Abstract
Hyperventilation has been linked to emotional distress in adults. This study investigates end-tidal carbon dioxide (ETCO2), respiratory rate (RR), and heart rate variability (HRV) in adolescent girls with emotional disorders and healthy controls. ETCO2, RR, HRV, and ratings of emotional symptom severity were collected in adolescent female psychiatric patients with emotional disorders (n = 63) and healthy controls (n = 62). ETCO2 and RR differed significantly between patients and controls. ETCO2, HR, and HRV were significant independent predictors of group status, that is, clinical or healthy, while RR was not. ETCO2 and RR were significantly related to emotional symptom severity and to HRV in the total group. ETCO2 and RR were not affected by use of selective serotonin reuptake inhibitors. It is concluded that emotional dysregulation is related to hyperventilation in adolescent girls. Respiratory-based treatments may be relevant to investigate in future research.
Keywords: Adolescents, Depression, Anxiety, Respiration, ETCO2
Adolescent major depressive disorder (MDD) is common worldwide and affects 8–20% of all youth (Kessler et al., 2007; Klein et al., 1999). The onset of puberty, especially in girls, is marked by a major increase in prevalence (Thapar, Collishaw, Pine, & Thapar, 2012). Moreover, MDD and anxiety disorders (AD) are frequently comorbid in adolescents (Angold, Costello, & Erkanli, 1999) with estimated comorbidity rates of 75% (Kouros, Quasem, & Garber, 2013). While this comorbidity may be explained in part by common variance between anxiety and depression assessment tools (Anderson & Hope, 2008), both MDD and AD reflect clinically important levels of subjective distress and negative affect (Watson et al., 1995).
Adolescents with depression are at higher risk of many negative health outcomes including increased risk of suicide, substance use disorder (Birmaher, Ryan, Williamson, Brent, & Kaufman, 1996), and physical illnesses such as cardiovascular disease, Type II diabetes, metabolic syndrome, and pathological cognitive aging (McIntyre et al., 2007; Wolkowitz, Reus, & Mellon, 2011). Various mechanisms have been proposed to explain the association between depression and systemic disease, such as the glucocorticoid cascade (Lee, Ogle, & Sapolsky, 2002) and an increased allostatic load (Juster, McEwen, & Lupien, 2010; McEwen, 2003).
We and others have also shown that vagal inhibitory tone, which is associated with autonomic self-regulation, is decreased in adolescents and adults with anxiety disorders and depression, indicating an impairment of the descending vagal pathways in these disorders (Henje Blom, Olsson, Serlachius, Ericson, & Ingvar, 2010; Lane et al., 2013; Rechlin, Weis, Spitzer, & Kaschka, 1994; Thayer & Lane, 2000). Conversely, increased influence of the myelinated vagus decreases the HR, inhibits fight and flight activity, decreases the hypothalamic-pituitary-adrenal axis response, inflammatory processes, and glucose regulation, and promotes social behavior (Porges, 2007; Thayer & Sternberg, 2006). The output from the prefrontal cortex and amygdala are under vagal inhibitory control by the myelinated vagus nerve. The high frequency domain of heart rate variability (HRV) includes the respiratory sinus arrhythmia (i.e., the variability of heart rate that is synchronized with the breathing rate). The high frequency domain of HRV is thought to best capture the vagal tone (Berntson, Cacioppo, & Grossman, 2007; Malik, 1996).
Respiratory patterns have been studied in relation to emotional disorders and are known to affect vagal tone. It has been debated whether hyperventilation is a response to perceived chronic stress and whether this respiratory pattern may sustain or even contribute to the allostatic load and the symptomatology of anxiety and depression (Bass, 1997; Folgering, 1999; Han, Stegen, De Valck, Clément, & Van de Woestijne, 1996; Masaoka & Homma, 2001). When breathing pattern changes in parallel with changes in metabolic activity, such as during exercise, sleep, or fever, circulating blood gases show little or no change. However, when psychological influences change breathing pattern in the absence of changes in metabolic activity, as may be the case in anxiety and depressed patients, blood gases do change. Hyperventilatory breathing during anxiety or emotional distress decreases partial pressure CO2 (pCO2, hypocapnia; Meuret, Wilhelm, & Roth, 2004), The decrease in pCO2 associated with hyperventilatory breathing results in respiratory alkalosis, as indicated by a decrease of end-tidal CO2 (ETCO2; the peak concentration of carbon dioxide at the end of the expiratory phase of a breath. ETCO2 is considered proportional to the arterial pH; Gardner, 1994). Hyperventilatory breathing and the resulting decreases in ETCO2 can be achieved by increasing breathing rate, breathing volume, rapid shallow breathing, or a combination of these patterns (Laffey & Kavanagh, 2002). Thus, while many often focus on respiratory rate (RR), this is only one of the factors contributing to decreases in ETCO2.
Research in the field of respiratory psychophysiology, as well as several Eastern traditions, claims that emotional states are expressed in breathing patterns, and that voluntary change of breathing patterns can alter emotional states (Boiten, 1998; Boiten, Frijda, & Wientjes, 1994; Brown & Gerbarg, 2005; Grossman, 1983; Ley, 1999; Tweeddale, Rowbottom, & McHardy, 1994; Van Diest, Thayer, Vandeputte, Van de Woestijne, & Van den Bergh, 2006). It is well known that acute hypocapnia can produce adverse physiological and medical effects and induce symptoms of anxiety and panic (Grossman, 1983; Laffey & Kavanagh, 2002), but the concept of a chronic hyperventilation syndrome is debated and currently lacks conclusive diagnostic criteria (Bass, 1997; Folgering, 1999). Studies based on self-assessment in pediatric and adolescent populations indicate a high prevalence of hyperventilation (Baranes, Rossignol, Stheneur, & Bidat, 2005; Gridina, Bidat, Chevallier, & Stheneur, 2013). A long-term follow-up study of children and adolescents with hyperventilation showed that 40% were still hyperventilating as adults, and many had signs and symptoms of chronic anxiety (Herman, Stickler, & Lucas, 1981). Despite these findings, whether breathing patterns and respiratory metabolism contribute to emotional dysregulation and the disease progression of anxiety and depression in adolescents is not established.
The primary aim of this study is to determine whether adolescent girls with emotional disorders show more hyperventilation, measured by ETCO2 and RR, than healthy controls, and whether ETCO2 and RR can be used to distinguish between the two groups. We hypothesize that adolescent girls with emotional disorders will have lower ETCO2 and higher RR than healthy controls, which would imply that breathing-based interventions may constitute a target for the development of new treatment models.
Secondary aims of the study are to investigate (a) the relationship between emotional symptom severity and respiratory parameters, (b) the relationship between respiratory parameters (RR and ETCO2) and autonomic regulation as measured by HRV, and (c) possible differences in RR and ETCO2 between subjects in the clinical group with and without antidepressive medication (selective serotonin reuptake inhibitors; SSRIs). Adolescent girls with MDD and/or AD were included in the clinical sample due to the previously described high comorbidity rates.
Method
Description of the Samples
A detailed description of the samples and the data collection procedures has been published in previous papers in which respiratory patterns were not analyzed (Henje Blom, Olsson, Serlachius, Ericson, & Ingvar, 2009; Henje Blom et al., 2010). In summary, the original clinical sample consisted of adolescent girls (n = 79) with a mean age of 16.8 years (range: 14.5–18.4 years) who were psychiatric patients and had a Development and Wellbeing Assessment (DAWBA, see below) validated clinical diagnosis of MDD and/or one or several ADs (general anxiety disorder, social phobia, specific phobia, panic disorder, separation anxiety, posttraumatic stress disorder) at the time of assessment. The subjects had on-going treatments (median duration of 11 months) at one of 13 open psychiatric clinics for children and adolescents located in the center of Stockholm, surrounding suburbs, or in smaller towns nearby. Patients with severe autism or psychotic symptoms were not recruited to the study. Six subjects were denied participation because the DAWBA was incomplete or could not confirm diagnoses of MDD and/or AD. All measurements were performed at the patient's clinic by the first author and an assistant and followed the same order in all subjects.
The original control sample consisted of adolescent girls (n = 66), with a mean age of 16.5 years (range: 15.9–17.7 years) recruited from four different high schools. The clinical patient sample and the controls were age matched and recruited from similar locations in and around Stockholm. The measurements of the control sample were carried out at the school nurses' offices by the first author and four nurses. Exclusion criteria for both samples were diabetes, thyroid dysfunction, pregnancy, and more than 5% missing or distorted data in any registered data segment. The exclusion procedure is described in Figure 1. The Regional and Central Ethics Committee at the Karolinska Institute approved the study. Informed consent was obtained from all subjects and at least one of their parents after the nature of the study had been fully explained to them.
Figure 1.
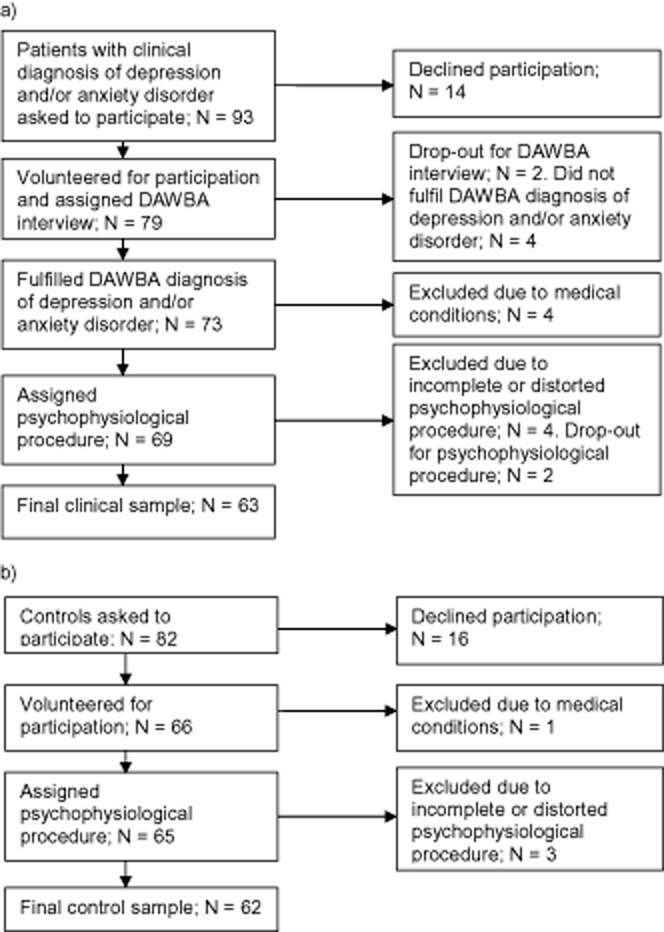
Exclusion procedure of (a) the clinical sample, and (b) the control sample.
Physiological Measurements
ETCO2, RR, and HR were measured continuously using an Air-Pas oxycapnograph (PBM, Stockholm, Sweden), a J&J Engineering I-330-C-2 physiological monitoring system (J&J Engineering, Poulsbo, WA), and cStress customized software (PBM). CO2 measured as a percentage in exhaled air was sampled from a nasal cannula (Ø = 5 mm) inserted 10 mm into the left nostril. ETCO2 was identified by an algorithm in the customized software as the peak of the CO2 concentration at the end of the exhalation phase. ETCO2 corresponds well to arterial pCO2 (Gardner, 1994). RR was calculated as breaths per minute from CO2 fluctuations, counting the peaks per minute.
The electrocardiogram (ECG) was recorded from electrodes placed on the left and right wrist with a sampling rate of 1024 Hz. Interbeat intervals were calculated online using an R-wave peak detection algorithm and stored on a PC for offline editing and calculation of HRV indices. The ECG recording time was 4 min and was preceded by 15 min of rest. The subjects were sitting upright in silence breathing spontaneously. No body movements were allowed during the procedure. None of the subjects had clinical signs or symptoms of infectious disease. Use of tobacco (snuff and cigarettes) or intake of tea, coffee, and caffeine containing soft drinks or beta stimulant asthma medication was not allowed 1 h prior to the measurements. All the recorded signals were scanned manually offline for distortions due to movement and irregular or distorted breaths caused by talk, movements, sighs, coughs, or yawns. In the CO2 signal, each breath was checked to reach an end-tidal plateau and was otherwise removed. Discarded interbeat intervals were replaced using a cubic spline interpolation. The standard deviation of the interbeat intervals (SDNN) was used as a time domain measure of HRV reflecting all of the cyclic components responsible for variability in the period of recording. Fourier analysis was performed on de-trended data in which the variability distributes as a function of frequency. The high frequency power component (HF; 0.15–0.4 Hz) and the low frequency power component (LF; 0.04–0.15 Hz) were used as frequency domain measures of HRV. The HF, LF, and SDNN were calculated and then logarithmically transformed. This 4-min HRV registration has previously been shown to generate stable results in repeated measures 6 months later in the control sample, supporting that short registrations without standardized interventions can capture HRV differences of possible predictive and clinical value (Henje Blom et al., 2009). We have previously published comparisons between HRV indices of the same samples (Henje Blom et al., 2010), and these data are the basis for the current investigations on associations between autonomic regulation and respiratory parameters.
Self-Assessment Scales and Diagnostic Validation
The Strengths and Difficulties Questionnaire (SDQ) is a widely used screening instrument for mental health problems in children and teenagers (Goodman, 2001). It is made up of 25 statements regarding psychological symptoms and behaviors, forming five subscales: hyperactivity/ inattention, emotional symptoms, conduct problems, peer problems, and prosocial behaviors. In this study, only the emotional scale (SDQ-em) was used. The SDQ-em does not differentiate between anxiety and depressive problems but gives a score of emotional symptom severity. Acceptable psychometric properties for the self-report version of SDQ for adolescents have been shown in previous studies, and SDQ-em has been shown to have valid ability to differentiate cases of MDD and AD in this age group (Blom, Larsson, Serlachius, & Ingvar, 2010).
The DAWBA (Goodman, Ford, Richards, Gatward, & Meltzer, 2000) is a frequently used internet-based structured interview compatible with the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). It focuses on current rather than lifetime problems.
Statistical Analyses
Groups were compared in a two-tailed fashion, with the unpaired two-sample t test. Associations between the SDQ-em, ETCO2, RR, and HRV indices (HF, LF, and SDNN) were assessed by Pearson's product-moment correlations. HF, LF, and SDNN had positively skewed distributions and were therefore logarithmically transformed to obtain normal distributions. Three logistic regression analyses with ETCO2, RR, HRV, and HR as independent variables and group status (clinical vs. control) as dependent variable were conducted. The three analyses were identical except that the HRV index was exchanged including HF in Model 1, LF in Model 2, and SDNN in Model 3. The three HRV indices are all highly intercorrelated (all rs > .8) and were therefore not entered together in one model due to multicollinearity. Probability levels of .05 or less were considered significant. Analyses were done in Statistica 11 (http://www.statsoft.com).
Results
ETCO2, RR, and the emotional symptom severity, as measured by SDQ-em scores, differed significantly between the clinical sample and controls; that is, ETCO2 was lower, but RR and SDQ-em scores were higher in the clinical sample as compared with the controls (Table 1). ETCO2, HR, and the three HRV indices (HF, LF, and SDNN), but not RR, were significant unique predictors of clinical status in the regression models (Table 2). ETCO2 showed the largest unique contribution to the prediction followed by HRV and HR. The models correctly classified 84–87% of the cases.
Table 1.
Mean Values and Standard Deviations (SD) of the Emotional Symptom Severity Score, End-Tidal CO2, and Respiratory Rate for the Control and Clinical Sample
| Control sample | Clinical sample | Cohen's d | t value | |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| n = 62 | n = 63 | |||
| SDQ-em | 3.7 (2.3) | 7.3 (1.6) | 1.8 | −9.8*** |
| ETCO2 (%) | 4.8 (0.3) | 4.2 (0.3) | 2.0 | 10.8*** |
| RR (BrPM) | 14.9 (4.4) | 16.4 (3.8) | 0.4 | −2.1* |
Note. SDQ-em = Strengths and Difficulties Questionnaire emotional subscale; ETCO2 = end-tidal CO2; RR = respiratory rate; BrPM = breaths per minute.
p < .05.
p < .001.
Table 2.
Three Logistic Regression Models with Group Status (Clinical vs. Control) as Dependent Variable
| n = 112 | Estimate | Odds ratio | p | AIC | Log odds ratio for classification of cases |
|---|---|---|---|---|---|
| Model 1 (HF) | 82.2 | 3.90 | |||
| ETCO2 | 6.36 | 578.54 | < .0001 | ||
| RR | 0.00 | 1.00 | .96 | ||
| HR | 0.11 | 1.12 | .004 | ||
| HF | 1.04 | 2.84 | .01 | ||
| Model 2 (LF) | 85.1 | 3.44 | |||
| ETCO2 | 6.34 | 566.90 | < .0001 | ||
| RR | 0.02 | 1.02 | .83 | ||
| HR | 0.08 | 1.09 | .02 | ||
| LF | 0.81 | 2.25 | .04 | ||
| Model 3 (SDNN) | 79.4 | 3.44 | |||
| ETCO2 | 6.30 | 546.30 | < .0001 | ||
| RR | 0.02 | 1.02 | .81 | ||
| HR | 0.12 | 1.13 | .002 | ||
| SDNN | 3.36 | 28.89 | .004 |
Note. The three models exchange heart rate variability (HRV) index as independent variable so that Model 1 includes HF; Model 2, LF; and Model 3, SDNN. End-tidal CO2 (ETCO2), respiratory rate (RR), and heart rate (HR) are included as independent measures in all models. Akaike information criterion (AIC) and log odds ratio for classification of cases are reported as measures of the relative quality of the models.
SDQ-em scores showed significant correlations to RR and ETCO2 in the combined group of clinical and control subjects, but not when the clinical and control samples were analyzed separately (Table 3). All indices of HRV (HF, LF, and SDNN) correlated significantly with ETCO2 and RR (inverse) in the total group. In the clinical sample, only a significant inverse correlation between RR and LF was found. In the control sample, significant inverse correlations were found between RR and all the HRV indices. There were no significant correlations between ETCO2 and any HRV measures in the clinical or in the control samples when analyzed separately (Table 2).
Table 3.
Pearson Product-Moment Correlation Coefficients (rs) Showing the Associations between the Emotional Symptom Severity Score and Heart Rate Variability on One Side and End-Tidal CO2 and Respiratory Rate on the Other
| Pearson's r | ||
|---|---|---|
| ETCO2 | RR | |
| SDQ-em (n) | ||
| Total (125) | −.44*** | .25** |
| Controls (62) | −.09 | .23 |
| Clinical group (63) | .24 | .08 |
| HF (n) | ||
| Total (112a) | .23* | −.24* |
| Controls (53) | .15 | −.43** |
| Clinical group (59) | −.01 | .02 |
| LF (n) | ||
| Total (112a) | .19* | −.44*** |
| Controls (53) | −.09 | −.53*** |
| Clinical group (59) | .12 | −.30* |
| SDNN (n) | ||
| Total (112a) | .22* | −.33*** |
| Controls (53) | −.06 | −.48*** |
| Clinical group (59) | .11 | −.11 |
Note. ETCO2 = end-tidal CO2; RR = respiratory rate; SDQ-em = Strengths and Difficulties Questionnaire emotional subscale; HF = high frequency; LF = low frequency; SDNN = standard deviation of the interbeat intervals.
13 subjects (10 in the clinical group and 3 in the control group) had distorted HR data.
p < .05.
p < .01.
p < .001.
In the clinical sample, ETCO2 and RR did not differ significantly between the subgroup taking SSRIs as compared with the subgroup without SSRI medication (ns = 21 and 40; both ps > .4). Hormonal contraceptives were more frequently used in the clinical sample compared with controls (21 of 62 compared with 5 of 63, χ2 = 12.5, p < .001). The significant differences between the clinical and control samples regarding ETCO2 and RR remained unchanged when subjects using hormonal contraceptives were excluded (ETCO2: t = 9.5, p < .001; RR: t = 2.5, p < .05; ns = 41 and 57). Information about contraceptives was missing from one girl in the control sample.
Discussion
The main finding of this study is that the clinical sample, consisting of adolescent girls with a diagnosis of MDD and/or AD, showed significantly lower ETCO2 and higher RR compared with the sample of healthy, age-matched controls. ETCO2 showed the largest unique contribution to the prediction of clinical group status (i.e., a diagnosis of MDD and/or AD). HR and HRV also significantly predicted clinical status but to a lesser degree, while RR did not. ETCO2 showed the largest difference between the clinical and control samples on all included measures (Cohen's d = 2.0), and RR showed only a small difference (Cohen's d = 0.4). The mean ETCO2 value for the clinical sample was below the suggested normal reference range (ETCO2 = 4.6–6.0%) and close to the cut-off suggested for hyperventilation syndrome (ETCO2 ≤ 4.0%; Bass & Gardner, 1985; Gardner, 1994; Wilhelm, Gevirtz, & Roth, 2001). The mean ETCO2 value for the control sample was within the normal range, and the mean RR was within the normal range in both groups. Significant correlations between emotional symptom severity, as measured with SDQ-em, and both ETCO2 and RR, respectively, were found in the total sample. No correlations between these variables were found when each sample was analyzed separately. This is probably due to the limited ranges observed for the symptom severity and physiological measures.
The regression analyses presented above also confirm the previously reported findings that all measured HRV indices (i.e., HF, LF, and SDNN) were significantly lower in the clinical sample compared with healthy controls, the effect sizes ranging from Cohen's d = 0.53 to 0.60 (Henje Blom et al., 2010). These new analyses expand on the previous findings by showing that the results remain even when controlling for RR, ETCO2, and HR. No significant difference of HR has been found between the clinical sample (73.2 beats per minute) and the controls (76.2 beats per minute). For detailed analyses of HRV, we refer to our previous publication (Henje Blom et al., 2010).
Significant correlations between autonomic regulation measured by LF, HF, and SDNN and RR were found in the total group and in the control sample, but in the clinical sample only between LF and RR. The most reliably described heart rate periodicities of HRV are the HF and LF domains. HF includes the spontaneous breathing rate (i.e., RSA), and LF is assumed to be related to the endogenous rhythm of blood pressure regulation via the baroreceptors and spontaneous vasomotor activity (Porges, 2007). One may speculate that an impairment of autonomic regulation is part of the pathophysiology of depression and anxiety that causes a decoupling between RR and HRV (Garcia, Koschnitzky, Dashevskiy, & Ramirez, 2013; Masaoka & Homma, 2001; Wang, Lü, & Qin, 2013). This would result in a diminished RSA (HF) as well as in a weaker relationship between RR and HF in the clinical sample. LF, on the other hand, is based on slower endogenous rhythms and is not as sensitive to changes of RR. The association between HRV and RR in the control sample is well documented in studies on adults and is believed to be related to longer exhalation time, which allows for a more extensive vagal cholinergic influence on the heart (Berntson et al., 1997; Pöyhönen, Syväoja, Hartikainen, Ruokonen, & Takala, 2004). We did not find any effect of SSRIs or hormonal contraceptives on respiratory parameters.
Our findings imply a difference in ETCO2 (but not in RR) between the groups that may be of clinical relevance. The finding that ETCO2 is the strongest and a unique predictor of clinical status (stronger than HR and HRV) is new and suggests that respiratory alkalosis is related to emotional dysregulation in adolescent girls. Changes of pCO2 have an effect on cerebral blood flow (Van den Bergh, Zaman, Bresseleers, Verhamme, & Van Diest, 2013) and cause alterations in the chemoreception in the brain stem (Azmitia, 1999; Hodges & Richerson, 2010; Kara, Narkiewicz, & Somers, 2003; Severson, Wang, Pieribone, Dohle, & Richerson, 2003), which may be possible mechanisms explaining the relationship between ETCO2 and emotional symptoms. One may also hypothesize that chronic changes of ETCO2 impact the allostatic load and that chronic emotional distress leads to increased RR and hyperventilation, which decreases autonomic regulation and causes respiratory alkalosis, in turn leading to increased emotional distress.
Our findings have to be regarded with several limitations in mind: (a) the cross-sectional design does not allow for any conclusions on causality and does not exclude possible confounding variables; (b) tidal volume was not measured, and consequently RSA could not be calculated taking it into account (Grossman & Taylor, 2007); (c) respiratory rate was derived from the raw CO2 signal instead of being measured directly from flow or respiratory movement recordings; (d) the significant correlations between ETCO2 and HRV that were shown for the total group may be dependent on the documented group differences in both variables; and (e) we did not have information on when in the menstrual cycle the physiological data were obtained, which would have been valuable because hormonal factors related to the menstrual cycle are known to influence breathing patterns and affect ETCO2 (England & Farhi, 1976).
Several studies have shown that regular practice of breathing techniques substantially reduced chemoreflex sensitivity (Spicuzza, Gabutti, Porta, Montano, & Bernardi, 2000) and reduced oxidative stress (Sharma et al., 2003) and depressive symptoms (Tweeddale et al., 1994). Unfortunately, these studies include a variety of different breathing techniques making it difficult to identify the possible active mechanism. Studies with better controlled forms of breathing training have been performed, especially targeting patients with panic disorder. Both voluntary hyper- and hypoventilation interventions have shown beneficial results, thus leading to the conclusion that the reduction of symptoms were not related to changes in ETCO2 (Kim, Wollburg, & Roth, 2012; Meuret, Ritz, Wilhelm, & Roth, 2005; Wollburg, Roth, & Kim, 2011). A few small studies on respiratory biofeedback with focus on the RSA have indicated effects on anxious and depressive symptoms (Patron et al., 2013; Sutarto, Wahab, & Zin, 2012). Future intervention studies may evaluate breathing practices aided by respiratory biofeedback as treatment in the increasing population of adolescent girls with emotional disorders.
In conclusion, adolescent girls with clinical depression and/or anxiety show signs of hyperventilation evidenced by significantly lower ETCO2 compared with healthy controls. This suggests that emotional dysregulation is related to hyperventilation in adolescent girls and that regular breathing training may be relevant to study as an intervention for emotional disorders in this age group.
References
- Anderson ER. Hope DA. A review of the tripartite model for understanding the link between anxiety and depression in youth. Clinical Psychology Review. 2008;28:275–287. doi: 10.1016/j.cpr.2007.05.004. doi: 10.1016/j.cpr.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40:57–87. [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue [Suppl 2] Neuropsychopharmacology. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Baranes T, Rossignol B, Stheneur C. Bidat E. [Hyperventilation syndrome in children] Archives de Pédiatrie. 2005;12:1742–1747. doi: 10.1016/j.arcped.2005.09.015. doi: 10.1016/j.arcped.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Bass C. Hyperventilation syndrome: A chimera? Journal of Psychosomatic Research. 1997;42:421–426. doi: 10.1016/s0022-3999(96)00365-0. [DOI] [PubMed] [Google Scholar]
- Bass C. Gardner WN. Respiratory and psychiatric abnormalities in chronic symptomatic hyperventilation. British Medical Journal. 1985;290:1387–1390. doi: 10.1136/bmj.290.6479.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M. van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Grossman P. Whither vagal tone. Biological Psychology. 2007;74:295–300. doi: 10.1016/j.biopsycho.2006.08.006. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA. Kaufman J. Childhood and adolescent depression: A review of the past 10 years. Part II. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1575–1583. doi: 10.1097/00004583-199612000-00008. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Blom EH, Larsson J-O, Serlachius E. Ingvar M. The differentiation between depressive and anxious adolescent females and controls by behavioural self-rating scales. Journal of Affective Disorders. 2010;122:232–240. doi: 10.1016/j.jad.2009.07.006. doi: 10.1016/j.jad.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Boiten FA. The effects of emotional behaviour on components of the respiratory cycle. Biological Psychology. 1998;49:29–51. doi: 10.1016/s0301-0511(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Boiten FA, Frijda NH. Wientjes CJ. Emotions and respiratory patterns: Review and critical analysis. International Journal of Psychophysiology. 1994;17:103–128. doi: 10.1016/0167-8760(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Brown RP. Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression. Part II—Clinical applications and guidelines. Journal of Alternative and Complementary Medicine. 2005;11:711–717. doi: 10.1089/acm.2005.11.711. doi: 10.1089/acm.2005.11.711. [DOI] [PubMed] [Google Scholar]
- England SJ. Farhi LE. Fluctuations in alveolar CO2 and in base excess during the menstrual cycle. Respiration Physiology. 1976;26:157–161. doi: 10.1016/0034-5687(76)90093-1. [DOI] [PubMed] [Google Scholar]
- Folgering H. [The pathophysiology of hyperventilation syndrome] Monaldi archives for chest disease. 1999;54:365–372. [PubMed] [Google Scholar]
- Garcia AJ, Koschnitzky JE, Dashevskiy T. Ramirez J-M. Cardiorespiratory coupling in health and disease. Autonomic Neuroscience: Basic & Clinical. 2013;175:26–37. doi: 10.1016/j.autneu.2013.02.006. doi: 10.1016/j.autneu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W. Measurement of end-tidal PCO2 and PO2. Biofeedback and Self-Regulation. 1994;19:103–113. doi: 10.1007/BF01776484. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R. Meltzer H. The development and well-being assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41:645–655. [PubMed] [Google Scholar]
- Gridina I, Bidat E, Chevallier B. Stheneur C. [Prevalence of chronic hyperventilation syndrome in children and teenagers] Archives de Pédiatrie. 2013;20:265–268. doi: 10.1016/j.arcped.2012.12.016. doi: 10.1016/j.arcped.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–300. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Grossman P. Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Han JN, Stegen K, De Valck C, Clément J. Van de Woestijne KP. Influence of breathing therapy on complaints, anxiety and breathing pattern in patients with hyperventilation syndrome and anxiety disorders. Journal of Psychosomatic Research. 1996;41:481–493. doi: 10.1016/s0022-3999(96)00220-6. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Olsson EMG, Serlachius E, Ericson M. Ingvar M. Heart rate variability is related to self-reported physical activity in a healthy adolescent population. European Journal of Applied Physiology. 2009;106:877–883. doi: 10.1007/s00421-009-1089-3. doi: 10.1007/s00421-009-1089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henje Blom E, Olsson EM, Serlachius E, Ericson M. Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99:604–611. doi: 10.1111/j.1651-2227.2009.01657.x. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman SP, Stickler GB. Lucas AR. Hyperventilation syndrome in children and adolescents: Long-term follow-up. Pediatrics. 1981;67:183–187. [PubMed] [Google Scholar]
- Hodges MR. Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: Contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. Journal of Applied Physiology. 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS. Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K. Somers VK. Chemoreflexes—Physiology and clinical implications. Acta Physiologica Scandinavica. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, De Graaf R, Demyttenaere K, Gasquet I. Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wollburg E. Roth WT. Opposing breathing therapies for panic disorder: A randomized controlled trial of lowering vs raising end-tidal PCO2. The Journal of Clinical Psychiatry. 2012;73:931–939. doi: 10.4088/JCP.11m07068. doi: 10.4088/JCP.11m07068. [DOI] [PubMed] [Google Scholar]
- Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH. Keller MB. Age of onset in chronic major depression: Relation to demographic and clinical variables, family history, and treatment response. Journal of Affective Disorders. 1999;55:149–157. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Kouros CD, Quasem S. Garber J. Dynamic temporal relations between anxious and depressive symptoms across adolescence. Development and Psychopathology. 2013;25:683–697. doi: 10.1017/S0954579413000102. doi: 10.1017/S0954579413000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffey JG. Kavanagh BP. Hypocapnia. The New England Journal of Medicine. 2002;347:43–53. doi: 10.1056/NEJMra012457. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- Lane RD, Weidenbacher H, Smith R, Fort C, Thayer JF. Allen JJB. Subgenual anterior cingulate cortex activity covariation with cardiac vagal control is altered in depression. Journal of Affective Disorders. 2013;150:565–570. doi: 10.1016/j.jad.2013.02.005. doi: 10.1016/j.jad.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO. Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disorders. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Ley R. The modification of breathing behavior. Behavior Modification. 1999;23:441–479. doi: 10.1177/0145445599233006. [DOI] [PubMed] [Google Scholar]
- Malik M. Heart rate variability. Annals of Noninvasive Electrocardiology. 1996;1:151–181. doi: 10.1111/j.1542-474X.1996.tb00275.x. [Google Scholar]
- Masaoka Y. Homma I. The effect of anticipatory anxiety on breathing and metabolism in humans. Respiration Physiology. 2001;128:171–177. doi: 10.1016/s0034-5687(01)00278-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CWY, Miranda A. Kennedy SH. Should depressive syndromes be reclassified as “metabolic syndrome type II”? Annals of Clinical Psychiatry. 2007;19:257–264. doi: 10.1080/10401230701653377. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH. Roth WT. Voluntary hyperventilation in the treatment of panic disorder—Functions of hyperventilation, their implications for breathing training, and recommendations for standardization. Clinical Psychology Review. 2005;25:285–306. doi: 10.1016/j.cpr.2005.01.002. doi: 10.1016/j.cpr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH. Roth WT. Respiratory feedback for treating panic disorder. Journal of Clinical Psychology. 2004;60:197–207. doi: 10.1002/jclp.10245. doi: 10.1002/jclp.10245. [DOI] [PubMed] [Google Scholar]
- Patron E, Messerotti Benvenuti S, Favretto G, Valfrè C, Bonfà C, Gasparotto R. Palomba D. Biofeedback assisted control of respiratory sinus arrhythmia as a biobehavioral intervention for depressive symptoms in patients after cardiac surgery: A preliminary study. Applied Psychophysiology and Biofeedback. 2013;38:1–9. doi: 10.1007/s10484-012-9202-5. doi: 10.1007/s10484-012-9202-5. [DOI] [PubMed] [Google Scholar]
- Porges S. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhönen M, Syväoja S, Hartikainen J, Ruokonen E. Takala J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiologica Scandinavica. 2004;48:93–101. doi: 10.1111/j.1399-6576.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Spitzer A. Kaschka WP. Are affective disorders associated with alterations of heart rate variability? Journal of Affective Disorders. 1994;32:271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI. Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nature Neuroscience. 2003;6:1139–1140. doi: 10.1038/nn1130. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Sharma H, Sen S, Singh A, Bhardwaj NK, Kochupillai V. Singh N. Sudarshan Kriya practitioners exhibit better antioxidant status and lower blood lactate levels. Biological Psychology. 2003;63:281–291. doi: 10.1016/s0301-0511(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Gabutti A, Porta C, Montano N. Bernardi L. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet. 2000;356:1495–1496. doi: 10.1016/S0140-6736(00)02881-6. doi: 10.1016/S0140-6736(00)02881-6. [DOI] [PubMed] [Google Scholar]
- Sutarto AP, Wahab MNA. Zin NM. Resonant breathing biofeedback training for stress reduction among manufacturing operators. International Journal of Occupational Safety and Ergonomics: JOSE. 2012;18:549–561. doi: 10.1080/10803548.2012.11076959. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS. Thapar AK. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF. Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF. Sternberg E. Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Tweeddale PM, Rowbottom I. McHardy GJ. Breathing retraining: Effect on anxiety and depression scores in behavioural breathlessness. Journal of Psychosomatic Research. 1994;38:11–21. doi: 10.1016/0022-3999(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Van den Bergh O, Zaman J, Bresseleers J, Verhamme P. Van Diest I. Anxiety, pCO2 and cerebral blood flow. International Journal of Psychophysiology. 2013;89:72–77. doi: 10.1016/j.ijpsycho.2013.05.011. doi: 10.1016/j.ijpsycho.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Van Diest I, Thayer JF, Vandeputte B, Van de Woestijne KP. Van den Bergh O. Anxiety and respiratory variability. Physiology & Behavior. 2006;89:189–195. doi: 10.1016/j.physbeh.2006.05.041. doi: 10.1016/j.physbeh.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lü W. Qin R. Respiratory sinus arrhythmia is associated with trait positive affect and positive emotional expressivity. Biological Psychology. 2013;93:190–196. doi: 10.1016/j.biopsycho.2012.12.006. doi: 10.1016/j.biopsycho.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME. McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. doi: 10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gevirtz R. Roth WT. Respiratory dysregulation in anxiety, functional cardiac, and pain disorders. Assessment, phenomenology, and treatment. Behavior Modification. 2001;25:513–545. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI. Mellon SH. Of sound mind and body: Depression, disease, and accelerated aging. Dialogues in Clinical Neuroscience. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollburg E, Roth WT. Kim S. Effects of breathing training on voluntary hypo- and hyperventilation in patients with panic disorder and episodic anxiety. Applied Psychophysiology and Biofeedback. 2011;36:81–91. doi: 10.1007/s10484-011-9150-5. doi: 10.1007/s10484-011-9150-5. [DOI] [PubMed] [Google Scholar]


