Abstract
Polygalacturonase (PG), one of the hydrolases responsible for cell wall pectin degradation, is involved in organ consenescence and biotic stress in plants. PG1 is composed of a catalytic subunit, PG2, and a non-catalytic PG1β subunit. OsBURP16 belongs to the PG1β-like subfamily of BURP-family genes and encodes one putative PG1β subunit precursor in rice (Oryza sativa L.). Transcription of OsBURP16 is induced by cold, salinity and drought stresses, as well as by abscisic acid (ABA) treatment. Analysis of plant survival rates, relative ion leakage rates, accumulation levels of H2O2 and water loss rates of leaves showed that overexpression of OsBURP16 enhanced sensitivity to cold, salinity and drought stresses compared with controls. Young leaves of Ubi::OsBURP16 transgenic plants showed reduced cell adhesion and increased cuticular transpiration rate. Mechanical strength measurement of Ubi::OsBURP16 plants showed that reduced force was required to break leaves as compared with wild type. Transgenic rice showed enhanced PG activity and reduced pectin content. All these results suggested that overexpression of OsBURP16 caused pectin degradation and affected cell wall integrity as well as transpiration rate, which decreased tolerance to abiotic stresses.
The cell wall is a barrier against biotic and abiotic stresses. Overexpression of stress-inducible OsBURP16, the beta-subunit of polygalacturonase 1, decreases pectin contents and cell adhesion in rice. Analyses of plant survival, ion leakage, H2O2 levels, and leaf water loss showed that these effects of overexpression were accompanied by enhanced sensitivity to cold, salinity and drought compared to the wild-type. Our data therefore provide new information on links between polygalacturonase activity and abiotic stress resistance in rice.
Keywords: abiotic stress, cell adhesion, pectin, polygalacturonase
Introduction
Polygalacturonase (PG), one of the cell wall hydrolases, is responsible for wall pectin degradation during fruit ripening. PG1 is composed of a 45 kD catalytic subunit (PG2) and a 38 kD non-catalytic β subunit (PG1β). Previous studies found tomato PG1β protein in cell wall extracts, and the cDNA of tomato PG1β was cloned (Zheng et al. 1992, 1994). The PG1β subunit was purified from PG1 in tomato as a heat-stable, acidic, heavily glycosylated 38 kD glycoprotein (Tucker et al. 1981). The PG1β subunit functioned as a PG converter to alter the biochemical and enzymatic properties of PG2, resulting in the formation of PG1 (Tucker et al. 1981). PG1β can bind to the cell wall and interact with the catalytic subunit PG2. The interaction of PG1β with PG2 converts the thermolabile PG2 into the thermostable PG1 (Peeters et al. 2004).
PGs are expressed in a wide range of tissues and developmental stages in plants, and are involved in a diverse range of processes including fruit ripening, leaf and flower abscission, pod and anther dehiscence, pathogen defense and plant–host interactions (Brummell & Harpster 2001; Atkinson et al. 2002; Swain et al. 2011). For example, the PG1 complex is involved in pectin metabolism by regulating pectin solubilization or degradation during fruit ripening in tomato (Watson et al. 1994; Zheng et al. 1994). Overexpression of PG in transgenic apple leads to various novel phenotypes, including silvery-colored leaves and premature leaf shedding due to reduced cell adhesion in leaf abscission zones (Atkinson et al. 2002). Arabidopsis dehiscence zone polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 (QRT2) are endo-PGs. ADPG1 and ADPG2 are essential for silique dehiscence, and QRT2 is essential for pollen grain separation (Ogawa et al. 2009). Few studies investigating PG or PG1β-like proteins in rice have been reported.
The BURP domain was named for the proteins BNM2, USP, RD22 and PG1β, which share the highly conserved sequence CH-X(10)-CH-X(23–26)-CH-X(8)-CH-W (Hattori et al. 1998). A previous report showed that plant BURP proteins can be divided into seven subfamilies. In rice, 17 OsBURP proteins have been found in the RD22-like, BURPV, BURPVI, BURPVII and PG1β-like subfamilies. All the OsBURP genes but OsBURP01 and OsBURP13 are induced by at least one stress treatment such as drought, salt, cold or ABA treatment. The PG1β-like subfamily consists of three members, OsBURP02, OsBURP14 and OsBURP16. OsBURP16 is responsive to ABA treatment and all the abiotic stress treatments including cold, salt and drought (Ding et al. 2009).
To address the function of OsBURP16, we cloned OsBURP16 from a cDNA library of cold-stressed rice. In this study, our results indicated that Ubi::OsBURP16 transgenic plants were hypersensitive to cold, salt and drought stress compared with wild-type Zhonghua 10 (ZH10). The PG activity was higher in transgenic plants than that of ZH10, which caused a decrease in adhesion of cell walls. These results suggest that OsBURP16 is involved in cell wall formation and abiotic stress response.
Materials and Methods
Plant transformation, growth conditions and stress treatments
The wild-type rice cultivar Zhonghua 10 (Oryza sativa L. ssp. japonica cv. Zhonghua 10; ZH10) was used in this work. Plasmid construction and rice transformation were conducted following methods described previously (Chen et al. 2011). The cDNA sequence of OsBURP16 (LOC_Os10g26940) was amplified from the cDNA library of ZH10 using gene-specific primer pairs 5′-GAA GAT CTT CGA CTT GTC TTT CCA GCA CAG C-3′ and 5′-GGG GTA CCC CAC ACA CGG ATA CAT CGC ACT-3′, and was cloned into the Bam HI and Kpn I sites of the pUN1301 vector and driven by the ubiquitin promoter (Ge et al. 2004). Transgenic plants of the T3 generation with positive β-glucuronidase (GUS) activity were used in the experiments.
To analyse the response to ABA treatment, T3-generation transgenic seedlings with positive GUS staining and wild-type seedlings were used. The seedlings were grown in the incubator under 16 h light/8 h dark at 30 °C during the day and 28 °C during the night. The light intensity was 540 μmol·m−2·s−1. Leaves of 2-week-old seedlings were sprayed with 0.1 mm ABA solution and sampled at 0, 1, 3, 6, 12 and 24 h after treatment for RNA isolation (Ding et al. 2009).
For cold stress, 2-week-old seedlings were treated at 4 °C for 72 h in a low-temperature chamber under 16 h light/8 h dark, and then the seedlings were transferred to a greenhouse to recover for one week. For RNA isolation the seedlings were sampled at 0, 1, 3, 6, 12 and 24 h after treatment at 4 °C.
For salt stress, germinated seeds were placed into 96-well plates without bottom and floated in Kimura B nutrient solution (Ma & Takahashi 1990) to grow for 2 weeks in an incubator under 16 h light/8 h dark (30 °C/28 °C). The light intensity was 540 μmol·m−2·s−1. After two weeks, the 96-well plates with seedlings were transferred into fresh Kimura B nutrient solution containing 200 mm NaCl and treated for 4 d under the same light and temperature conditions. Four days later, the NaCl–Kimura B solution was replaced by fresh Kimura B nutrient solution, and plants were allowed to recover for one week. For RNA isolation, the seedlings were sampled at 0, 1, 3, 6, 12 and 24 h after treatment in the 200 mm NaCl–Kimura B solution.
For drought stress assays, water was withheld from 2-week-old seedlings grown in pots that contained the same weight of soil. Following drought treatment, the plants were watered normally for 7 d. The 2-week-old seedlings were irrigated with 20% polyethylene glycol (PEG) 6000 followed by sampling at 0, 1, 3, 6, 12 and 24 h for total RNA isolation.
The recovery of the plants was photographed. Plants that had green and healthy young leaves were considered to have survived, and the survival rates were determined (Ouyang et al. 2010). About 50 plants of each line were used in each stress experiment, and the experiments were performed at least three times.
Southern blot and transcript measurement
The Southern blot assay of transgenic rice plants was performed according to our previous report (Chen et al. 2011). Genomic DNA was isolated from 2-week-old rice seedlings, and 20 μg DNA was digested with EcoR I or Hind III. The digested DNA fragments were separated on a 0.7% agarose gel through electrophoresis and then blotted onto a nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membrane was pre-hybridized in SuperHyb Solution (Tiandz, Beijing, China) at 65 °C for 4 h. PCR was used to generate [α-32P] dCTP-labelled GUS, using the forward primer 5′-CAA CTG GAC AAG GCA CTA GC-3′ and reverse primer 5′-AGC GTC GCA GAA CAT TAC AT-3′. After pre-hybridization, the membrane was hybridized with [α-32P] dCTP-labelled GUS in SuperHyb Solution at 65 °C for 20 h and then washed with 2 × saline–sodium citrate (SSC) plus 0.1 % sodium dodecyl sulphate (SDS) washing buffer at 65 °C for 20 min and washed twice with 1 × SSC plus 0.1% SDS at 65 °C for 15 min. The membranes were exposed to X-ray film (Kodak Co Ltd, Xiamen, China) at −70 °C for 3–7 d, and then the signals were obtained using autoradiography.
To determine the transcriptional levels, quantitative RT-PCR (qRT-PCR) was performed. Total RNA was isolated by using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Rice cDNA was synthesized from 2 μg RNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA). qRT-PCR was performed on an Mx3000P instrument (Agilent Technologies Company, Shanghai, China) with SYBR Green Master Mix (Toyobo Co Ltd, Japan) according to the manufacturer's instructions. Gene expression levels were normalized to that of ACTIN1 (LOC_Os03g50885). Primers for ACTIN1 were 5′-TGG CAT CTC TCA GCA CAT TCC-3′ (forward) and 5′-TGC ACA ATG GAT GGG CCA GA-3′ (reverse). Primers for OsBURP16 were 5′-GTA CCG TAC TGC CAA ATG GG-3′ (forward) and 5′-AGC TGG TAA TCT TGC CAC CA-3′ (reverse). Primers for OsTPP1 were 5′-ACG ACT TTC TGC CAA TGA TCG-3′ (forward) and 5′-GTT GCA CCT TCA ATT CCA CTG A-3′ (reverse). Primers for OsTPS1 were 5′-CAA GGT GGA GCA GGG ATT CTC-3′ ′ (forward) and 5′-TGC GAT GTT CTT CCA ATT AAA ATG T-3′ (reverse). As ACTIN1 can be induced by abiotic stresses (Supporting Information Fig. S1b), we used the OsCIPK06 gene as the internal control for the stress treatment experiments, as it is known not to be induced by ABA or abiotic stresses (Xiang et al. 2007). Primers for OsCIPK06 were 5′-CGT GGT TCA AGA AGA CGT CCA-3′ (forward) and 5′-AGC AGG ATC GGC AAA GGC-3′ (reverse). Three biological replications were performed, and the results were consistent.
Measurement of relative electrolyte leakage and diaminobenzidine staining for H2O2
Relative ion leakage was assayed following the method of Song et al. (2011). Four-week-old seedlings from wild type and three Ubi::OsBURP16 transgenic lines (OE3, OE4 and OE6) were transferred into a 4 °C chamber, and another set of plants was grown in a greenhouse as control. After 60 or 108 h, 0.1 g of leaves was harvested for measurement from each of six plants. Leaf fragments were immersed in 6 mL deionized water and shaken at 200 r.p.m. at 25 °C for 2 h, and electrical conductivity was determined (C1). The samples were then boiled for 20 min, and the total conductivity was determined again (C2) after cooling to room temperature. Relative ion leakage (%) was calculated as C1/C2 × 100.
DAB staining was performed following the method of Ouyang et al. (2010). Two-week-old plants were treated with 200 mm NaCl solution for 2 d, and the leaves were detached and immersed in 1% 3,3′-diaminobenzidine (DAB) solution in deionized water (pH 3.8). After 30 min under vacuum the samples were incubated at room temperature for 48 h in the dark. The samples were then bleached by boiling in ethanol in order to reveal brown spots, which are indicative of the reaction of DAB with H2O2.
Water loss, stomatal conductance and transpiration rate measurements
Leaves of wild-type and Ubi::OsBURP16 plants grown under normal conditions for 2 weeks were detached and weighed immediately on a piece of weighing paper, and then placed on a laboratory table and weighed at designated time intervals (Chen et al. 2006). Three replicates were performed for each line. The extent of water loss was assessed by calculating measured mass as a percentage of the initial mass of the plants.
The fully expanded second leaves of seedlings at the tillering stage (counting from the top of the plant) were used for stomatal conductance and transpiration rate measurements using a portable gas analysis system (Li-Cor 6400, Lincoln, NE, USA). The measurements were performed under the following conditions: a constant water concentration of 10.25 ± 0.02 mmol·mol−1, a constant temperature of 29.50 ± 0.02 °C and a constant CO2 concentration of 420.20 ± 0.02 μmol·mol−1. The light intensity, which was expressed by the effective radiation of photosynthesis, was 1100 ± 50 μmol·m−2·s−1 in the environment and 1500 ± 50 μmol·m−2·s−1 in the measuring room. At least four leaves from each sample group were measured in this experiment, and the same results were obtained.
Epidermis observation
Young leaves of 2-week-old plants were cut into 1.5 cm sections, boiled in deionized water for 10 min, and then transferred into 95% ethanol and boiled for 1 h. The material was put into hot (96 °C) 85% lactic acid for 8 min. After the leaves became transparent, they were observed under differential interference contrast microscopy. In order to make sure cells were alive before staining with propidium iodide, fresh leaves or roots of 2-week-old plants were directly collected without treatment to remove pigments.
To count the number of stomata, leaves from 2-week-old plants were fixed in FAA fixative (89 mL ethanol, 6 mL acetic acid, 5 mL formaldehyde) overnight at room temperature and then dehydrated through an ethanol series. The samples were then replaced by isoamyl acetate, dried to a critical point in CO2 and mounted on stubs. After the samples were sputter-coated with gold, images were obtained with a scanning electron microscope (Hitachi S-4800 FESEM, Hitachi, Tokyo, Japan). For the stomatal aperture measurement, leaves of 25-day-old plants were detached and immediately fixed by liquid nitrogen. The stomatal pictures were obtained using an environmental scanning electron microscope (Hitachi S-3000 N).
Histochemical staining for pectin and immunolocalization of methyl – ester pectin
Leaves from 2-week-old plants were excised and fixed in FAA, which was fully infiltrated into the leaf tissues under vacuum. The samples were then dehydrated with an ethanol series and embedded in paraplast (Sigma, St. Louis, MO, USA). The embedded material was cut into 8 μm sections and stained with 0.01% (w/v) ruthenium red for general localization of pectin (Atkinson et al. 2002; Yong et al. 2003). The stained samples were observed by light microscopy. Data were analysed with ImageJ software, and 10 sections of each experimental group were analysed.
Fresh roots of 3-day-old seedlings behind the root apex were sectioned to 1 − 3 mm and were directly collected into a fixative solution containing 4% paraformaldehyde in 50 mm PIPES, 5 mm MgSO4, and 5 mm EGTA, pH 6.9 (Yang et al. 2008) for 1 h in 2 mL Eppendorf tubes. Then the 1−3 mm samples were cut by hand to the thickness of about 0.1−0.5 mm and were washed twice with phosphate buffered saline (PBS) and blocked with 0.2% bovine serum albumin (BSA) in PBS for 30 min at room temperature. All of these procedures were done on glass slides that had already been coated with polylysine. Homogalacturonan (HG), consisting of linear chains of 1,4-linked α-d-galacturonic acid residues, is one of the major components of pectin. Some carboxyl groups of HG are methyl-esterified. To visualize the total HG content, base de-esterification by treating with 0.1 m NaCO3 for 30 min before adding the JIM5 antibody was needed. Monoclonal antibodies JIM5 and JIM7 (diluted 1:10 in PBS containing 0.2% BSA) were then added for 2 h. After washing three times in PBS, goat anti-rat IgG (Sigma) fluorescein isothiocyanate conjugate (diluted 1:50 in PBS containing 0.2% BSA) was added and incubated for 2 h. Before being mounted on glass slides, samples were washed three times with PBS. The samples were then mounted on glass slides and examined under a laser scanning confocal microscope (LSM 510, Zeiss, Jena, Germany) by using a digital X-Y scan system. Control samples were also examined.
Pectin chemical analysis and PG activity assay
The method of cell wall preparation was highly similar to the protocols outlined previously (Ahmed & Labavitch, 1977, Bouton et al. 2002). Cell walls were prepared from leaves of 2-week-old plants that were grown under normal conditions. Approximately 100 mg of leaves were boiled in 1 mL of 96% ethanol for 20 min. The homogenate was centrifuged at 1000 g for 10 min, and the supernatant was discarded. The residue was washed three times each with 70% ethanol, 96% ethanol and acetone. The samples were then exposed to vacuum overnight at 40 °C for air-drying. The prepared cell wall samples were dissolved in sulphuric acid and uronic acids and then quantified by colorimetry utilizing meta-hydroxydiphenyl (Ahmed & Labavitch, 1977).
The protocol for extracting PG was modified from Zheng et al. (1992). Leaves (2 g) of 2-week-old plants were ground with liquid nitrogen and were homogenized in 6 mL of ice-cold 0.1 m sodium citrate buffer (pH 6.0). The extracts were filtered through four layers of miracloth (Amresco, Solon, OH, USA), incubated at 4 °C for 3 h and then centrifuged at 10 000 g for 20 min at 4 °C. The supernatants were filtered through miracloth again, and proteins were subsided at 4 °C by the slow addition of solid ammonium sulphate to a final saturation of 80%, and stirred for 1 h at 4 °C. The supernatant was discarded after centrifugation at 10 000 g for 20 min at 4 °C, while the precipitate was dissolved in 2 mL of 1 m NaCl and dialysed against 1 m NaCl at 4 °C for 24 h. After dialysis, the solution was utilized for the PG activity assays. PG activity was measured according to Atkinson et al. (2002).
For the measurement of PG activity and pectin content after drought stress, 2-week-old ZH10 plants were treated with 20% PEG 6000 for 12 h before measurement, while other 2-week-old ZH10 plants were treated with fresh culture solution as control.
Breaking force test
To examine the mechanical strength in the leaves of the transgenic and wild-type plants, the middle sections of the third leaves (6 cm length) of 2-week-old plants were collected and immediately used for measurement. The force required to break the leaves was measured with a digital force/length tester (5848 Microtester, Instron, Norfolk County, MA, USA) according to a previous report (Ning et al. 2011). At least 20 leaves of each sample group were measured in this experiment, and the same results were obtained.
Results
Expression patterns of OsBURP16
OsBURP16 is one of the three members in the PG1β-like gene subfamily. It contains two exons and one intron and encodes a peptide with 344 amino acid residues (Ding et al. 2009). There is high amino acid similarity between OsBURP16 and the known BURP domain containing protein LePG1β in tomato (Fig. 1a). We predicted likely transmembrane helices in the structures of OsBURP16 and the previously characterized protein LePG1β using TMHMM Server v. 2.0 and found that the two proteins may both mainly be localized outside the cell membrane (Fig. 1b). Analysis using the Rice eFP Browser (http://bar.utoronto.ca/efprice/cgi-bin/efpWeb.cgi) showed that OsBURP16 was mainly expressed in young leaves (Supporting Information Fig. S1a). Our quantitative RT-PCR (qRT-PCR) assays showed OsBURP16 was expressed in various tissues, mainly in leaf lamina, leaf cushion and internodes (Fig. 1c). These results show that OsBURP16 is highly expressed in young leaves.
Figure 1.
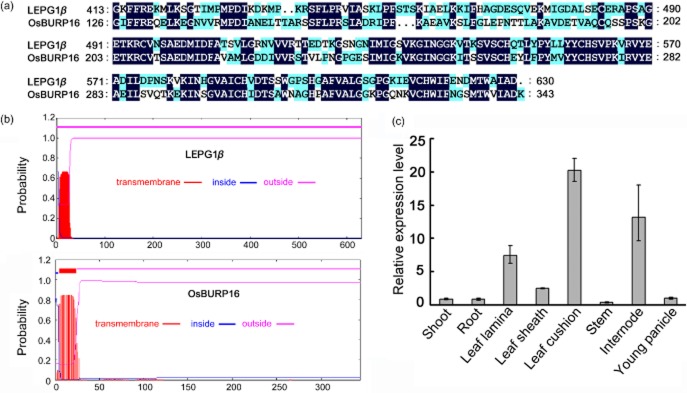
Characterization and expression pattern of OsBURP16 in rice. (a) Amino acid sequence alignment of the BURP domain of OsBURP16 with LePG1β, a PG1β subunit of tomato (Lycopersicon esculentum L.). (b) Prediction of transmembrane helices in OsBURP16 and LePG1β (a PG1β subunit of tomato), performed with the software TMHMM Server v. 2.0 online (http://www.cbs.dtu.dk/services/TMHMM). (c) The tissue expression patterns of OsBURP16. The expression level in shoot was set at 1, and the ACTIN1 gene was used as the internal control. Data represent means ± SD of three biological replicates.
The effects of ABA and abiotic stresses on the expression of OsBURP16 were monitored by qRT-PCR to investigate the gene's role in responses to environmental factors. Under 4 °C cold stress, the transcription level of OsBURP16 increased by about 1.5-fold compared with the untreated plants at 6 h after commencing the cold treatment, and then decreased to the level observed prior to treatment (Supporting Information Fig. S1c). For the drought stress analysis, the expression level of OsBURP16 was up-regulated at 12 h and then decreased to the level of untreated seedlings (Supporting Information Fig. S1c). The expression levels of OsBURP16 also clearly increased at 3 h in response to treatment with 200 mm NaCl. Treatment with 100 μm ABA caused a decrease in the transcriptions of OsBURP16 (Supporting Information Fig. S1c). These results suggest that OsBURP16 is involved in responses to ABA and abiotic stresses.
Decreased pectin content and cold tolerance in OsBURP16 transgenic plants
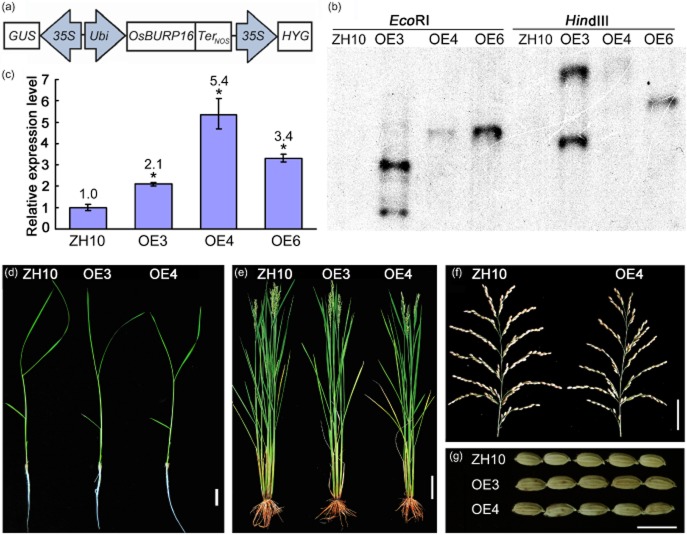
To investigate the function of OsBURP16, cDNA of OsBURP16 was cloned into the vector pUN1301, which is driven by the maize ubiquitin (Ubi) promoter (Fig. 2a). This construct was introduced into the wild-type rice cultivar Zhonghua 10 (ZH10) by Agrobacterium-mediated transformation. Three independent Ubi::OsBURP16 transgenic lines (OE3, OE4 and OE6) were confirmed by Southern blot analysis (Fig. 2b). qRT-PCR analysis revealed that the transcription levels of OsBURP16 were increased 2.1-, 5.4- and 3.4-fold in the T3 progeny of OE3, OE4 and OE6, respectively (Fig. 2c). There was no obvious difference in the morphology of Ubi::OsBURP16 transgenic rice plants compared with ZH10 at different developmental stages under normal conditions (Fig. 2d–g). As such, these three independent transgenic lines (OE3, OE4 and OE6) with relatively high expression levels of OsBURP16 were chosen for subsequent experiments. Attempts to generate mutants or RNAi plants of OsBURP16 have so far not been successful, but are ongoing.
Figure 2.
Generation of Ubi::OsBURP16 transgenic rice. (a) Diagram of the Ubi::OsBURP16 construct that was used for the generation of transgenic plants. (b) Southern blot analysis of overexpression transgenic rice lines. ZH10, wild type Zhonghua10; OE3, OE4, OE6 are the overexpression transgenic lines 3, 4 and 6. Rice genomic DNA was digested by EcoRI in the left four lanes and by HindIII in the right four lanes. (c) The expression levels of OsBURP16 in OsBURP16-overexpressing lines (OE3, OE4, OE6) were determined by qRT-PCR. The expression level in ZH10 was normalized as 1. The number in each column indicates the expression levels of OsBURP16 in each transgenic line. Data represent means ± SD of three biological replicates. (d–g) Phenotypes of 2-week-old seedlings (d), plants at heading stage (e), spikelets (f) and seeds (g) of ZH10 and OsBURP16 line OE3 and OE4. Scale bar = 2 cm (d), 10 cm (e), 5 cm (f) and 1 cm (g).
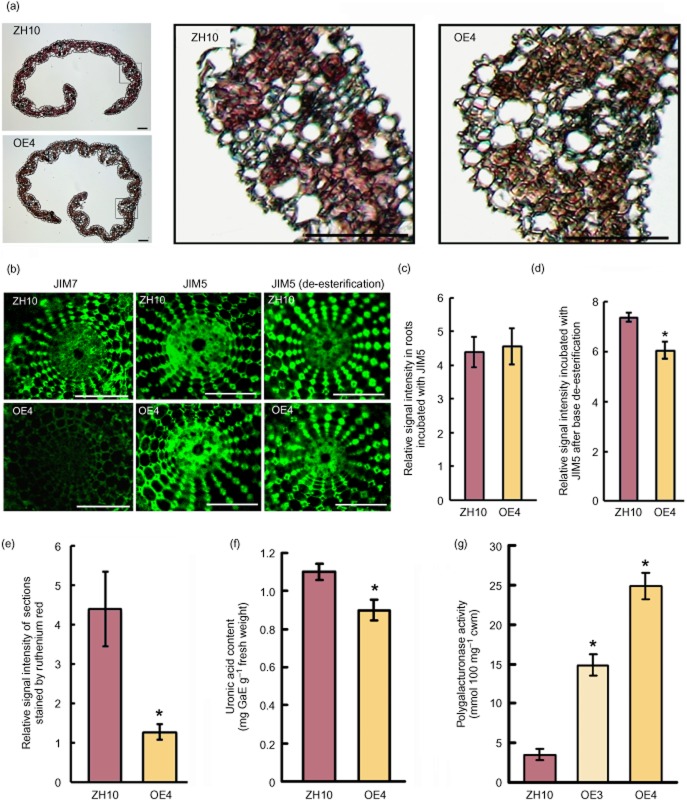
As pectin is well known as an important component of the adhesive material between plant cells (Bouton et al. 2002), the young leaves were stained with pectin-specific ruthenium red (Atkinson et al. 2002). The results showed that the young leaves of transgenic plants stained more weakly than ZH10 (Fig. 3a,e). Pectin content was also determined. As shown in Fig. 3f, the uronic acid content in ZH10 and OsBURP16 transgenic plants was 1.1 mg·g−1 and 0.9 mg·g−1 fresh weight, respectively. This decrease can be partly taken as an indication of the lower galacturonic acid (GalUA) content in the Ubi::OsBURP16 seedlings' leaves. GalUA is the sugar monomer used in the formation of the homogalacturan chains of pectin (Brummell & Harpster 2001). These results suggest that increased pectin solubilization or degradation may occur in the leaves of Ubi::OsBURP16 seedlings as compared with the wild type.
Figure 3.
Pectin and uronic acid content of cell wall fractions from OsBURP16-overexpressing transgenic and wild-type plants. (a) Cross sections of leaves of OsBURP16-overexpressing and ZH10 plants stained by ruthenium red. The black frames are enlarged in the right of the picture. Scale bar = 20 μm. (b) Immunolocalization of high- and low-methyl-ester pectin (JIM7 and JIM5, respectively) after base de-esterification in root hand sections of OsBURP16 OE4 and ZH10 seedlings. Scale bars = 100 μm. (c) Relative signal intensity of immunolocalization of low-methyl-ester pectin (JIM5) shown in (b) with ImageJ software. Ten slides each were measured. (d) Relative signal intensity of immunolocalization of low-methyl-ester pectin (JIM5) after base de-esterification shown in (b) (ImageJ software). Ten slides each were measured; asterisk indicates significant difference at P ≤ 0.05 compared with the wild type by Student's t-test. (e) Relative signal intensity of sections shown in (a) (ImageJ software). Ten slides each were measured; asterisk indicates significant difference at P ≤ 0.05 compared with ZH10 by Student's t-test. (f) Uronic acid content determined by colorimetry in wild-type and Ubi::OsBURP16 rice. The y-axis presents values for galacturonic acid equivalents (GaE) per 1 g fresh weight. Data represent means ± SD of three biological replicates. The asterisk indicates significant difference at P ≤ 0.05 compared with ZH10 by Student's t-test. (g) PG activity in leaves of ZH10 and OsBURP16-overexpressing rice (OE3 and OE4). PG activity was measured as millimoles of reducing groups per 100 mg of cell wall material (mmol·100 mg−1 cwm). Each column represents means ± SD (n = 3) of the independent measurement. Asterisks indicate significant difference at P ≤ 0.05 compared with ZH10 by Student's t-test.
Studies have found that the properties of the cell wall and the action of pectin methylesterases on matrix properties may be important factors for cell adhesion in plants (Knox et al. 1990; Knox 1992; Willats et al. 2001). The degree of pectin methylation was reported to be an important factor affecting the properties of the cell wall (Yang et al. 2010). Additionally, pectin content and the degree of pectin methylation were reported to be related to aluminium stress in rice and maize (Eticha et al. 2005; Yang et al. 2008). PEG treatment not only reduces the total cell wall pectin content, but can also decrease the degree of methylation of pectin in root tips (Yang et al. 2010). We measured low-methyl-ester pectin (JIM5 epitope) and high-methyl-ester pectin (JIM7 epitope) in roots of Ubi::OsBURP16 and wild type by immunolocalization. The results showed that high-methyl-ester pectin content was decreased in Ubi::OsBURP16 plants, while low-methyl-ester pectin content did not change (Fig. 3b,c). We also did the immunolocalization of low-methyl-ester pectin after base de-esterification to visualize the total HG content in root hand sections of OsBURP16-overexpressing transgenic seedlings (OE4) and wild-type ZH10 plants (Fig. 3b,d); the results showed that the total HG content was significantly reduced, which is consistent with the lower GalUA content in the OE4 plants (Fig. 3f), and all these results might partly be taken as an indication of lower pectin content.
PGs are essential for the degradation of pectin, catalysing random hydrolysis of α-1,4-glycosidic linkages in polygalacturonic acid (Swain et al. 2011). PG activity in young leaves was measured. In young leaves of the OE3 and OE4 lines, PG activity was increased by more than three- and sixfold, respectively, as compared with PG activity from ZH10 (Fig. 3g). This result indicates that overexpression of OsBURP16 can promote the activity of PG and the degradation of pectin in the cell wall.
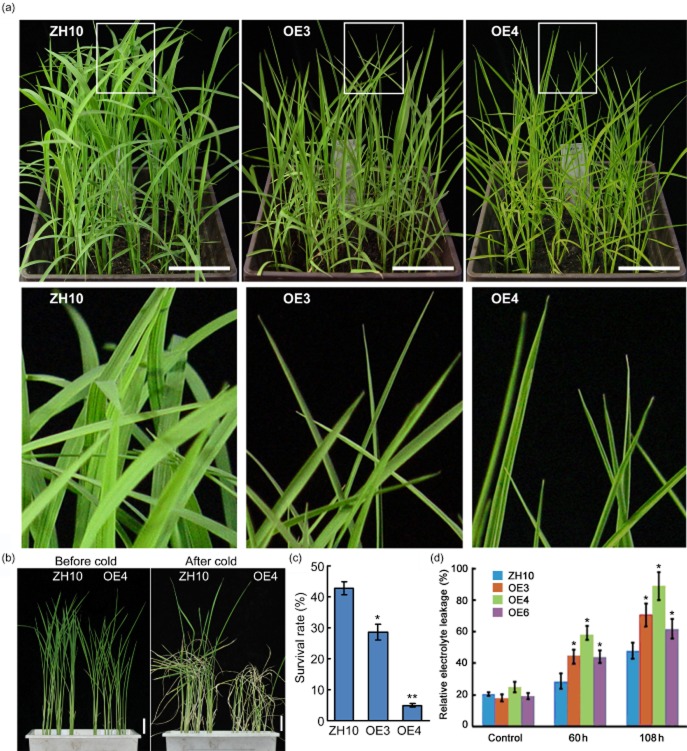
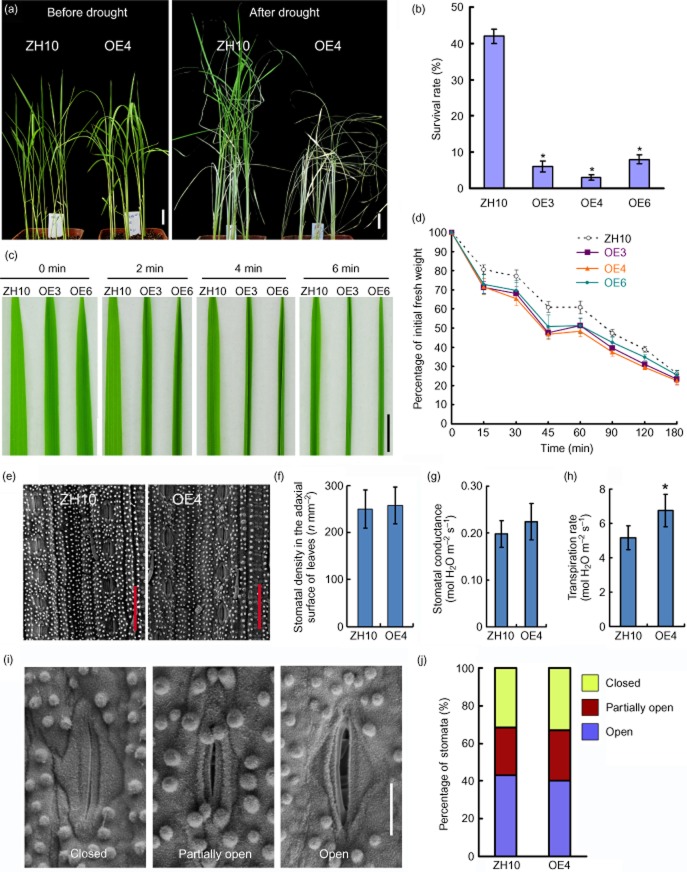
The cold tolerance of Ubi::OsBURP16 plants was tested in the same manner as the analysis of OsBURP16 expression under cold stress. OsBURP16 transgenic lines of T3 progeny and wild-type seedlings at the trefoil stage were exposed to temperatures of 4 °C. After treatment for 8 h, leaves of line OE3 and line OE4 started rolling, while the ZH10 leaves remained normal (Fig. 4a). When the seedlings were exposed to 4 °C for 72 h and allowed to recover for 7 d in normal conditions, the survival rate of the ZH10 plants was 42%, whereas the survival rates of OE3 and OE4 were only 28% and 4% (Fig. 4b,c), respectively.
Figure 4.
Performance of Ubi::OsBURP16 transgenic rice under cold stress. (a) Seedlings of OsBURP16-overexpressing lines (OE) and wild-type (ZH10) at trefoil stage were treated at 4 °C for 8 h. The leaves of lines OE3 and OE4 started rolling, while the leaves of ZH10 remained normal. The boxed parts in the top panel are enlarged as a detail (lower panel). Scale bar = 5 cm. (b) Two-week-old seedlings were treated at 4 °C for 72 h, and then allowed to recover for 7 d. Scale bars = 5 cm. (c) Survival rates of the cold-treated plants after 7 d of recovery. Data represent means ± SD (n = 16 for each replicate) of three independent experiments. One asterisk and two asterisks indicate significant differences at P ≤ 0.05 and P ≤ 0.01 compared with ZH10 by Student's t-test, respectively. (d) Relative ion leakage in rice leaves after 4 °C treatment for 60 h and 108 h. Data represent means ± SD of three biological replicates. One asterisk indicates significant difference at P ≤ 0.05 compared with corresponding ZH10 by Student's t-test.
To evaluate the effects of cold stress on cell membranes, 4-week-old seedlings were treated at 4 °C for 60 or 108 h, and the relative ion leakage was measured. Ubi::OsBURP16 lines showed higher relative electrolyte leakage than ZH10 at both 60 and 108 h. With no cold treatment, the relative ion leakage rates of OE3, OE4, OE6 and ZH10 were 18%, 25%, 19% and 21%, respectively. Following treatment at 4 °C for 60 h, the relative ion leakage rates of OE3, OE4, OE6 and ZH10 were 45%, 58%, 44% and 29%, respectively (Fig. 4d). These results indicate that the cell membrane stability is reduced in Ubi::OsBURP16 transgenic lines compared with the wild type.
Sensitivity of OsBURP16 transgenic rice seedlings to salt stress
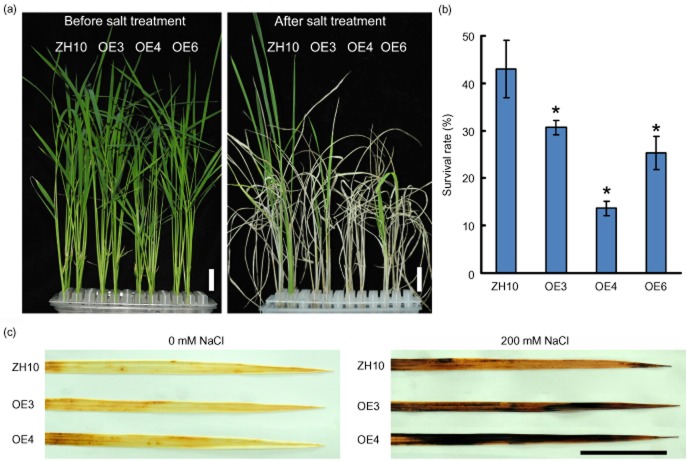
To measure the response of Ubi::OsBURP16 transgenic rice seedlings to salt stress, 2-week-old transgenic and wild-type seedlings were treated with 200 mm NaCl for 4 d. The survival rates were calculated following 7 d of recovery in normal growth conditions. As shown in Fig. 5b, approximately 44% of wild-type plants survived, while the survival rates of Ubi::OsBURP16 lines OE3, OE4 and OE6 were 31%, 14% and 26%, respectively (Fig. 5a,b).
Figure 5.
Performance of Ubi::OsBURP16 transgenic rice under salt stress. (a) Plants grown for 2 weeks were treated with 200 mm NaCl for 4 d and then allowed to recover for 7 d. Scale bars = 2 cm. (b) Survival rates of the salt-treated plants after 7 d of recovery. Each column represents mean ± SD (n = 16 for each replicate) of three independent experiments. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student's t-test. (c) 3,3′-Diaminobenzidine (DAB) staining of rice leaves from plants with 0 mm or 200 mm NaCl treatment for 2 d. The brown regions on the leaves are areas with high rates of DAB reaction with H2O2. Scale bar = 2 cm.
Abiotic stress usually causes the generation of reactive oxygen species, such as H2O2, which is an important molecule in stress signaling in plants (Zhu 2001). The stress tolerance of Ubi::OsBURP16 transgenic seedlings can be further assessed through the detection of H2O2. Rice seedlings were treated with 200 mm NaCl for 2 d and were then subjected to DAB staining to detect H2O2 content in leaves. The untreated leaves of OE3, OE4 and ZH10 showed similar staining patterns. When treated with 200 mm NaCl for 2 d, the leaves of OE3 and OE4 exhibited more dark brown areas than those of the wild type ZH10 (Fig. 5c). These results suggest that OsBURP16 overexpression resulted in the accumulation of more reactive oxygen species in young leaves, and that the transgenic seedlings were more sensitive to salt stress than the wild type.
Decreased drought tolerance due to overexpression of OsBURP16 with increased cuticular transpiration
A series of experiments were performed to determine the effect of overexpression of OsBURP16 on plant tolerance to drought stress. Two-week-old plants were deprived of water for 20 d and allowed to recover at normal water supply levels for 2 weeks (Fig. 6a). Following the recovery period, the survival rate of the wild-type plants was 42%, while those of the three Ubi::OsBURP16 lines were all less than 10% (Fig. 6b).
Figure 6.
Performance of Ubi::OsBURP16 transgenic rice under drought stress.(a) ZH10 and OsBURP16 transgenic line 4 (OE4) plants grown for 2 weeks were deprived of water for 20 d, and then allowed to recover for 2 weeks. Scale bar = 2 cm. (b) Survival rates of the drought-treated plants after 2 weeks of recovery. Each column is mean ± SD (n = 16 for each replicate) of three independent experiments. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student's t-test. (c) Detached leaves of three-leaf-stage seedlings of ZH10, OE3 and OE6 were photographed at 0, 2, 4 and 6 minutes after detachment. Scale bar = 1 cm. (d) Dehydration of 4-week-old leaves of ZH10, OE3, OE4 and OE6. At room temperature (25 ± 2 °C), leaves were weighed at the given times from 15 min until 180 min after leaves were detached. Data represent means ± SD of three biological replicates. (e) The stomatal densities of ZH10 and OE4 leaves were observed by scanning electron microscopy. Bars = 50 μm. (f) Numbers of stomata of ZH10 and OE4 leaves. Data are given as means ± SD (n = 10). Stomatal conductance (g) and transpiration rate (h) of ZH10 and OE4 leaves were measured. Data represent means ± SD of three biological replicates. Asterisk indicates significant difference at P ≤ 0.05 compared with the wild type by Student's t-test. (i) Environmental scanning electron microscopy images of three levels of stomatal opening. Bar = 10 μm. (j) The percentage of stomata at each of three degrees of openness in ZH10 and OE4 plants (n = 82 stomata for ZH10; n = 84 stomata for OsBURP16 OE4 plants).
To observe the water loss rate in vitro, leaves were exposed to the air at room temperature and photographed at different times. It was found that the leaves of the overexpression lines began to wither earlier than those of the wild type. For example, leaves of Ubi::OsBURP16 lines curled at 2 min, while the wild-type leaf curled at 6 min (Fig. 6c). Water loss is consistent with this phenotype: when the detached leaves of transgenic plants were air-dried for 45 min, the fresh weights of OE3, OE4 and OE6 leaves were less than 50% of their initial weights. Contrastingly, the fresh weight of ZH10 leaves was more than 60% of initial weight (Fig. 6d). The stomatal aperture controls CO2 uptake and water loss to the atmosphere, thus playing an important role in abiotic stress response. The loss-of-function mutant of OsSRO1c showed increased stomatal aperture and sensitivity to drought and faster water loss compared with the wild-type plant, whereas OsSRO1c overexpression led to decreased stomatal aperture and reduced water loss, indicating the direct correlation between the stomatal aperture and drought tolerance (You et al. 2013). Then the stomatal density and apertures of Ubi::OsBURP16 and ZH10 leaves were observed by scanning electron microscopy (Fig. 6e,i). No obvious differences in stoma numbers or stomatal apertures were observed between Ubi::OsBURP16 and ZH10 (Fig. 6f,j). We further investigated the stomatal conductance and the transpiration rate of ZH10 and OE4. The cuticle covers the leaf surface and acts as an effective barrier against water loss (Burghardt & Riederer 2003). Both cuticular transpiration and stomatal transpiration affect water loss (Karbulkova et al. 2008). The OE4 seedlings showed a higher transpiration rate than the wild-type seedlings (Fig. 6h), which indicates higher cuticular transpiration rates, as there was no obvious difference in the stomatal conductance and stomatal apertures (Fig. 6g,i,j). The results from the survival rate, leaf curling, water loss rate, stomatal density and transpiration rate analyses all showed that Ubi::OsBURP16 plants have less ability to retain water compared with ZH10.
As drought stress can induce the expression of OsBURP16, we measured the pectin content as well as the PG activity after 12 h treatment with 20% PEG 6000. The results showed that PG activity was clearly increased while pectin content was clearly decreased (Supporting Information Fig. S2a,b). This result might partly indicate that higher levels of OsBURP16 can promote the activity of PG and the degradation of pectin in the cell wall.
All of the above results from experiments with the Ubi::OsBURP16 lines – cold sensitivity, higher electrolyte leakage rates, salt sensitivity, increased accumulation of reactive oxygen species, drought sensitivity, enhanced water loss and transpiration rate – indicate that OsBURP16 overexpression transgenic seedlings are more sensitive to abiotic stress than wild-type seedlings.
Reduced cell adhesion strength in OsBURP16 overexpression plants
As OsBURP16 is mainly expressed in young leaves (Fig. 1c), we observed the cell–cell adhesion in the epidermis of 2-week-old transgenic rice leaves using differential interference contrast microscopy. In contrast to the wild type, the intercellular spaces of leaves in the transgenic plants were larger and appeared as transparent structures, while there was no obvious difference in their roots (Fig. 7a and Supporting Information Fig. S3). Since the spaces between the cells were larger in Ubi::OsBURP16 transgenic plants than in the wild type, we measured the mechanical force required to break the leaves. The assay results showed that less force was required to break the leaves of Ubi::OsBURP16 plants than those of the wild type. Additionally, the extension length of Ubi::OsBURP16 leaves was shorter than that of the wild type (Fig. 7b). These results may indicate that the cell adhesion strength is reduced in Ubi::OsBURP16 transgenic plants.
Figure 7.
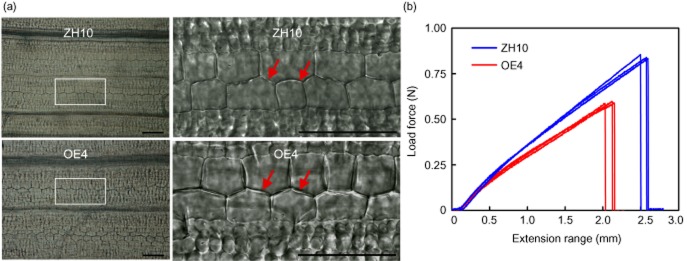
Phenotypic characterization of the third leaves in Ubi::OsBURP16 transgenic rice. (a) Cellular observation of the third leaves of 2-week-old ZH10 and OsBURP16-overexpressing plants (OE4). The regions outlined in white are shown as details to the right. Scale bar = 50 μm. (b) Mechanical strength measurement of the third leaves of OsBURP16 OE4 and ZH10. Measurements of the breaking force and extension length were performed in triplicate.
Changes in stress-related genes in OsBURP16 overexpression plants
It is well known that accumulation of osmoregulatory substances promotes tolerance to abiotic stresses. Trehalose, an osmoregulatory substance in plants, is produced from glucose by trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP). Overexpression of a heterologous TPS gene from microorganisms (Jang et al. 2003) or OsTPP1 triggers an abiotic stress pathway and confers salt and cold stress tolerance in rice (Pramanik & Imai 2005; Ge et al. 2008). In fact, we measured the transcript levels of OsTPS1 and OsTPP1, and both of them decreased significantly in OsBURP16 overexpression seedlings (Supporting Information Fig. S2c).
Discussion
Encoding of a β subunit of PG by OsBURP16 in rice
A previous study showed that the transcription levels of OsBURP16 respond to ABA and abiotic stresses including drought, salt and cold (Ding et al. 2009), but the study did not elucidate the functions of this gene. In this study, we report that Ubi::OsBURP16 plants were more sensitive than wild type to abiotic stresses including drought, salt and cold (Figs 6). Although overexpression of some stress-induced genes can enhance stress tolerance, overexpression of others can have an opposing effect. For example, the rice MAPK phosphatase gene IBR5 is induced by PEG, ABA and H2O2, but its overexpression decreases drought stress tolerance (Li et al. 2012). OsMAPK33 is a drought-stress-inducible gene, and its overexpression enhances sensitivity to salt stress through unfavourable ion homeostasis (Lee et al. 2011). MAIF1, a rice F-box domain gene, is induced rapidly and strongly by ABA and abiotic stresses. Overexpression of MAIF1 reduces rice ABA sensitivity and abiotic stress tolerance (Yan et al. 2011). These genes, together with OsBURP16, are all induced by abiotic stresses and yet play negative roles in abiotic stress response; further investigations of the products of these genes or their downstream genes are still needed to expound their mechanisms in detail.
OsBURP16 encodes a putative precursor of PG1β, one of the subunits that regulates PG activity (Tucker et al. 1981). Our results indicate that overexpression of OsBURP16 leads to an increase in PG activity and a decrease in pectin content. The results for both immunolocalization and ruthenium red staining showed a consistent decrease in pectin content in the leaves of transgenic plants (Fig. 3). These results suggest that OsBURP16 acts as a PG1β subunit to regulate the activity of PG, as is the case in tomato (Zheng et al. 1992) and apple (Atkinson et al. 2002).
Effects on cell adhesion, cuticular transpiration and decreased tolerance to several stresses associated with reduced pectin content due to OsBURP16 overexpression
The β subunit is a heat- and pressure-stable protein and is known to confer enhanced thermal resistance properties to PG (Peeters et al. 2004). PG hydrolyses pectin to generate oligogalacturonides, which affect the texture and rigidity of cell walls (Atkinson et al. 2002; Pelloux et al. 2007; Ogawa et al. 2009). Pectin functions as an adhesive in cell wall formation, and pectin content directly influences cell wall integrity.
To evaluate the adhesion of cell walls between two adjacent cells, we performed observations under differential interference contrast microscopy and measured the mechanical force required to break the leaf. The results indicated that the leaves of Ubi::OsBURP16 lines had larger spaces between adjacent cells and were easier to break than the wild-type leaves (Fig. 7). We can conclude that overexpression of the PG1 β-subunit gene OsBURP16 resulted in increased PG activity (Fig. 3g) and might also result in a decrease in cell wall adhesion and integrity; however, it is possible that the decrease in force required to break the leaf is not directly related to reduced cell–cell adhesion.
The cell wall is not only the first barrier against biotic stress but is also involved in abiotic stress responses. For example, the Arabidopsis mutant sos5, which appears to have defects in the middle lamella of the cell wall and the primary cell wall, is hypersensitive to salt stress (Shi et al. 2003). Overexpression of the cell wall protein proline-rich protein 3 (PRP3) enhances cell wall integrity and cold tolerance in rice (Gothandam et al. 2010). Cold acclimation induces an increased pectin content in cell walls and increased resistance to freezing (Solecka et al. 2008). Abiotic stresses induce expression of PGIP, which encodes a PG-inhibiting protein to prevent the degradation of pectin by PG (Ahsan et al. 2005). The Arabidopsis AtMYB41 gene encodes an R2R3-MYB transcription factor that regulates the modification of cell wall and cuticle integrity. In AtMYB41-overexpression lines, more rapid water loss compared with wild type results from discontinuity in the cuticle (Cominelli et al. 2008). In 35S::AtMYB41 lines, ADPG1 and ADPG2 are up-regulated 12- and 10-fold, respectively (see supporting information in Cominelli et al. 2008). Both ADPG1 and ADPG2 encode PG in Arabidopsis (Ogawa et al. 2009). Mutation of QUASIMODO1, a gene that encodes a glycosyltransferase required for pectin synthesis, results in reduced pectin content, loss of cell adhesion and faster water loss than in wild type (Bouton et al. 2002). All of these data suggest that cell wall integrity and pectin content are associated with tolerance to abiotic stresses. These reports support our conclusions that overexpression of OsBURP16 promotes PG activity (Fig. 3g), hydrolysing pectin (Fig. 3f) and affecting the integrity of the cell wall (Fig. 7), which may lead to more sensitivity to abiotic stresses than in wild-type plants.
Young rice seedlings are particularly sensitive to low temperature stress, which causes retardation of seedling development, yellowing, withering, reduced tillering and stunted growth (Chinnusamy et al. 2007). Tracing the phenomenon of leaves' becoming yellow and withering is helpful for estimating the stress tolerance of rice seedlings (Su et al. 2010). Leaves of Ubi::OsBURP16 transgenic lines rolled immediately compared with wild type under cold or drought conditions, and showed more rapid water loss than that of wild type under drought stress (Fig. 4). Both cuticular transpiration and stomatal transpiration affect water loss (Karbulkova et al. 2008). The OE4 seedlings showed higher transpiration rate than wild-type seedlings (Fig. 6h), which indicates higher cuticular transpiration rate, as there was no obvious difference in the stomatal conductance and stomatal apertures (Fig. 6g,i,j). When the seedlings were treated with NaCl, Ubi::OsBURP16 plants accumulated more reactive oxygen species than did wild-type plants (Fig. 5). The transgenic lines showed lower survival rates than ZH10 under all three abiotic stresses (Figs 6). The above studies suggest that the lower survival rate, rapid leaf curling and high water loss rate of Ubi::OsBURP16 plants may be the result of the high cuticular transpiration rate caused by the reduced cell adhesion.
In conclusion, we found that overexpression of the precursor of PG1 β-subunit resulted in an increase of PG activity, which in turn increased the degradation of pectin in the cell wall, thereby reducing cell adhesion. The Ubi::OsBURP16 plants, with looser, less cohesive cells, were more sensitive to abiotic stress than wild-type plants. Overexpression of OsBURP16 then decreases pectin contents and cell adhesion and increases abiotic stress sensitivity in rice. Based on the present results, OsBURP16 may play a role in regulating abiotic stresses in rice, a hypothesis that could be tested in future studies using a loss of function approach.
Acknowledgments
This work was supported by the Research Program from the Chinese Ministry of Agriculture (2014ZX08009-003-002), an Innovation Grant from the Chinese Academy of Sciences (CAS) (KSCX2-EW-J-1) and the National Natural Science Foundation of China (31070695). We thank Prof. Taihua Zhang (Institute of Mechanics, CAS) for help with breaking force measurements and Rongxi Jiang (Institute of Botany, CAS) for the rice transformation. We would like to thank Dr J. H. Snyder for examining the English usage in the manuscript as well as Jingquan Li (Key Lab of Plant Molecular Physiology, Institute of Botany, CAS) for help in using the confocal microscope and Yanbao Tian (Institute of Genetics and Developmental Biology, CAS) for help in using the scanning electron microscope. There are no conflicts of interest to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site
Expression pattern analysis of OsBURP16 and ACTIN1. (a) The tissue expression pattern of OsBURP16 was predicted by the Rice eFP website (http://bar.utoronto.ca/efprice/cgi-bin/efpWeb.cgi). The color scale illustrates the microarray signal level. (b) The expression patterns of OsACTIN1 under different abiotic stresses were predicted by the Rice eFP website (http://bar.utoronto.ca/efprice/cgi-bin/efpWeb.cgi) in 7-day-old seedlings. The color scale illustrates the microarray signal level. (c) Expression profiles of OsBURP16 under cold, drought and salt stresses and ABA treatment in 2-week-old wild-type plants. OsCIPK06 was used as the internal control. The x-axis represents the time courses of the stress treatments. Data represent means ± SD of three biological replicates.
Pectin content and PG activity in leaves of ZH10 before and after drought stress as well as relative expression levels of stress-related genes in ZH10 and Ubi::OsBURP16 transgenic line 4 (OE4) seedlings. (a) Uronic acid content, determined by colorimetry in leaves of ZH10 before and after drought stress. The y-axis presents values for galacturonic acid equivalents (GaE) per 1 g fresh weight. Data represent means ± SD of three biological replicates. The asterisk indicates significant difference at P ≤ 0.05 compared with ZH10 before drought stress by Student's t-test. (b) PG activity in leaves of wild type (ZH10) before and after drought stress. PG activity was measured as millimoles of reducing groups per 100 mg of cell wall material (mmol·100 mg−1 cwm). Each column represents means ± SD of three biological replicates. Asterisks indicate significant difference at P ≤ 0.05 compared with ZH10 before drought stress by Student's t test. (c). Relative expression levels of stress-related genes in ZH10 and Ubi::OsBURP16 transgenic line 4 (OE4) seedlings. OsTPP1, trehalose-6-phosphate phosphatase gene 1 (LOC_Os02g44230); OsTPS, trehalose-6-phosphate synthase gene 1 (LOC_Os08g34580). OsCIPK06 was used as the internal control. Data represent means ± SD of three biological replicates. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student's t-test.
Cellular observation of leaves or roots of 2-week-old ZH10 and OsBURP16-overexpressing plants (OE4) by staining with propidium iodide (PI). (a) Leaf sheath cells of ZH10 and OsBURP16-overexpressing plants (OE4). Scale bars = 50 μm. (b) Root cells of ZH10 and OsBURP16-overexpressing plants (OE4). Scale bars = 50 μm.
References
- Ahmed AER. Labavitch JM. A simplified method for accurate determination of cell wall uronide content. Journal of Food Biochemistry. 1977;1:361–365. [Google Scholar]
- Ahsan N, Yoon HS. Jo J. Molecular cloning of a BcPGIP cDNA from Brassica campestris and its expression to several stresses. Plant Science. 2005;169:1081–1089. [Google Scholar]
- Atkinson RG, Schroder R, Hallett IC, Cohen D. MacRae EA. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiology. 2002;129:122–133. doi: 10.1104/pp.010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F. Truong HN. QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. The Plant Cell. 2002;14:2577–2590. doi: 10.1105/tpc.004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA. Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology. 2001;47:311–340. [PubMed] [Google Scholar]
- Burghardt M. Riederer M. Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. Journal of Experimental Botany. 2003;54:1941–1949. doi: 10.1093/jxb/erg195. [DOI] [PubMed] [Google Scholar]
- Chen N, Xu YY, Wang X, Du C, Du JZ, Yuan M. Chong K. OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant, Cell & Environment. 2011;34:52–64. doi: 10.1111/j.1365-3040.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- Chen ZZ, Zhang HR, Jablonowski D, Zhou XF, Ren XZ, Hong XH. Gong ZH. Mutations in ABO1/ELO2, a subunit of holo-elongator, increase abscisic acid sensitivity and drought tolerance in Arabidopsis thaliana. Molecular Cell Biology. 2006;26:6902–6912. doi: 10.1128/MCB.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J. Zhu JK. Cold stress regulation of gene expression in plants. Trends in Plant Science. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Cominelli E, Sala T, Calvi D, Gusmaroli G. Tonelli C. Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. The Plant Journal. 2008;53:53–64. doi: 10.1111/j.1365-313X.2007.03310.x. [DOI] [PubMed] [Google Scholar]
- Ding XP, Hou X, Xie KB. Xiong LZ. Genome-wide identification of BURP domain-containing genes in rice reveals a gene family with diverse structures and responses to abiotic stresses. Planta. 2009;230:149–163. doi: 10.1007/s00425-009-0929-z. [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A. Horst WJ. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell & Environment. 2005;28:1410–1420. [Google Scholar]
- Ge L, Chen H, Jiang JF, Zhao Y, Xu ML, Xu YY. Chong K. Overexpression of OsRAA1 causes pleiotropic phenotypes in transgenic rice plants, including altered leaf, flower, and root development and root response to gravity. Plant Physiology. 2004;135:1502–1513. doi: 10.1104/pp.104.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge LF, Chao DY, Shi M, Zhu MZ, Gao JP. Lin HX. Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta. 2008;228:191–201. doi: 10.1007/s00425-008-0729-x. [DOI] [PubMed] [Google Scholar]
- Gothandam KM, Nalini E, Karthikeyan S. Shin JS. OsPRP3, a flower specific proline-rich protein of rice, determines extracellular matrix structure of floral organs and its overexpression confers cold-tolerance. Plant Molecular Biology. 2010;72:125–135. doi: 10.1007/s11103-009-9557-z. [DOI] [PubMed] [Google Scholar]
- Hattori J, Boutilier KA, Campagne MMV. Miki BL. A conserved BURP domain defines a novel group of plant proteins with unusual primary structures. Molecular and General Genetics. 1998;259:424–428. doi: 10.1007/s004380050832. [DOI] [PubMed] [Google Scholar]
- Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH. Kim JK. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiology. 2003;131:516–524. doi: 10.1104/pp.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbulkova J, Schreiber L, Macek P. Santrucek J. Differences between water permeability of astomatous and stomatous cuticular membranes: effects of air humidity in two species of contrasting drought-resistance strategy. Journal of Experimental Botany. 2008;59:3987–3995. doi: 10.1093/jxb/ern238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP. Cell adhesion, cell separation and plant morphogenesis. The Plant Journal. 1992;2:137–141. [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C. Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim BG, Kwon TR, Jeong MJ, Park SR, Lee JW. Park SC. Overexpression of the mitogen-activated protein kinase gene OsMAPK33 enhances sensitivity to salt stress in rice (Oryza sativa L.) Journal of Biosciences. 2011;36:139–151. doi: 10.1007/s12038-011-9002-8. [DOI] [PubMed] [Google Scholar]
- Li YG, Feng DR, Zhang DL, Su JB, Zhang Y, Li ZQ. Wang JF. Rice MAPK phosphatase IBR5 negatively regulates drought stress tolerance in transgenic Nicotiana tabacum. Plant Science. 2012;188:10–18. doi: 10.1016/j.plantsci.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Ma JF. Takahashi E. Effect of silicon on the growth and phosphorus uptake of rice. Plant and Soil. 1990;126:115–119. [Google Scholar]
- Ning J, Zhang BC, Wang NL, Zhou YH. Xiong LZ. Increased leaf angle1, a Raf-like MAPKKK that interacts with a nuclear protein family, regulates mechanical tissue formation in the lamina joint of rice. The Plant Cell. 2011;23:4334–4347. doi: 10.1105/tpc.111.093419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S. Swain SM. Arabidopsis dehiscence zone polygalacturonase1 (ADPG1), ADPG2 and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. The Plant Cell. 2009;21:216–233. doi: 10.1105/tpc.108.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B. Chen SY. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. The Plant Journal. 2010;62:316–329. doi: 10.1111/j.1365-313X.2010.04146.x. [DOI] [PubMed] [Google Scholar]
- Peeters L, Fachin D, Smout C, van Loey A. Hendrickx ME. Influence of beta-subunit on thermal and high-pressure process stability of tomato polygalacturonase. Biotechnology and Bioengineering. 2004;86:543–549. doi: 10.1002/bit.20134. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C. Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Pramanik MHR. Imai R. Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Molecular Biology. 2005;58:751–762. doi: 10.1007/s11103-005-7404-4. [DOI] [PubMed] [Google Scholar]
- Shi HZ, Kim Y, Guo Y, Stevenson B. Zhu JK. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. The Plant Cell. 2003;15:19–32. doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecka D, Zebrowski J. Kacperska A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Annals of Botany. 2008;101:521–530. doi: 10.1093/aob/mcm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SY, Chen Y, Chen J, Dai XY. Zhang WH. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta. 2011;234:331–345. doi: 10.1007/s00425-011-1403-2. [DOI] [PubMed] [Google Scholar]
- Su CF, Wang YC, Hsieh TH, Lu CA, Tseng TH. Yu SM. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiology. 2010;153:145–158. doi: 10.1104/pp.110.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM. Kay P, Ogawa M. Preventing unwanted breakups using polygalacturonases to regulate cell separation. Plant Signaling & Behavior. 2011;6:93–97. doi: 10.4161/psb.6.1.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker GA, Robertson NG. Grierson D. The conversion of tomato-fruit polygalacturonase isoenzyme 2 into isoenzyme 1 in vitro. European Journal of Biochemistry. 1981;115:87–90. doi: 10.1111/j.1432-1033.1981.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Watson CF, Zheng L. DellaPenna D. Reduction of tomato polygalacturonase beta subunit expression affects pectin solubilization and degradation during fruit ripening. The Plant Cell. 1994;6:1623–1634. doi: 10.1105/tpc.6.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, Orfila C, Limberg G, Buchholt HC, van Alebeek GJ, Voragen AG. Knox JP. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. Journal of Biological Chemistry. 2001;276:19404–19413. doi: 10.1074/jbc.M011242200. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Huang Y. Xiong L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiology. 2007;144:1416–1428. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YS, Chen XY, Yang K, Sun ZX, Fu YP, Zhang YM. Fang RX. Overexpression of an F-box protein gene reduces abiotic stress tolerance and promotes root growth in rice. Molecular Plant. 2011;4:190–197. doi: 10.1093/mp/ssq066. [DOI] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P. Zheng SJ. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Eticha D, Rao IM. Horst WJ. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.) Journal of Experimental Botany. 2010;61:3245–3258. doi: 10.1093/jxb/erq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong WD, Xu YY, Xu WZ, Wang X, Li N, Wu JS. Zhu ZQ. Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2. Planta. 2003;217:261–270. doi: 10.1007/s00425-003-0994-7. [DOI] [PubMed] [Google Scholar]
- You J, Zong W, Li XK, Ning J, Hu HH, Li XH. Xiong LZ. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. Journal of Experimental Botany. 2013;64:569–583. doi: 10.1093/jxb/ers349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Heupel RC. DellaPenna D. The beta subunit of tomato fruit polygalacturonase isoenzyme 1: isolation, characterization, and identification of unique structural features. The Plant Cell. 1992;4:1147–1156. doi: 10.1105/tpc.4.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Watson CF. DellaPenna D. Differential expression of the two subunits of tomato polygalacturonase isoenzyme 1 in wild-type and rin tomato fruit. Plant Physiology. 1994;105:1189–1195. doi: 10.1104/pp.105.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression pattern analysis of OsBURP16 and ACTIN1. (a) The tissue expression pattern of OsBURP16 was predicted by the Rice eFP website (http://bar.utoronto.ca/efprice/cgi-bin/efpWeb.cgi). The color scale illustrates the microarray signal level. (b) The expression patterns of OsACTIN1 under different abiotic stresses were predicted by the Rice eFP website (http://bar.utoronto.ca/efprice/cgi-bin/efpWeb.cgi) in 7-day-old seedlings. The color scale illustrates the microarray signal level. (c) Expression profiles of OsBURP16 under cold, drought and salt stresses and ABA treatment in 2-week-old wild-type plants. OsCIPK06 was used as the internal control. The x-axis represents the time courses of the stress treatments. Data represent means ± SD of three biological replicates.
Pectin content and PG activity in leaves of ZH10 before and after drought stress as well as relative expression levels of stress-related genes in ZH10 and Ubi::OsBURP16 transgenic line 4 (OE4) seedlings. (a) Uronic acid content, determined by colorimetry in leaves of ZH10 before and after drought stress. The y-axis presents values for galacturonic acid equivalents (GaE) per 1 g fresh weight. Data represent means ± SD of three biological replicates. The asterisk indicates significant difference at P ≤ 0.05 compared with ZH10 before drought stress by Student's t-test. (b) PG activity in leaves of wild type (ZH10) before and after drought stress. PG activity was measured as millimoles of reducing groups per 100 mg of cell wall material (mmol·100 mg−1 cwm). Each column represents means ± SD of three biological replicates. Asterisks indicate significant difference at P ≤ 0.05 compared with ZH10 before drought stress by Student's t test. (c). Relative expression levels of stress-related genes in ZH10 and Ubi::OsBURP16 transgenic line 4 (OE4) seedlings. OsTPP1, trehalose-6-phosphate phosphatase gene 1 (LOC_Os02g44230); OsTPS, trehalose-6-phosphate synthase gene 1 (LOC_Os08g34580). OsCIPK06 was used as the internal control. Data represent means ± SD of three biological replicates. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student's t-test.
Cellular observation of leaves or roots of 2-week-old ZH10 and OsBURP16-overexpressing plants (OE4) by staining with propidium iodide (PI). (a) Leaf sheath cells of ZH10 and OsBURP16-overexpressing plants (OE4). Scale bars = 50 μm. (b) Root cells of ZH10 and OsBURP16-overexpressing plants (OE4). Scale bars = 50 μm.