards to symptoms and routine laboratory tests. Blood samples were drawn at first contact with healthcare and thereafter repeated sampling was performed approximately every other day during the acute phase. The patients were followed up after 1–3 months. Written and oral informed consent was obtained from all patients and the study was approved by the regional ethics committee of Umeå University, Umeå, Sweden.
Criteria for severe illness
Patients who met two or more of the following criteria were considered to have a moderate/severe illness: treated in the intensive care unit, dialysis, radiologically verified thrombosis, need for platelet transfusion, moderate/severe hypotension (systolic blood pressure ≤ 90 mmHg and intravenous fluid treatment at any given time during hospitalization), and bleeding of moderate/major importance; i.e. gastrointestinal bleeding, macroscopic haematuria and metrorrhagia 23.
Real-time reverse transcription-PCR
RNA extraction and real-time reverse transcription-PCR were performed as previously described 24. Briefly, the RNA was reverse-transcribed followed by a real-time PCR with primers and probe detecting the PUUV S-segment.
Immunofluorescence
To detect PUUV-specific IgM, IgA and IgG serum antibodies, an immunofluorecence analysis was performed as previously described 24. Briefly, patient serum was added to spot-slides covered with fixed Vero E6 cells infected with the local strain PUUV Umeå/hu. For IgM analysis, sera were pretreated with Reumatoid factor-absorbent (Virion\Serion GmbH, Würzburg, Germany) to eliminate possible interference by rheumatoid factor. Presence of PUUV-specific antibodies was determined by use of fluorescein-conjugated rabbit anti-human IgM, IgA and IgG (F0317, F0204 and F202, respectively by DAKO A/S Glostrup, Denmark). Sera were diluted to the end-point titre (Fig. 1).
Fig 1.
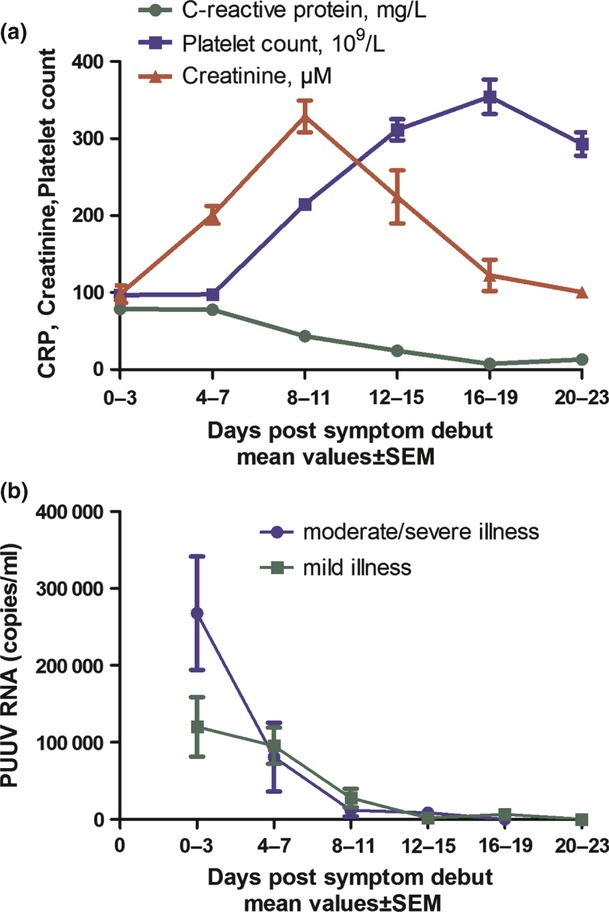
Laboratory findings and viral RNA kinetics in 105 patients with Puumala hantavirus (PUUV) infection. (a) C-reactive protein (CRP), creatinine and platelet count presented during the first 3 weeks after disease onset. (b) The kinetics of PUUV RNA load in plasma samples from patients with mild versus moderate/severe illness. The figure represents mean values for each time interval and the error bars are standard error of the mean (SEM). In total, 341 plasma samples from the 105 patients were analysed. The proportions of samples from patients with moderate/severe versus mild illness were: (0–3 days) 4 vs 16 samples, (4–7 days) 14 vs 92, (8–11 days) 21 vs 87, (12–15 days) 7 vs 30, (16–19 days) 6 vs 23, and (20–23 days) 0 vs 16.
Statistical analysis
The clinical symptoms were described using percentages, whereas laboratory findings were presented as medians and range. Mann–Whitney U test was used to compare laboratory findings between patients with mild versus severe illness.
Binary logistic regression was performed to analyse associations with disease severity. Levels of PUUV RNA, PUUV-specific antibody titres and white blood cell count (all first available samples) were investigated as predictors for disease severity. Associations of severe disease with age, sex and the number of days after disease onset when samples were collected were also investigated. A multivariate model was constructed by first conducting univariate logistic regression analysis for each factor separately and then by including all significant predictive factors simultaneously in a model. The same procedure was applied to find predictors for airway symptoms, need for oxygen treatment and bleeding manifestations. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA). Limit of significance was set at 0.05.
Results
Clinical characteristics and laboratory findings
Older patients (>50 years) had a significantly higher number of hospital days compared with younger patients (mean 5.7 and 2.8 days, respectively, p 0.045). No difference was found in the hospitalization rate or time spent in hospital between the sexes (p 0.662, p 0.204, respectively) in the study group. All 105 patients had signs of acute HFRS. There were no fatal cases, but 15 of the 105 patients (14%) were defined as having moderate/severe illness (Table 1). Of all patients, 26% had one or more symptoms from the lower respiratory tract and 12% were given oxygen treatment because of desaturation. Five (4.8%) patients were treated in the intensive care unit for symptoms such as respiratory failure, anuria, thrombosis or confusion/depression of consciousness.
Table 1.
Demographics of the study population and clinical symptoms
| Characteristics | All patients (n = 105) | Moderate/severe illness (n = 15) |
|---|---|---|
| Age, mean and range | 50 (40–63) | 58 (43–73) |
| Gender, female/male (n) | 56/49 | 8/7 |
| Hospital care | 72% | 100% |
| Days of hospital care (mean and range) | 4 (0–55) | 12 (1–55) |
| Fever | 98% | 100% |
| Headache | 74% | 47% |
| Backache | 51% | 33% |
| Nausea/vomiting | 51% | 53% |
| Respiratory symptoms | 41% | 67% |
| Lower respiratory symptoms | 26% | 53% |
| Abdominal pains | 35% | 40% |
| Blurred vision | 21% | 33% |
| Renal impairment (creatinine > normal range) | 88% | 87% |
| Renal impairment (creatinine >4 times normal) | 33% | 60% |
| Haemorrhagic manifestations | 28% | 33% |
| Thrombocytopenia (<100 × 109/L) | 71% | 93% |
| Thrombocytopenia (<50 × 109/L) | 34% | 80% |
Respiratory symptoms included all symptoms from both the upper and lower respiratory tract. Lower respiratory tract symptoms were defined as dyspnoea, hypoxia and obstructivity. Creatinine levels reported in (μM) were used as a marker for renal impairment, when described as over normal range or more than four times normal for women and men, respectively.
A majority of the patients had thrombocytopenia (Tables1 and 2) and almost one-third showed bleeding manifestations. Epistaxis was most frequent, but also haematemesis, melaena, visible haematuria, petechial rash and haematoma were noted. Eight patients received platelet infusion, either for bleeding in combination with fever, or as a prophylactic treatment because of pronounced thrombocytopenia with platelet counts less than 20 × 109 to 30 × 109/L. Acute renal failure was seen in one-third of the patients (defined as a creatinine level more than four times normal). One patient had haemodialysis during the hospital stay for renal impairment and volume overload.
Table 2.
Laboratory findings in association with disease severity
| All patients (n = 105) | Mild illness (n = 90) | Moderate/severe illness (n = 15) | p-value | Normal range | |
|---|---|---|---|---|---|
| Max. CRP (mg/L) | 68 (11–254) | 65 (11–236) | 82 (29–254) | 0.083 | <3 mg/L |
| Min. platelets (109/L) | 72 (12–397) | 79 (19–397) | 35 (12–182) | <0.001 | Females 165–387, Males 145–348 |
| Max. WBC (109/L) | 9.5 (4–50) | 9.2 (4–22) | 14.3 (8–50) | <0.001 | 3.5–8.8 |
| Max. neutrophils (109/L) | 5.8 (2.8–18.3) | 5.6 (2.8–13.1) | 7.2 (5.3–18.3) | <0.001 | 1.8–6.3 |
| Max. lymphocytes (109/L) | 2.1 (0.6–6.2) | 2.1 (0.6–6.2) | 2.6 (0.6–6.0) | 0.927 | 1.0–3.5 |
| Max. monocytes (109/L) | 0.9 (0.4–3.2) | 0.9 (0.4–2.4) | 1.1 (0.4–3.2) | 0.352 | 0.3–1.2 |
| Max. creatinine (μM) | 232 (60–1634) | 222 (60–1548) | 370 (136–1634) | 0.01 | Females 45–90, Males 60–105 |
| Min. sodium (mM) | 136 (107–146) | 136 (121–146) | 130 (113–144) | 0.064 | 137–145 |
| Max. potassium (mM) | 4.4 (3.3–6.3) | 4.5 (3.3–6.3) | 4.4 (3.8–5.0) | 0.346 | 3.6–4.6 |
| Max. ALT (μkat/L) | 1.2 (0.33–7.81) | 1.2 (0.3–7.8) | 1.2 (0.3–3.6) | 0.797 | Females <0.75, Males <1.1 |
| Max. AST (μkat/L) | 1.0 (0.35–4.45) | 1.0 (0.35–4.5) | 1.2 (0.6–3.5) | 0.209 | Females <0.6, Males <0.75 |
| Max. LD (μkat/L) | 5.6 (2.6–11.8) | 5.4 (2.6–11.8) | 7.0 (4.2–10.3) | 0.008 | <4.2 |
| IgG titre | 1:320 (neg–1:5120) | 1:320 (neg–1:5120) | 1:40 (neg–1:1280) | 0.023 |
CRP, C-reactive protein; WBC, white blood cell count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LD, lactate dehydrogenase.
Laboratory values were given as medians, range within parentheses. The p-value was calculated using Mann–Whitney U test.
The median IgG titre is presented as median and range. Negative samples represent titres <1 : 40.
Serum creatinine peaked at around day 8 after symptom debut (Fig. 1a). The minimum platelet count was noted in the first days after disease onset, whereas it normalized at the time of maximum creatinine levels. C-reactive protein, a marker for inflammation, reached maximum levels after a few days (Fig. 1a) and lactate dehydrogenase, corresponding to tissue damage, was also increased during the acute infection (data not shown).
Kinetics of PUUV RNA load
Puumala virus RNA was detected in 90% of the patient samples collected at day 0–3 after onset of disease (mean 1.17 × 105 PUUV RNA copies/mL) and in 81% of the patient samples collected at day 4–7 (Fig. 1b). In most patients, PUUV RNA was detected until day 9 after symptom debut. In total, 80% of the patients had detectable RNA in at least one sample and the RNA-negative patients had their first sample drawn at a significantly later day (p <0.001) of disease compared with the positive (mean day 10.3 and 5.4, respectively).
The humoral immune response upon acute infection
The first available serum sample from all patients was analysed for PUUV-specific IgM, IgA and IgG (Fig. 2); 93% were IgM positive, 86% were IgA positive and 80% were IgG positive. (Fig. 2a–c). Four patients were negative for all three types of antibodies in their first samples, they all had their first sample taken at day 1–3 and they all seroconverted in follow-up samples. Seventeen patients were positive for IgM but negative for IgG in their first sample, they all seroconverted for IgG in follow-up samples.
Fig 2.
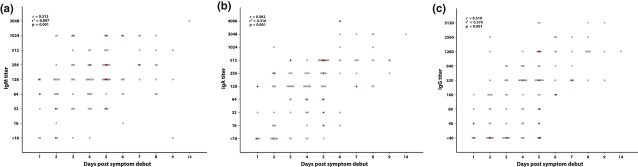
Kinetics of Puumala hantavirus (PUUV) -specific humoral immune response. Each patient's first available serum sample is represented by a dot. Antibody titres were significantly (p <0.05) higher in late samples then in early samples, r, r² and p-value were calculated using linear regression (log10 value of the titre). Moderate/severe patient samples are marked by red squares. (No samples were collected day 10–13).
All samples analysed after day 5 of disease had detectable IgA and IgG (Fig. 2b,c). Only three patients were IgG and IgA positive but IgM negative (sampling days 2, 4 and 9). No patient was positive for IgA exclusively.
Association between antibody response and viral load
There was a statistically significant inverse association between presence of PUUV-specific IgG and IgA antibodies and low PUUV RNA load (p 0.014 and p 0.001, respectively). No such association could be seen for IgM (see Supplementary material, Figures S1 and S2).
Associations with disease severity
Patients with moderate/severe illness had significantly lower platelets, higher creatinine, higher lactate dehydrogenase, higher white blood cell counts and neutrophils compared with patients with mild disease (Table 2).
Potential predictors for disease severity were investigated. Univariate logistic regression revealed that high age, low PUUV-specific IgG response and higher white blood cell count were significantly associated with moderate/severe disease (Table 3). We further combined all significant factors in a multivariate analysis, which confirmed that low PUUV-specific IgG-titre and higher white blood cell count were significantly associated with moderate/severe disease, whereas age was not (p 0.248; Table 3). The difference in IgG titres and WBC counts observed between mild and moderate/severe illness is also presented in Table 2 and in Supplementary material, Figures S3 and S4. High viral load was not significantly associated with severe disease (p 0.53). Although there was a tendency for higher viral load in patients with moderate/severe illness 0–3 days after disease onset, it was not significantly associated with disease severity (Table 3). We also investigated if long-lasting presence of PUUV RNA in blood was associated with more severe disease. Of the patients, 46% had at least one positive sample during the second and third weeks of disease; however, long lasting viraemia was not associated with severe disease (p 0.735 Pearson chi-squared test).
Table 3.
Regression analysis of predictors for moderate/severe disease
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Age | 1.047* | 1.004–1.092 | 1.029 | 0.980–1.080 |
| Sex | 1.000 | 0.334–2.991 | – | – |
| PUUV RNA levela in plasma | 1.099 | 0.816–1.479 | – | – |
| PUUV-specific IgM titrea in serum | 1.167 | 0.527–2.582 | – | – |
| PUUV-specific IgA titrea in serum | 1.042 | 0.579–1.874 | – | – |
| PUUV-specific IgG titrea in serum | 0.588* | 0.376–0.920 | 0.442** | 0.245–0.798 |
| White blood cell count | 1.369*** | 1.145–1.639 | 1.418*** | 1.168–1.722 |
| Day of disease when the sample was collected | 0.914c | 0.755–1.107c | – | – |
| 0.916d | 0.703–1.194d | – | – | |
| 0.901e | 0.694–1.171e | – | – | |
Levels were measured in each patient's first available sample.
Bold text indicate significance
p <0.05
p <0.01
p <0.001.
Log scale (10 log).
Scale: 1 unit = 1 × 109/L.
Plasma sample for Puumala hantavirus (PUUV) RNA level. Subdivision into time intervals 0–3, 4–7 and 8–11 days was not significantly associated with moderate/severe illness (p 0.12, p 0.454 and p 0.747, respectively). Confidence intervals not applicable because of small samples sizes.
Serum sample for antibody titre.
Serum sample for white blood cell count.
Airway symptoms and bleeding diathesis are frequent in hantavirus-infected patients so these symptoms were evaluated in the same multivariate model as the disease severity classification. Higher age (OR = 1.039, 95% CI = 1.008–1.071, p 0.013), and a lower IgG titre (OR = 0.680, 95% CI = 0.471–0.980, p 0.039) were significantly associated with airway symptoms. Similarly, higher age (OR = 1.10, 95% CI = 1.035–1.167, p 0.002) and lower IgG titre (OR = 0.566, 95% CI = 0.330–0.970, p 0.038) were associated with the need for oxygen treatment. Bleeding manifestations showed no significant associations with any potential predictors in the model (data not shown).
Discussion
The aim of our study was to investigate the possible association between PUUV RNA load and humoral immune response with disease severity and from this, to gain further knowledge of disease pathogenesis and possible treatment alternatives. The majority of patients analysed had PUUV RNA in plasma for over 1 week after disease onset. Interestingly, the PUUV RNA load per se was not significantly associated with a more severe clinical outcome, suggested in previous studies on HFRS and HCPS patients infected with other hantaviruses 13–16. Differences in virulence for different hantaviruses could be an explanation: compared with other hantaviruses causing HFRS and HCPS 13,14,16, we observed a 10–100 times lower RNA load, suggesting that PUUV-infected patients in general have lower levels of viraemia than patients with other hantavirus infections. PUUV RNA was detected in plasma for up to 3 weeks after disease onset; however, the duration of PUUV RNA was not associated with disease severity. There was a tendency for a higher viral load in patients with moderate/severe illness during the first 0–3 days, although the sampling size was too small to find a significant association to disease severity. From these findings, one could speculate that antivirals may be beneficial during the first few days after disease onset. The immune response has also been implicated in the pathogenesis of hantavirus infections 1,2. Accordingly, we found that higher WBC counts and especially higher neutrophils were significantly associated with more severe disease.
Importantly, a low IgG response in the admission sample was significantly associated with disease severity. These findings are supported by previous studies on patients infected with Sin Nombre virus, showing association between low IgG titres 19,20 and neutralizing antibodies 19 in severe disease. In our study, one limitation was that neutralizing antibodies were not analysed. We detected an inverse association between hantavirus-specific IgG and IgA antibody titres and viral load, as previously indicated for IgG 13,24 and salivary IgA 25. However, in contrast to IgG titres, we did not observe any association with disease severity for IgA and IgM titres. Our data imply the importance of anti-PUUV-specific IgG antibodies for virus clearance and protection against severe disease. It has previously been shown that case-fatality rate increases with age during HFRS caused by PUUV and Seoul virus 26,27. In line with these observations, we here show that high age is a risk factor for severe HFRS caused by PUUV infection. Female sex has been reported to be a risk factor for lethal outcome of HFRS caused by Seoul virus 27. However, we did not find an association between sex and disease severity or hospitalization frequency for PUUV-caused HFRS.
Hantaviruses in the Americas cause cardiac and respiratory symptoms, features of HCPS. In our study of patients with HFRS, respiratory symptoms were frequently observed, supporting the similarities to HCPS 2,28. We did not include these symptoms as a criterion for severe disease, but we found that a low PUUV-specific IgG response was associated with airway symptoms and need of oxygen treatment in the HFRS patients.
A low IgG response against PUUV was associated with moderate/severe disease. Previous studies in animal models that mirror HFRS and HCPS have shown that serum containing neutralizing antibodies can prevent disease development 29,30. Interestingly, in an unpublished ongoing clinical trial in South America, patients with HCPS have been treated with fresh plasma from survivors that contains high levels of neutralizing antibodies. Preliminary data suggest a reduction in mortality in these passively immunized patients with HCPS (referred by Dolgin 5).
In conclusion, our results suggest a relationship between a strong specific IgG response and a favourable clinical outcome of patients. Although further studies are needed, our results support the use of passive immunization as a potential treatment for HFRS/HCPS.
Acknowledgments
We thank the patients for their participation and the study nurse Maria Casserdahl, and the other staff at the Clinic of Infectious Diseases, Umeå University Hospital, for assistance in the study. We want also to thank Irene Eriksson and Maj Bylund, Department of Clinical Microbiology, Virology, Umeå University for technical assistance.This work was supported by grants from the Swedish Heart-Lung Foundation, the Kempe foundation, the County Council of Västerbotten, the Medical Faculty of Umeå University, the County Councils of Northern Sweden, the Swedish Research Council, and the Lars Kleberg foundation.
Transparency Declarations
The authors declare no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Association between Puumala hantavirus (PUUV) viral load and antibody responses.
Association between PUUV viral load and antibody responses.
Predictors for disease severity. Patients with moderate/severe disease had significantly lower IgG titers in their first available serum sample than patients with mild disease.
Predictors for disease severity. Patients with moderate/severe disease had significantly higher white blood cell counts in their first available serum sample than patients with mild disease.
References
- 1.Vapalahti O, Mustonen J, Lundkvist A, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–661. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YK, Lee SC, Kim C, Heo ST, Choi C, Kim JM. Clinical and laboratory predictors of oliguric renal failure in haemorrhagic fever with renal syndrome caused by Hantaan virus. J Infect. 2007;54:381–386. doi: 10.1016/j.jinf.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Libraty DH, Makela S, Vlk J, et al. The degree of leukocytosis and urine GATA-3 mRNA levels are risk factors for severe acute kidney injury in Puumala virus nephropathia epidemica. PLoS ONE. 2012;7:e35402. doi: 10.1371/journal.pone.0035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolgin E. Hantavirus treatments advance amidst outbreak in US park. Nat Med. 2012;18:1448. doi: 10.1038/nm1012-1448a. [DOI] [PubMed] [Google Scholar]
- 6.Rusnak JM, Byrne WR, Chung KN, et al. Experience with intravenous Ribavirin in the treatment of hemorrhagic fever with renal syndrome in Korea. Antiviral Res. 2009;81:68–76. doi: 10.1016/j.antiviral.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korva M, Saksida A, Kunilo S, Vidan Jeras B, Avsic-Zupanc T. HLA-associated hemorrhagic fever with renal syndrome disease progression in Slovenian patients. Clin Vaccine Immunol. 2011;18:1435–1440. doi: 10.1128/CVI.05187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102:1030–1041. doi: 10.1160/TH09-09-0640. [DOI] [PubMed] [Google Scholar]
- 9.Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindgren T, Ahlm C, Mohamed N, Evander M, Ljunggren HG, Bjorkstrom NK. Longitudinal analysis of the human T cell response during acute hantavirus infection. J Virol. 2011;85:10252–10260. doi: 10.1128/JVI.05548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadeghi M, Eckerle I, Daniel V, Burkhardt U, Opelz G, Schnitzler P. Cytokine expression during early and late phase of acute Puumala hantavirus infection. BMC Immunol. 2011;12:65. doi: 10.1186/1471-2172-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saksida A, Wraber B, Avsic-Zupanc T. Serum levels of inflammatory and regulatory cytokines in patients with hemorrhagic fever with renal syndrome. BMC Infect Dis. 2011;11:142. doi: 10.1186/1471-2334-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saksida A, Duh D, Korva M, Avsic-Zupanc T. Dobrava virus RNA load in patients who have hemorrhagic fever with renal syndrome. J Infect Dis. 2008;197:681–685. doi: 10.1086/527485. [DOI] [PubMed] [Google Scholar]
- 14.Terajima M, Hendershot JD, 3rd, Kariwa H, et al. High levels of viremia in patients with the hantavirus pulmonary syndrome. J Infect Dis. 1999;180:2030–2034. doi: 10.1086/315153. [DOI] [PubMed] [Google Scholar]
- 15.Xiao R, Yang S, Koster F, Ye C, Stidley C, Hjelle B. Sin nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J Infect Dis. 2006;194:1403–1409. doi: 10.1086/508494. [DOI] [PubMed] [Google Scholar]
- 16.Yi J, Xu Z, Zhuang R, et al. Hantaan virus RNA load in patients having hemorrhagic fever with renal syndrome: correlation with disease severity. J Infect Dis. 2012;207:1457–1461. doi: 10.1093/infdis/jis475. [DOI] [PubMed] [Google Scholar]
- 17.Huggins JW, Hsiang CM, Cosgriff TM, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 18.Mertz GJ, Miedzinski L, Goade D, et al. Placebo-controlled, double-blind trial of intravenous Ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin Infect Dis. 2004;39:1307–1313. doi: 10.1086/425007. [DOI] [PubMed] [Google Scholar]
- 19.Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182:43–48. doi: 10.1086/315657. [DOI] [PubMed] [Google Scholar]
- 20.MacNeil A, Comer JA, Ksiazek TG, Rollin PE. Sin Nombre virus-specific immunoglobulin M and G kinetics in hantavirus pulmonary syndrome and the role played by serologic responses in predicting disease outcome. J Infect Dis. 2010;202:242–246. doi: 10.1086/653482. [DOI] [PubMed] [Google Scholar]
- 21.de Carvalho Nicacio C, Bjorling E, Lundkvist A. Immunoglobulin A responses to Puumala hantavirus. J Gen Virol. 2000;81:1453–1461. doi: 10.1099/0022-1317-81-6-1453. [DOI] [PubMed] [Google Scholar]
- 22.Hedman K, Vaheri A, Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet. 1991;338:1353–1356. doi: 10.1016/0140-6736(91)92235-t. [DOI] [PubMed] [Google Scholar]
- 23.Sundberg E, Hultdin J, Nilsson S, Ahlm C. Evidence of disseminated intravascular coagulation in a hemorrhagic fever with renal syndrome-scoring models and severe illness. PLoS ONE. 2011;6:e21134. doi: 10.1371/journal.pone.0021134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evander M, Eriksson I, Pettersson L, et al. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J Clin Microbiol. 2007;45:2491–2497. doi: 10.1128/JCM.01902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson L, Rasmuson J, Andersson C, Ahlm C, Evander M. Hantavirus-specific IgA in saliva and viral antigen in the parotid gland in patients with hemorrhagic fever with renal syndrome. J Med Virol. 2011;83:864–870. doi: 10.1002/jmv.22040. [DOI] [PubMed] [Google Scholar]
- 26.Hjertqvist M, Klein SL, Ahlm C, Klingstrom J. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis. 2010;16:1584–1586. doi: 10.3201/eid1610.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein SL, Marks MA, Li W, et al. Sex differences in the incidence and case fatality rates from hemorrhagic fever with renal syndrome in China, 2004–2008. Clin Infect Dis. 2011;52:1414–1421. doi: 10.1093/cid/cir232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmuson J, Pourazar J, Linderholm M, Sandstrom T, Blomberg A, Ahlm C. Presence of activated airway T lymphocytes in human Puumala hantavirus disease. Chest. 2011;140:715–722. doi: 10.1378/chest.10-2791. [DOI] [PubMed] [Google Scholar]
- 29.Brocato R, Josleyn M, Ballantyne J, Vial P, Hooper JW. DNA vaccine-generated duck polyclonal antibodies as a postexposure prophylactic to prevent hantavirus pulmonary syndrome (HPS) PLoS ONE. 2012;7:e35996. doi: 10.1371/journal.pone.0035996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingström J, Stoltz M, Hardestam J, Ahlm C, Lundkvist A. Passive immunization protects cynomolgus macaques against Puumala hantavirus challenge. Antivir Ther. 2008;13:125–133. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between Puumala hantavirus (PUUV) viral load and antibody responses.
Association between PUUV viral load and antibody responses.
Predictors for disease severity. Patients with moderate/severe disease had significantly lower IgG titers in their first available serum sample than patients with mild disease.
Predictors for disease severity. Patients with moderate/severe disease had significantly higher white blood cell counts in their first available serum sample than patients with mild disease.


