Abstract
Purpose
Significantly increasing heart transplantations have been performed in Taiwan in the past decades, but the trends of maintenance immunosuppression for heart transplant recipients have not been well known. In this study, we aimed to explore the trends of maintenance immunosuppressive therapy and common complications for heart transplant recipients.
Methods
We retrospectively analyzed ambulatory prescriptions in 488 heart transplant recipients for the period 2000–2009. Patient complications after heart transplantation were also identified.
Results
The annual number of new heart transplant recipients ranged from 18 to 68. The 5-year survival rate was 77.9%. The total number of regimens was 10 in 2000, and increased to 28 in 2009. Most prescriptions were immunosuppressive combinations (95.5%–89.5%). The majority of immunosuppressive regimens were a triple regimen: cyclosporine, mycophenolic acid and corticosteroid in 2009. Cyclosporine was a predominant calcineurin inhibitor with a decreasing trend from 73.9% to 59.1%, whereas the use of tacrolimus significantly increased from 11.9% to 38.4%. Mycophenolic acid was the most frequently used antimetabolite (60.1%–80.3%), while the use of azathioprine was reduced (21.6%–2.3%). From 2008, the launch of everolimus initiated a new era in the utilization of mammalian target of rapamycin inhibitors for maintenance immunosuppression.
Conclusions
Cyclosporine remained the most frequently used calcineurin inhibitors, and tacrolimus increased gradually. Mycophenolic acid was the most popular antimetabolite rather than azathioprine. The rapidly increased everolimus combined regimen may change the patterns of maintenance immunosuppression. The increasing number of combination therapies indicates an active role of everolimus and a tendency of complex tailored individual therapies. © 2014 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Keywords: heart transplantation, immunosuppressive therapy, prescription patterns, pharmacoepidemiology
INTRODUCTION
Heart transplantation (HT) has become a legitimate destination therapy for patients with end-stage heart failure in Taiwan after the Human Organ Transplantation Act was enacted in 1987. Since the early legislation for human organ transplantation and with advanced surgical techniques, a considerable number of HTs has been performed in Taiwan in the past decades, compared with other Southeast Asian countries.1,2 The post-HT survival rate has been significantly improved worldwide due to the advancement of transplantation techniques and the availability of new immunosuppressive agents.3 Nevertheless, clinicians are more concerned about immunosuppression regimens to prevent graft failure and minimize undesirable adverse effects from long-term immunosuppression therapy. After the availability of several new immunosuppressive agents, including tacrolimus (TAC) (launched in Taiwan in 1998), mycophenolic acid (MPA) (in 1998), sirolimus (in 2002) and everolimus (in 2008), the trends of maintenance immunosuppression for heart transplant recipients in Taiwan are still not well known. In this study, our primary aim was to explore the trends of immunosuppressive therapy maintenance for HT recipients in Taiwan by analyzing the data from the Taiwan National Health Insurance Research Database (NHIRD). Furthermore, we evaluated the common complications after HT accompanied with long term immunosuppressive therapy.
METHODS
Data source
The Taiwan National Health Insurance (NHI) program has offered comprehensive medical care since 1995, covering nearly 99% of all of the inhabitants in Taiwan.4 The Bureau of NHI granted public access to the Taiwan National Health Insurance Research Database (NHIRD) in 1999 for the purpose of healthcare research. The dataset contains patient identification code, gender, age, date of admission and discharge, date of ambulatory visits, prescription details, diagnosis codes and procedure codes according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). Because all HT recipients need post operative intensive care, we could retrieve all of the HT information from the use of critical care contained in the NHIRD, which has comprehensive details of healthcare utilization and enrollment information for all beneficiaries who have ever received intensive care. Therefore we could enroll all heart transplant recipients.
Study population
From the database, patients with diagnosis of HT at hospital discharge or ambulatory visits were identified by the diagnostic ICD-9-CM codes (3751–3755) and specific reimbursement claim codes for heart implantation. Furthermore, the validation of HT diagnosis was confirmed by the prescriptions involving immunosuppressive agents after hospital discharge. Patients who had ever received solid organ transplants (heart, lung, kidney, liver, pancreas, and spleen) more than once were excluded. Patient complications after HT were also identified by the ICD-9-CM coding, such as new malignancies (140–209), post-transplant lymphoproliferative disorder (238.77), complication after HT (996.83) and newly diagnosed renal dysfunction (580–589). Complications after HT were followed up from first diagnosed to the end of the study, which was 31 December in 2009. For prescription trend analysis, all ambulatory prescriptions of immunosuppressant agents for patients who received HT between 2000 and 2009 were analyzed. All medications were included in our prescription dataset but everolimus only included from 2008. The rejection event was defined as any admission with a diagnosis of complications after HT (ICD-9 code 996.8) along with the use of muromonab-CD3 (OKT3), antithymocyte globulin (ATG) or high dose methylprednisolone during the same admission.
Statistical analyses
Statistical analyses were performed using the Statistical Analysis Systems, version 9.3 (SAS Institute, Cary, NC, USA). Descriptive statistics are presented as percentages. The rejection rate and survival rate were computed by using the Kaplan–Meier method. Chi-square for trend was applied for the prescription rates and time trends during the 10-year period of our inquiry. A p-value <0.05 was considered statistically significant.
RESULTS
We identified 488 HT recipients who had received ambulatory prescriptions involving immunosuppressive agents after their HT during the study period. In our study group, the number of new HT recipients ranged from 18 to 68 per year. Most of the HT recipients were male (81.8%), and the mean age at the time of their first HT was 45.3 ± 15.2 years. Moreover, 96.7% of the patients had comorbids with cardiovascular diseases (ICD-9-CM code 410–414, 420–429), 28.3% with diabetes mellitus, 27.5% with chronic liver disease, 7.6% with chronic renal disease and 2.7% with prior malignancy before their transplantation. After transplantation, complications of HT were noted by the ICD-9-CM coding, such as new malignancies (140–209), post-transplant lymphoproliferative disorder (238.77), complication after HT (996.83) and newly diagnosed renal dysfunction (580–589). More than half of the recipients (N = 259, 53.1%) had complications related to HT and the average onset time was 1.24 ± 1.61 year after transplant. Forty-nine patients (10.0%) were newly diagnosed with chronic kidney disease which was the second most common complication after HT. The onset time of chronic kidney disease was 2.72 ± 2.10 years. Post-transplant malignancies occurred in 33 patients (6.8%) with average onset time of 1.89 ± 1.37 year (Table 1). The first two frequent malignancies after transplantation were nodular lymphoma (N = 6) and female breast cancer (N = 5). The 1-year, 3-year and 5-year overall survival rates of HT recipients were 93.6%, 84.3% and 77.9%, respectively (Figure 1). The rejection rate in the first year was 16.4% and the second year was 25% (9% increment), and the third year was 29% (4% increment).
Table 1.
Patients had complications and the onset time of complications
| Complications (ICD-9-CM code) | Onset time (year) | Number of patients at time of occurring complications after heart transplantation | ||||
|---|---|---|---|---|---|---|
| Total | <1 year | 1–3 years | 4–6 years | >6 years | ||
| Complications after heart transplantation (996.83) | 1.24 ± 1.61 | 259 | 163 | 65 | 23 | 8 |
| Chronic kidney disease (580–9) | 2.72 ± 2.10 | 49 | 12 | 17 | 15 | 5 |
| Post-transplant malignancy (140–209) | 1.89 ± 1.37 | 33 | 11 | 17 | 5 | 0 |
ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification.
Figure 1.
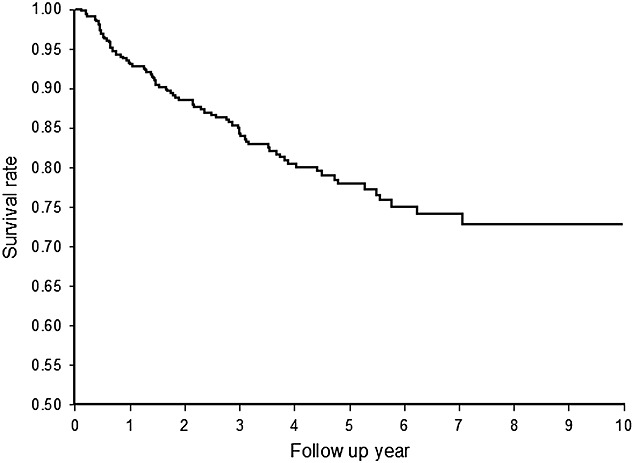
Survival rate among adult heart transplant recipients during 2000–2009 (1 year, 93.6%; 3 year, 84.3%; 5 year, 77.9%)
Regarding prescription patterns for maintenance immunosuppression, calcineurin inhibitors (CNI) were the majority among the immunosuppressive agents (Figure 2). Cyclosporine (CSA) remained the most frequently used CNI; however, the use of TAC increased significantly from 11.9% to 38.4%, while the use of CSA was reduced from 73.9% to 59.1%. The use of azathioprine (AZA) continuously decreased (21.6%–2.3%) during the study period, while the new antimetabolite immunosuppressant MPA was popular in heart transplant recipients, peaking at 80.3% in 2004 and dropped to 60.1% in 2009. After the new mammalian target of rapamycin inhibitors (mTORi) everolimus was approved for HT recipients, the use of mTORi increased significantly after 2008. Corticosteroid was still indispensable, though its use showed a decreasing trend from 96.6% to 63.8%.
Figure 2.
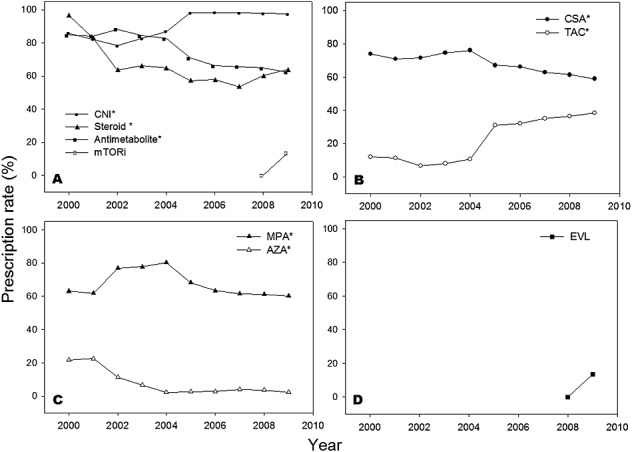
Prescription trends of maintenance immunosuppressive agents during 2000–2009. (A) Trends of 4 classes of immunosuppressive agents. (B) Trends of two calcineurin inhibitors. (C) Trends of two antimetabolites. (D) Trends of mammalian target of rapamycin inhibitors. AZA, azathioprine; CNI, calcineurin inhibitors; CSA, cyclosporine; EVL, everolimus; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors; TAC, tacrolimus. * Indicates p-value <0.05 under the chi-square for trend
From 2000 to 2009, the number of regimens increased from 10 to 28 (Table 2) but immunosuppressant combinations were prescribed in most prescriptions (95.5% in 2000 and 89.5% in 2009). Single drug regimen only accounted for 4.5% and 10.5% of total prescriptions in 2000 and 2009, respectively. In 2009, the most frequently used single drug regimen was cyclosporine. For the prescription trend in combination therapies, the prescription number for dual-drug regimens increased from 23.9% in 2000 to 50%, reached a plateau during 2005–2008, but followed by a drop to 43.3% in 2009. The number of triple-drug regimens decreased from 71.6% in 2000 to a trough at 34.5% in 2007, followed by a significant upward trend to 44.7% in 2009. As the result, the utilizations of dual- and triple-drug regimens were comparable in 2009 (Figure 3).
Table 2.
Patterns of immunosuppressive regimens among heart transplant recipients at ambulatory visits, 2000–2009
| Year | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|---|---|---|---|
| Total regimens | 10 | 15 | 15 | 16 | 15 | 16 | 15 | 15 | 17 | 28 |
| Total prescriptions | 176 | 737 | 969 | 1207 | 1769 | 2390 | 2974 | 3362 | 3875 | 4345 |
| Single-drug regimens (%) | 4.54 | 11.80 | 20.02 | 17.90 | 18.15 | 12.38 | 13.72 | 17.19 | 14.66 | 10.52 |
| STE | 2.27 | 4.07 | 2.58 | 2.49 | 2.60 | 1.30 | 0.77 | 1.22 | 1.44 | 1.43 |
| CSA | 0.57 | 1.63 | 3.30 | 5.72 | 7.01 | 7.45 | 8.44 | 10.95 | 9.42 | 5.96 |
| TAC | 0 | 1.36 | 1.34 | 2.07 | 2.60 | 3.26 | 3.90 | 4.46 | 3.74 | 2.72 |
| MPA | 1.70 | 4.75 | 12.59 | 7.13 | 5.31 | 0.33 | 0.61 | 0.57 | 0.36 | 0.30 |
| AZA | 0 | 0 | 0.21 | 0.50 | 0.62 | 0.04 | 0 | 0 | 0 | 0 |
| EVL | 0 | 0.12 | ||||||||
| Two-drug regimens (%) | 23.87 | 27.14 | 29.82 | 31.07 | 29.34 | 48.95 | 50.37 | 48.33 | 47.79 | 43.33 |
| CSA-based | ||||||||||
| +STE | 9.66 | 6.24 | 4.33 | 4.31 | 4.52 | 12.38 | 16.91 | 14.19 | 16.08 | 11.05 |
| +MPA | 1.14 | 8.01 | 17.44 | 15.91 | 17.58 | 22.85 | 18.43 | 16.75 | 12.41 | 10.13 |
| +AZA | 0 | 1.22 | 1.24 | 2.15 | 0.96 | 0.88 | 0.67 | 0.51 | 0.62 | 0.55 |
| +EVL | 0 | 3.38 | ||||||||
| TAC-based | ||||||||||
| +STE | 2.84 | 2.58 | 0.21 | 1.08 | 0.57 | 4.77 | 3.80 | 3.54 | 4.70 | 5.39 |
| +MPA | 0 | 0.14 | 0.31 | 0.41 | 0.90 | 7.41 | 8.37 | 10.71 | 10.97 | 9.14 |
| +AZA | 0 | 0 | 0 | 0 | 0.11 | 0.54 | 1.88 | 2.56 | 2.35 | 1.17 |
| +EVL | 0 | 1.84 | ||||||||
| Others | ||||||||||
| MPA + STE | 10.23 | 3.66 | 4.54 | 5.47 | 4.69 | 0.13 | 0.30 | 0.09 | 0.57 | 0.53 |
| AZA + STE | 0 | 5.29 | 1.75 | 1.74 | 0 | 0 | 0 | 0 | 0.10 | 0.02 |
| MPA + EVL | 0 | 0.09 | ||||||||
| EVL + STE | 0 | 0.05 | ||||||||
| Three-drug regimens (%) | 71.59 | 61.06 | 50.15 | 51.04 | 52.52 | 38.66 | 35.91 | 34.47 | 37.55 | 44.65 |
| CSA-based | ||||||||||
| +MPA + STE | 40.91 | 39.08 | 37.15 | 44.57 | 45.34 | 22.72 | 21.69 | 20.14 | 22.53 | 22.62 |
| +AZA + STE | 21.59 | 14.79 | 8.05 | 2.07 | 0.68 | 0.79 | 0.13 | 0.45 | 0.23 | 0.32 |
| +MPA + EVL | 0 | 0.14 | ||||||||
| +EVL + STE | 0.13 | 3.94 | ||||||||
| TAC-based | ||||||||||
| +MPA + STE | 9.09 | 6.11 | 4.95 | 4.31 | 6.50 | 14.73 | 13.99 | 13.41 | 14.30 | 14.96 |
| +AZA + STE | 0 | 1.09 | 0 | 0.08 | 0 | 0.42 | 0.10 | 0.48 | 0.36 | 0.18 |
| +MPA + EVL | 0 | 0.64 | ||||||||
| +EVL + STE | 0 | 1.80 | ||||||||
| Others | ||||||||||
| MPA + STE + EVL | 0 | 0.05 | ||||||||
| Four-drug regimens (%) | 1.50 | |||||||||
| CSA + MPA + EVL + STE | 0 | 0.99 | ||||||||
| TAC + MPA + EVL + STE | 0 | 0.51 | ||||||||
AZA, azathioprine; CSA, cyclosporine; EVL, everolimus; MPA, mycophenolic acid; STE, corticosteroids; TAC, tacrolimus.
Figure 3.
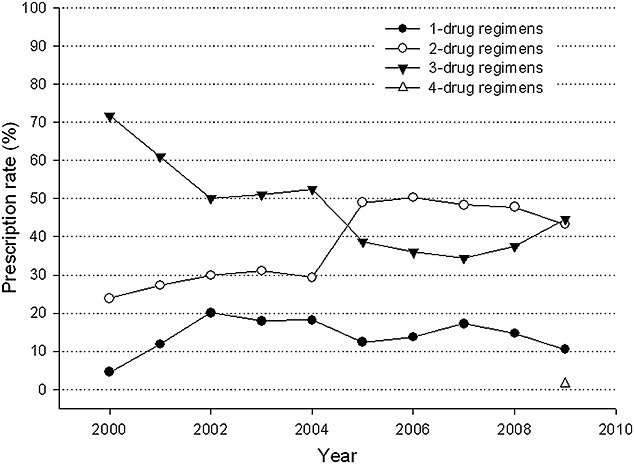
Prescription trends of different combination regimens of maintenance immunosuppression among heart transplant recipients, 2000–2009
The most popular triple-drug regimen was still the combination of CSA with MPA and corticosteroid (40.91% in 2000 to 22.62% in 2009). Comparing the change of dual-drug regimens between 2000 and 2009, CSA and steroid combinations has replaced MPA and steroids combinations as the most frequently used dual-drug regimen (Table 2).
CNI-based regimens accounted for 95.9% of dual-drug regimens and 99.97% of triple-drug regimens. In 2000, cyclosporine-based regimens were the most common treatment among dual- and triple-drug regimens (45.2% and 87.3%, respectively) whereas tacrolimus-based regimens only accounted for 11.9% of dual-drug regimens and 12.7% of triple-drug regimen. In 2009, cyclosporine-based regimens increased in dual-drug regimens (57.9%), but decreased in triple-drug regimens (60.5%). Notably, prescriptions of tacrolimus-based regimens increased to 40.5% among dual-drug regimens and 39.4% among triple-drug regimens in 2009.
The use of MPA in dual-drug regimens was 59.5% in 2000 and 45.9% in 2009, while in triple-drug regimens it increased from 69.8% in 2000 to 86.0% in 2009. AZA was seldom used in dual-drug regimens (0% in 2000 and 4.0% in 2009). Among triple-drug regimens, those with AZA decreased from 30.2% in 2000 to 1.1% in 2009.
After everolimus was reimbursed by NHI in 2008, we observed that the utilization of everolimus increased among patients with different follow-up durations. The rates were 14.5% (18/124) among patients within 1 year after transplant, 19.6% (29/148) among patients with 1–3 years follow-up, and 22.6% (36/159) among patients with 4–6 years follow-up. Five new triple-drug regimens involving mTORi were observed up to 2009. Regimens involving everolimus accounted for 0.3% of total triple-drug regimens in 2008, increasing to 14.7% in 2009. Until 2009, 12.4% of dual-drug regimens included enerolimus. Four-drug regimens first appeared in 2009, all of them contained everolimus.
We further analyzed prescription patterns for patients with different follow-up durations. According to the follow-up durations after transplantation, the patterns of immunosuppression combinations were shown in Figure 4. For patients receiving transplantation within 1 year, the most common maintenance therapies were triple-drug combinations, which were CNI + MPA + steroids combinations. For patients receiving transplantation more than 1 year, the most common maintenance therapies were dual-drug combinations, including CSA and MPA, TAC and MPA, CSA and steroids.
Figure 4.
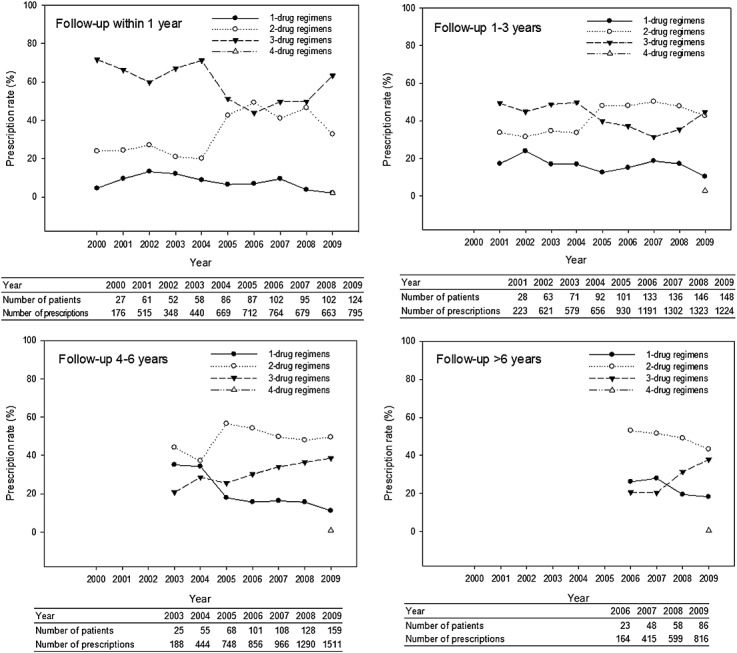
Prescription trends of different combination regimens of maintenance immunosuppression among heart transplant recipients with different follow-up duration after transplant
DISCUSSION
Intensive multiple immunosuppressant regimens have become the mainstay of maintenance immunosuppression for HT recipients. Unlike other solid organ transplantations, graft failure after HT results in significant mortality.5 Therefore, four classifications of immunosuppressive agents are currently used as long-term prophylaxis of organ rejection for post-HT patients: CNIs (cyclosporine and tacrolimus), antimetabolites (azathioprine and mycophenolic acid), mTOR inhibitors (sirolimus and everolimus) and corticosteroids. In this study, 19 out of 28 regimens contained CNI, and more than 90% of immunosuppression prescriptions contained CNI. It concluded that CNI is the key component of maintenance immunosuppression for HT patients. Cyclosporine was the most frequently used CNI among HT recipients (73.9% in 2000 to 59.1% in 2009), although it has been gradually replaced by tacrolimus (11.9% in 2000 to 38.4% in 2009). In recent years, most heart transplant centers around the world have changed their regimens from CSA to TAC and from AZA to MPA.6 In 2013 the International Society for Heart and Lung Transplantation (ISHLT) registry reported the use of CSA decreasing while TAC increasing (from 2000 to 2012, 75.2% to 13.0% vs. 22.8% to 81.4%, respectively), and the use of MPA increased while AZA decreased (68.5% to 85.4% and 20.0% to 3.2%, respectively).6 Likewise, the utilization of TAC and MPA observed in Taiwan was consistent with global trends. ISHLT registry data showed that the 1-year, 3-year and 5-year survival rates of adult HT recipients during 2003–2010 were 84.5%, 78.2% and 72.5%, respectively.3 Notably, in our result, the 1-year, 3-year and 5-year overall survival rates of HT recipients were 93.6%, 84.3% and 77.9%, respectively.
Post-transplant morbidities including acute rejection, cardiac allograft vasculopathy (CAV), renal failure and malignancy may lead to death.3,7 Owing to the complexity of patients' profiles after HT, physicians have continuously tried different regimens to achieve optimal maintenance immunosuppression. Grimm et al. conducted a large, controlled, multicenter study which showed that the TAC-based regimen was associated with a lower rate of acute rejection compared with the CSA-based regimen.8 A growth of TAC-based regimen in our study reflects the clinical effectiveness of TAC for immunosuppression. Drug selection for long-term immunosuppressive therapy is usually influenced by considering the drug-related clinical adverse effects. Several adverse effects from CSA and TAC were also revealed from clinical trials. Kobashigawa et al. showed that CSA-based treatment led to more hyperlipidemia and hypertension reactions than TAC-based treatment did, while the latter led to more post-transplant diabetes mellitus.9
Cardiac allograft vasculopathy (CAV) is another complication related to post-HT mortality.7,10 Approximately 5–10% of recipients experienced complication with CAV within 1 year after transplantation and nearly 50% of recipients developed atherosclerosis within 5 years.11 For CAV prevention, strategies must be adopted early, including early diagnosis of CAV by intravascular ultrasound, coronary angiography, and introduction of statins, vasodilators and optimal immunosuppressants.12 Unlike the controversial effects of CNIs on CAV,13 the benefit of mTORi has been proven in preventing CAV among HT recipients.14,15 In this study, we observed that more new triple-drug and quadruple-drug combinations containing mTORi were prescribed after the availability of everolimus. This observation indicated that physicians prefer mTOR inhibitors for the prevention of CAV among HT recipients in Taiwan. Mycophenolic acid was proved having protective effect on CAV progress by inhibiting the inflammation cascade. Kobashigawa et al. also reported that regimens containing MPA might slow the onset and progression of CAV.16–18
Post-transplant malignancy has a negative impact on long-term survival of HT recipients. According to the ISHLT 29th Report in 2012, malignancy contributed to more than 20% of the deaths among HT recipients 5 years after transplantation.3 Skin cancer, post-transplant lymphoproliferative disorder (PTLD) and solid organ tumors are the most noted malignancies among heart transplant recipients.19–21 Numerous trials have suggested that immunosuppressive therapy is likely the cause of post-transplant malignancy; particularly, CNI may enhance tumor growth via promoting the release of growth factors.22–25 AZA also was reported to exhibit a higher incidence of post-transplant malignancy compared with MPA.26 However, certain immunosuppressive agents may have preventive effect on the development of post-transplant malignancy. Recent evidence also suggested that mTORi was associated with a lower incidence of post-transplant malignancies by its anti-proliferative activity and minimizing dose of CNI use.27,28 Everolimus, mTORi, can act synergistically with CSA to achieve maintenance of immunosuppression; thus, combining everolimus with a lower dose of CSA can avoid compromise of immunosuppression. This combination can reduce the risk of post-transplant malignancies by reducing overexposure to CSA.23,29 In 2012, regimens of everolimus with a CNI used in Taiwanese patients after HT have been reported leading to a safe and effective clinical outcome.30,31 Currently, everolimus is recognized as a promising adjuvant agent for heart transplant patients in immunosuppression therapy. Sirolimus, another mTORi, has a similar effect on the reduction risk of malignancy, and it has been used for HT recipients in other countries;3,22 however sirolimus is not applied in Taiwanese recipients due to the limitation of the reimbursed indication.
Renal dysfunction represents a frequent complication after organ transplantation.32 From the ISHLT 29th Report in 2012, the prevalence of severe renal impairment was 6% at 1 year and 16% at 5 years after transplantation.3 Certain immunosuppressive regimens may associate with post-transplant nephropathy, especially CNI. For patients co-morbid with renal impairment, two studies have shown the avoidance of renal dysfunction by applying protocols of either mTORi or MPA with low dose CNI combination therapy.33,34 In comparative trials, switching from AZA to MPA or introducing mTORi in combination with low-dose CNI has shown the benefit of renal function improvement.35,36 In this study, the increasing utilizations of MPA and mTORi may show that physicians were likely to prescribe medications which have lower risk of developing adverse effects.
This study had some limitations. First, for immunosuppressive drugs without reimbursement, charged by self-pay only, the prescribed records could not be obtained from NHIRD. Second, we only analyzed the common complications after HT which have specific ICD-9-CM codes, including new malignancies (140–209), post-transplant lymphoproliferative disorder (238.77), complication after HT (996.83), and newly diagnosed renal dysfunction (580–589). However, some specific complications related to HT such as CAV could not be further analyzed in this study due to lack of specific diagnosis code in ICD-9-CM coding system.
In conclusion, CNI-based combination regimens are still the most common maintenance therapy for HT recipients. Cyclosporine was the most commonly used CNI, but its use was gradually replaced by tacrolimus. MPA was substituted for AZA as the most widely prescribed antimetabolite. However, the mTOR inhibitors, everolimus offering unique benefits of preventions of CAV and malignancy, may lead to a new trend in maintenance immunosuppression in the near future. In this study, there was a marked increase in the numbers of immunosuppressive regimen for post-HT, indicating a growing trend of tailoring immunosuppressive treatment based on patients' complexity.
Conflict of interest
The authors declare that they have no competing interests regarding the content of this article.
KEY POINTS.
The number of immunosuppressive regimens for heart transplant recipients increased, indicating a growing trend of tailoring immunosuppressive treatment based on patient individualization.
Calcineurin inhibitors are the key component of maintenance immunosuppression for heart transplant patients in Taiwan.
The rapidly increased use of everolimus may change the trends in maintenance immunosuppression.
ETHIC STATEMENT
All identifiable information was encrypted across the dataset by the Bureau of NHI. This study was exempted from a full review by the Institutional Review Board of the Taipei Veterans General Hospital.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, the Department of Health or the National Health Research Institutes. This study was supported by grants from the Taipei Veterans General Hospital.
REFERENCES
- 1.Sivathasan C. Heart transplantation in Singapore. Ann Acad Med Singapore. 2009;38:309–6. [PubMed] [Google Scholar]
- 2.Chu SH. 2nd Asia Pacific Congress of Heart Failre. Singapore: Raffles City Convention Centre; 2005. Lessons from Heart Transplant Programmes in Asia Pacific. 9–12th Janurary. [Google Scholar]
- 3.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report-2012. The Journal of heart and lung transplantation: the official publication of the Int Soc Heart Transpl. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Wu TY, Majeed A, Kuo KN. An overview of healthcare system in Taiwan. London J Prim Care. 2010;3:115–119. doi: 10.1080/17571472.2010.11493315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraund S, Pethig K, Franke U, et al. Ten year survival after heart transplantation: palliative procedure or successful long term treatment? Heart. 1999;82:47–51. doi: 10.1136/hrt.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; Focus Theme: Age. The Journal of heart and lung transplantation: the official publication of the Int Soc Heart Transpl. 2012;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Mastrobuoni S, Dell'Aquila AM, Azcarate PM, Rabago G, Herreros J. Long-term survival (>20 years) following heart transplantation. J Cardiovasc Surg (Torino) 2012;53:677–684. [PubMed] [Google Scholar]
- 8.Grimm M, Rinaldi M, Yonan NA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transpl: official journal of the Am Soc Transpl Surgeons. 2006;6:1387–1397. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 9.Kobashigawa JA, Patel J, Furukawa H, et al. Five-year results of a randomized, single-center study of tacrolimus vs microemulsion cyclosporine in heart transplant patients. The Journal of heart and lung transplantation: the official publication of the Int Soc Heart Transpl. 2006;25:434–439. doi: 10.1016/j.healun.2005.11.452. [DOI] [PubMed] [Google Scholar]
- 10.Kobashigawa JA, Tobis JM, Starling RC, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Kobashigawa J. What is the optimal prophylaxis for treatment of cardiac allograft vasculopathy? Curr Control Trials Cardiovasc Med. 2000;1:166–171. doi: 10.1186/cvm-1-3-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 13.Segovia J, Gomez-Bueno M, Alonso-Pulpon L. Treatment of allograft vasculopathy in heart transplantation. Expert Opin Pharmacother. 2006;7:2369–2383. doi: 10.1517/14656566.7.17.2369. [DOI] [PubMed] [Google Scholar]
- 14.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 15.Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–2700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 16.Kobashigawa JA, Miller LW, Russell SD, et al. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transpl: official journal of the Am Soc Transpl Surgeons. 2006;6:1377–1386. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 17.Kobashigawa JA, Tobis JM, Mentzer RM, et al. Mycophenolate mofetil reduces intimal thickness by intravascular ultrasound after heart transplant: reanalysis of the multicenter trial. Am J Transpl: official journal of the Am Soc Transpl Surgeons. 2006;6:993–997. doi: 10.1111/j.1600-6143.2006.01297.x. [DOI] [PubMed] [Google Scholar]
- 18.Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transpl: the official publication of the Int Soc Heart Transpl. 2005;24:517–525. doi: 10.1016/j.healun.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Chen PL, Chang HH, Chen IM, et al. Malignancy after heart transplantation. J Chin Med Assoc. 2009;72:588–593. doi: 10.1016/S1726-4901(09)70434-4. [DOI] [PubMed] [Google Scholar]
- 20.Crespo-Leiro MG, Alonso-Pulpon L, Vazquez de Prada JA, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transpl: Official J Am Soc Transpl Am Soc Transpl Surgeons. 2008;8:1031–1039. doi: 10.1111/j.1600-6143.2008.02196.x. [DOI] [PubMed] [Google Scholar]
- 21.Yagdi T, Sharples L, Tsui S, Large S, Parameshwar J. Malignancy after heart transplantation: analysis of 24-year experience at a single center. J Card Surg. 2009;24:572–579. doi: 10.1111/j.1540-8191.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 22.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 23.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay F, Fernandes M, Habbab F, deB Edwardes MD, Loertscher R, Meterissian S. Malignancy after renal transplantation: incidence and role of type of immunosuppression. Ann Surg Oncol. 2002;9:785–788. doi: 10.1007/BF02574501. [DOI] [PubMed] [Google Scholar]
- 25.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371–1373. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transp: the Official Publication Intern Soc Heart Transpl. 2006;25:1186–1191. doi: 10.1016/j.healun.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Valantine H. Is there a role for proliferation signal/mTOR inhibitors in the prevention and treatment of de novo malignancies after heart transplantation? Lessons learned from renal transplantation and oncology. J Heart Lung Transp: the Official Publication Intern Soc Heart Transpl. 2007;26:557–564. doi: 10.1016/j.healun.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Basu A, Liu T, Banerjee P, et al. Effectiveness of a combination therapy using calcineurin inhibitor and mTOR inhibitor in preventing allograft rejection and post-transplantation renal cancer progression. Cancer Lett. 2012;321:179–186. doi: 10.1016/j.canlet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmkuhl HB, Mai D, Dandel M, et al. Observational study with everolimus (Certican) in combination with low-dose cyclosporine in de novo heart transplant recipients. J Heart Lung Transp: the Official Publication Intern Soc Heart Transpl. 2007;26:700–704. doi: 10.1016/j.healun.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Wang SS, Chou NK, Chi NH, et al. Clinical experience of tacrolimus with everolimus in heart transplantation. Transplant Proc. 2012;44:907–909. doi: 10.1016/j.transproceed.2012.01.094. [DOI] [PubMed] [Google Scholar]
- 31.Wang SS, Chou NK, Chi NH, et al. Heart transplantation under cyclosporine or tacrolimus combined with mycophenolate mofetil or everolimus. Transplant Proc. 2008;40:2607–2608. doi: 10.1016/j.transproceed.2008.08.072. [DOI] [PubMed] [Google Scholar]
- 32.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 33.Gullestad L, Iversen M, Mortensen SA, et al. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89:864–872. doi: 10.1097/TP.0b013e3181cbac2d. [DOI] [PubMed] [Google Scholar]
- 34.Potena L, Bianchi IG, Magnani G, et al. Cyclosporine lowering with everolimus or mycophenolate to preserve renal function in heart recipients: a randomized study. Transplantation. 2010;89:263–265. doi: 10.1097/TP.0b013e3181c42b95. [DOI] [PubMed] [Google Scholar]
- 35.Zuckermann A, Keogh A, Crespo-Leiro MG, et al. Randomized controlled trial of sirolimus conversion in cardiac transplant recipients with renal insufficiency. Am J Transpl: Official J Am Soc Transpl Am Soc Transpl Surgeons. 2012;12:2487–2497. doi: 10.1111/j.1600-6143.2012.04131.x. [DOI] [PubMed] [Google Scholar]
- 36.Aleksic I, Baryalei M, Busch T, et al. Improvement of impaired renal function in heart transplant recipients treated with mycophenolate mofetil and low-dose cyclosporine. Transplantation. 2000;69:1586–1590. doi: 10.1097/00007890-200004270-00012. [DOI] [PubMed] [Google Scholar]


