Abstract
A number of studies have suggested that avian brood size is individually optimized. Yet, optimal reproductive decisions likely vary owing to among-individual differences in environmental sensitivity. Specifically, ‘proactive’ individuals who do not track environmental changes may be less able to produce optimal brood sizes than ‘reactive’ individuals who have more precise local environmental knowledge. To test this, we quantified exploratory behaviour (a proxy for proactivity) in a great tit (Parus major) population, manipulated brood sizes (reduced, control, enlarged) and evaluated whether individuals of dissimilar coping style differed in their level of optimization. If reactive females behaved optimally, any deviation from their original brood size should lower fitness, whereas this should not be the case for proactive females. Reactive females indeed performed best at their natural brood size, whereas proactive females performed best when raising an enlarged brood. These findings imply that proactive females produced sub-optimal brood sizes. We speculate that proactive females might (i) take decisions based on biased perception of their environment, (ii) face energetic constraints in offspring production and/or (iii) be more willing to invest into current reproduction when given the option. Our findings provide experimental evidence for coping style-related differences in optimal reproductive decisions and life-history strategies.
Keywords: animal personality, coping styles, phenotypic plasticity, information use, individual optimization, Parus major
1. Introduction
Life-history theory predicts that parents should produce brood sizes that maximize their overall fitness [1,2]. However, when resources are limited, parents need to trade-off the allocation of these resources into competing costly activities. For example, optimal reproductive investment requires trading-off the number versus quality of produced offspring [3,4] and investment into current versus future reproduction [5,6]. Brood size (the number of offspring produced per breeding attempt) is optimized at an individual level when parents assess the current state of their environment and adjust their breeding decisions in a way that maximizes fitness (‘individual optimization hypothesis'; [7,8]). In birds, the idea of clutch size optimization has been extensively tested by manipulating parental reproductive output (such as clutch and brood sizes) to investigate whether their reproductive decision maximized fitness (reviewed in [9]). Some of those manipulations have yielded evidence for optimization hypothesis, while other studies showed that individuals appeared to behave in a non-adaptive manner [10–15]. Such mixed results are important for the development of life-history theory and underline the need to investigate the origin of apparent non-adaptive reproductive decisions.
Life-history theory is based on the premise that individuals behave in an optimal manner [1,2]. Viewed from a reaction norm perspective, this means that individuals are expected to express adaptive phenotypic plasticity in response to environmental change, i.e. any deviation from the reaction norm favoured by selection should reduce fitness (figure 1a). However, a decade of stress physiology research suggests that individuals differ fundamentally in whether their behavioural decisions are informed by environmental input [16]. Individuals that rely primarily on internal routines are referred to as having a ‘proactive’ coping style, whereas individuals that instead modify their behaviour as a function of the environment have a ‘reactive’ coping style [17]. Thus, individuals differ in their environmental sensitivity, i.e. the extent to which they track environmental changes. This is important in the context of the optimization hypothesis because it suggests that individuals may differ in their ability to take optimal reproductive decisions. Following this logic, we expect that individuals with a more reactive coping style (who are more sensitive to environmental stimuli) will produce a clutch that is optimally tuned to the local circumstances, whereas individuals with a proactive coping style (who ignore environmental stimuli) may be more likely to take reproductive decisions that are currently locally sub-optimal.
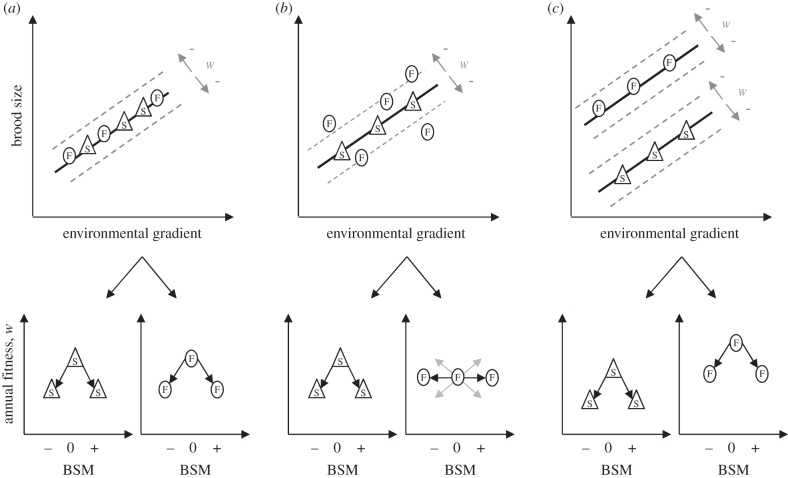
Figure 1.
Conceptual representation of three scenarios depicting how coping styles (S, slow explorers/reactive individuals; F, fast explorers/proactive individuals) differ in reproductive decisions (top panels) and depicting the predicted consequences of a brood size manipulation (BSM, reduced brood size (−), control (0) and enlarged brood size (+)) on annual fitness (w). Although exploration and proactivity are considered as continuous traits, for simplification we depict here the two extremes of the distribution (fast versus slow explorers). In (a) (individual optimization hypothesis), all individuals should closely follow the optimal brood size reaction norms (black lines) favoured by selection (top panels). As a consequence, any BSM should lower fitness (bottom panels). In (b), there are personality-related differences in environmental sensitivity. Slow explorers should produce an optimal brood size because they have precise information on the state of their local environment. By contrast, fast explorers are expected to deviate more from the optimal reaction norm because their information is less precise (top panel). Consequently, a BSM should result in reduced fitness for slow explorers (bottom left) and in either a lower or higher fitness in fast explorers, depending on whether their brood size was above or below the optimum (bottom right). In (c), all individuals behave in an optimal manner but due to general differences in life-history strategies, the intercepts of the brood size reaction norms differ between slow and fast explorers. Fast explorers should invest more heavily in current reproduction and are thus expected to have larger broods per breeding attempt, whereas reactive individuals should invest less in current reproduction and thus have smaller broods per breeding attempt. Similarly to (a), any BSM should lower fitness (bottom panels).
In this paper, we experimentally tested predictions of coping style theory by applying an ‘individual optimization’ paradigm to a population of great tits (Parus major). The experimental approach consists of comparing females whose manipulated reproductive output (brood size) is either below, above or equal to their chosen output, and asking which treatment group performs best for each coping style. Coping styles affect the expression of suites of behavioural traits such as sampling, speed of exploration, routine formation and risk-taking, some of which are readily assayed in the wild [18,19]. Using a field-based novel environment test, we quantified exploratory behaviour as a proxy for coping style. We have previously shown that in our study population this behaviour has a cross-year repeatability of 0.37 [20]. Additionally, fast-exploring individuals were both more willing to take risks and less responsive to environmental changes (characteristics of a proactive coping style), whereas slow-exploring individuals were more shy and responsive to change (characteristics of a reactive coping style) [20].
The aim of this study is to test whether females of different exploratory behaviour (a proxy for proactivity) also differ in their level of reproductive optimization. Because male great tits have little indirect phenotypic effects on female clutch size decisions [21], we focus exclusively on female exploratory behaviour. We consider three scenarios. First, if all females behaved in an optimal manner (individual optimization hypothesis), brood size manipulations (BSMs) should lower annual fitness (number and quality of offspring), regardless of female exploration score (figure 1a). Second, if females differed in their environmental sensitivity, we predict that the brood size of slow explorers should closely follow the brood size-environment reaction norm favoured by selection (adaptive phenotypic plasticity) because their behavioural decisions are informed by more precise information about the environment. Therefore, any manipulation of their brood size should decrease their annual fitness (figure 1b). By contrast, fast explorers may show greater variation around the optimal reaction norm because they take decisions without closely tracking the environment (they rely more on routines). Consequently, BSM may have no overall effect on annual fitness of fast explorers, because it may lower or increase annual fitness, depending on whether the natural brood size was above versus below the optimal brood size (figure 1b). Finally, females may optimize their reproductive decisions, but differences in life-history strategies may cause individuals with different exploratory behaviours to differ in their average brood size (variation in the intercept of their brood size reaction norm) [22]. Specifically, theory predicts that proactive individuals (i.e. fast explorers) should generally invest more in current reproduction compared with reactive individuals (i.e. slow explorers) because proactivity facilitates access to resources necessary for reproduction but simultaneously increases risk of mortality [22,23]. Consequently, fast-exploring females should produce larger broods per breeding attempt compared with slow-exploring females (figure 1c). We tested this non-exclusive mechanism by investigating whether speed of exploration was negatively correlated with pre-manipulation clutch and brood size.
2. Material and methods
(a). Study area and study species
The study was carried out in a great tit population inhabiting mixed deciduous forest patches in the Herrsching-Starnberg area of Bavaria, southern Germany (47°58′ N, 11°14′ E). The study site consists of 12 nest-box plots with 50 boxes each, established in autumn 2009.
(b). Data collection
We checked nest-boxes weekly from the beginning of April onwards, and recorded lay date (back-calculated assuming that one egg was laid per day) and clutch size. Shortly before the expected hatch date, we checked nest-boxes daily to determine hatch date (day 0). At day 2, we weighed the complete brood (±0.1 g). At day 3, we swapped nestlings between nests according to the experimental protocol detailed below. We marked nails of swapped nestlings either with nail polish or by clipping them for later identification. At day 6, we weighed nestlings, bled them and ringed them with an aluminium ring. At day 7, we caught both parents with a spring trap in the nest-box, tested them for exploratory behaviour (detailed below), weighed, bled, measured and ringed them. If we failed to catch females on day 7 (N = 62 females, 15.12%), another catching attempt was made on day 9. Catching probability was independent from the females' exploration score (General Linear Mixed Model (GLMM) with standardized exploration score as fixed effect and plot as random effect; female exploration β (95% credible interval (CI) = 0.12 (−0.44, 0.49)). At day 14, we took standard body measurements (body mass, tarsus, wing length) of the nestlings. From day 19 onwards, we checked boxes every second day to determine the fledge date. Nests were emptied and checked for deceased chicks. Further weekly checks gave information about the incidence of second or replacement clutches and the success of these clutches.
(c). Experimental set-up
We performed BSMs in 2 years (2010 and 2011) using first broods only (defined as clutches initiated within 30 days of the earliest clutch in that year [24]). We forced individuals to raise either a reduced brood (−3 nestlings), a control brood (manipulated but no net change in brood size) or an enlarged brood (+3 nestlings). A change of ±3 nestlings is sufficient to significantly alter reproductive investment while staying within the natural range of brood sizes [25]. Nestlings of similar age (day 3) and similar mean mass were transferred between nests (mean nestling mass difference ± s.d. = 0.41 ± 0.41 g, n = 106 duos). We exchanged nestlings such that in all experimental nests, including controls, half of the nestlings were from another brood. We assigned brood size treatments randomly, independently of the nest's original brood size (GLMM with BSM category as fixed effect and plot as random effect; BSM: 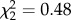 , p = 0.79, n = 210). In total, we obtained complete information (female's identity and exploration score) for 72 reduced nests, 66 control nests and 72 enlarged nests.
, p = 0.79, n = 210). In total, we obtained complete information (female's identity and exploration score) for 72 reduced nests, 66 control nests and 72 enlarged nests.
(d). Exploratory behaviour
We measured exploratory behaviour for all breeding birds when their nestlings were 7 or 9 days old (see above) using a cage test adapted from the classic ‘novel environment’ test [19,26]. A full description of the procedure can be found in Stuber et al. [20]. Briefly, directly following capture, the focal bird was placed into a darkened compartment connected to an ‘exploration cage’ (a 61 L × 39 W × 40 H (cm) solid white plastic box fitted with three perches and a mesh front part) and left to acclimatize for 1 min. The bird was then released into the cage, and its behaviour filmed for 2 min using a video camera placed 2 m in front of the cage. Following the test, standard morphometric measurements and blood samples were taken (detailed above) and the bird was released in the vicinity of its nest-box. Exploratory behaviour was then scored from the videos as the total number of flights between locations and hops within a location [26]. Inter-observer correlations were on average greater than 0.85 (10 random videos, scored by eight observers [20]).
The BSM was conducted 4 days prior to the parental exploration test (see above). Female exploratory behaviour did not differ between BSM categories, suggesting that their behaviour was not affected by the manipulation (GLMM with BSM fitted as fixed effect factor and plot as random effect: 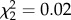 , p = 0.99). Repeated measures of exploration (e.g. assayed during second breeding attempts) were not included in the analyses.
, p = 0.99). Repeated measures of exploration (e.g. assayed during second breeding attempts) were not included in the analyses.
Exploratory behaviour in great tits is commonly assayed during winter [27]; unfortunately, only a subset of our breeding birds (31% of all birds breeding in 2010 and 2011) could be captured while roosting in a nest-box during winter, which increases the likelihood of personality-related sampling bias [28]. Therefore, to avoid such bias, we assayed exploratory behaviour during the breeding season; this test enabled behavioural quantification of all breeding birds. We have previously shown that spring and winter exploratory behaviours were not correlated, which suggests that they may measure two different underlying traits or different aspects of exploratory behaviour (as discussed in Stuber et al. [20]). However, the lack of correlation does not influence the interpretation of our results because both traits predict environmental sensitivity in the same direction, i.e. they are both proxies of coping style.
(e). Analyses of optimization of reproduction
We used a two-step approach to address whether brood size was optimal for the average female great tit in our population, and whether individuals differed in their level of optimization.
First, we provide a detailed general description of how the BSM affected fitness gains: the probability to succeed (to produce at least one fledgling) and, for nests that did succeed, the number of fledglings (measured as the brood size at day 14) and fledgling mass (average nestling mass at day 14). Variation in the number of recruits per nest was too low to be meaningfully analysed (mean number of recruits per nest ± s.d. = 0.07 ± 0.32 in 2010 and 0.29 ± 0.57 in 2011), probably due to the small size of our study areas causing the majority of fledglings to settle outside of them. We also quantified effects of the BSM related to the costs of reproduction: the probability of producing a second clutch (given that the first brood was successful) and the probability of surviving locally (i.e. breeding in the following year). Analyses of these different components of fitness altogether allowed us to infer whether females behaved in an optimal manner.
Second, we used phenotypic selection analyses [29,30] to test whether female optimization depended on coping style. Based on the results of the first step (see §3), we used two measures of relative fitness (defined as individual fitness divided by the fitness of the average individual in a focal year [29,30]) that were affected by the BSM: the relative number of fledglings and relative fledgling mass. All broods were used in the analyses, including those that failed to fledge any young.
(f). Statistical analyses
We used GLMMs to test whether brood size was optimized for the average individual in our population, by fitting a focal fitness component (see above) as the response variable. Pre-manipulation brood size (day 3), BSM category, year and the interaction between year × BSM category were included as fixed effects in all models to test for year-specific effects of the manipulation and to control for unmeasured environmental effects (given that the ecological conditions differed strongly between the two study years; see §3). Because mass varies over the day [31], we added ‘time of measurement since sunset’ as a covariate in the analyses of mass. BSM category (−3 nestlings, control, +3 nestlings) and year (2010 and 2011) were fitted as fixed factors with ‘control’ and ‘2010’, respectively, set as reference categories. All continuous fitness components were standardized (i.e. expressed in standard deviation units), and pre-manipulation brood size and time were fitted as covariates centred around the annual population mean [32]. Random intercepts were included for plot, nest-box and female identity. Analyses of the number of fledglings and mean fledgling mass were analysed using a Gaussian error distribution. Analyses of the probability to succeed, the probability of producing a second clutch and female local survival probability (i.e. probability of breeding in the following year) were analysed using a binomial error distribution.
To test whether types of females differed in the level of individual optimization, we performed phenotypic selection analyses by calculating standardized selection gradients among treatment groups. The strength and direction of phenotypic selection on exploratory behaviour were estimated by regressing the focal measure of fitness against an individual's relative trait value [29,30]. Measures of fitness were standardized and expressed as relative fitness (divided by the within-year mean fitness), and exploratory behaviour was standardized within year and expressed in standard deviation units. Using a GLMM with a Gaussian error distribution, variation in relative fitness was analysed in relation to the BSM (fitted as factor), standardized exploratory scores and the interaction between the BSM and standardized exploratory scores. Plot, nest-box and female identity were fitted as random effects in all models. Correcting the models for clutch size or brood size before manipulation did not affect the conclusions (results not shown). Year and interactions with year were not significant (y-variables were standardized within year) and are not discussed further because we did not have a priori hypotheses regarding effects of year.
The GLMMs were constructed in R v. 2.15.2 [33] with the ‘glmer’ function of the ‘lme4’ package. We used the ‘sim’ function of the ‘arm’ package to simulate values of the posterior distribution of the model parameters [34]. 95% CI around the mean (β) were extracted based on 1000 simulations [35]. The statistical significance of fixed effects and interactions were assessed based on the 95% CI around the mode. We considered an effect to be ‘significant’ in the frequentist sense when the 95% CI did not overlap with 0. The limits of a 95% CI were obtained as the 2.5 and 97.5% quantiles of the posterior distribution of parameter estimates from the full model.
3. Results
(a). Optimization of brood size
The BSM strongly affected the offspring but not the maternal components of fitness. Analyses revealed that both fledgling number and fledging mass were affected by the BSM. Compared with controls, enlarged broods (+3) fledged more nestlings of lower mass, whereas reduced broods (−3) fledged fewer nestlings of higher mass (table 1), revealing a trade-off between quality and quantity of offspring. Females with large pre-manipulation brood sizes, furthermore, produced more fledglings (table 1). Breeding conditions were better in 2011, leading to heavier and more fledglings per nest in that year. Enlarged broods produced significantly more fledglings in 2011 compared with 2010 (table 1). Furthermore, in 2011 females were more likely to succeed in producing at least one fledgling. However, maternal fitness components (i.e. the probability of producing a second clutch and female local survival probability) did not differ between manipulation categories (table 1). Overall, our results suggest that brood size was not optimized at the population level because females in the control treatment did not have the highest short-term fitness (see figures in electronic supplementary material, S1).
Table 1.
Model summary examining the effects of the brood size manipulation (BSM) on five components of fitness in a wild great tit population. Estimated effects sizes (β) are reported with their 95% credible intervals (CI) and sample size (number of observations). Significant effects are denoted in bold face. Non-applicable estimates are indicated with ‘NA’.
| mean fledgling mass | number of fledglings | p(succeed) | p(2nd clutch) | p(local survival) | |
|---|---|---|---|---|---|
| fixed effects | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| intercept | −0.56 (−0.92, −0.15) | −0.30 (−0.60, −0.03) | 0.52 (−0.27, 1.59) | 0.07 (−0.05, 0.15) | −0.32 (−0.93, 0.47) |
| BSMa | |||||
| reduced | 0.51 (0.18, 1.07) | −0.58 (−0.93, −0.26) | 0.09 (−0.74, 1.53) | 0.03 (−0.09, 0.15) | 0.33 (−0.66, 1.26) |
| enlarged | −0.61 (−1.08, −0.18) | 0.33 (−0.02, 0.74) | −0.26 (−1.60, 0.61) | 0.02 (−0.10, 0.13) | −0.91 (−1.98, 0.03) |
| original brood size | −0.06 (−0.15, 0.03) | 0.24 (0.17, 0.30) | −0.02 (−0.28, 0.20) | 0.00 (−0.02, 0.02) | −0.03 (−0.23, 0.17) |
| time of day | 0.0007 (−0.0004, 0.0018) | NA | NA | NA | NA |
| yearb | 0.83 (0.40, 1.26) | 0.86 (0.47, 1,12) | 2.09 (0.27, 3.36) | −0.02 (−0,13, 0.11) | −0.36 (−1.14, 0.86) |
| year × BSM | |||||
| 2011 × reduced | −0.02 (−0.55, 0.64) | −0.32 (−0.77, 0.09) | −1.10 (−2.72, 1,32) | 0.15 (−0.07, 0.27) | 0.86 (−0.48, 2.33) |
| 2011 × enlarged | 0.48 (−0.23, 0.92) | 0.44 (0.01, 0.96) | −0.31 (−2.08, 1.92) | 0.02 (−0.15, 0.19) | 1.20 (−0.05, 2.77) |
| random effects | σ2 (95% CI) | σ2 (95% CI) | σ2 (95% CI) | σ2 (95% CI) | σ2 (95% CI) |
| female | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | NAc | 0.32 (0.28, 0.41) |
| nest-box | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.36 (0.29, 0.41) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| plot | 0.08 (0.03, 0.11) | 0.03 (0.01, 006) | 0.57 (0.20, 0.90) | 0.006 (0.002, 0,014) | 0.00 (0.00, 0.00) |
| residual | 0.56 (0.44, 0.68) | 0.32 (0.26, 0.40) | 1 | 1 | 1 |
| sample size | 152 | 162 | 210 | 206 | 206 |
a ‘Control’ is used as reference category.
b ‘2010’ is used as reference category.
c Variance was non-estimable.
(b). Individual optimization and proactive behaviour
If variation in coping style explains differences in the level of optimization, we predict a significant interaction of BSM × exploration score on relative fitness (figure 1b). Our analyses confirmed that this was the case (overall effect on relative fledgling number: BSM × exploration: 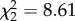 , p = 0.01; overall effect on relative fledgling mass: BSM × exploration:
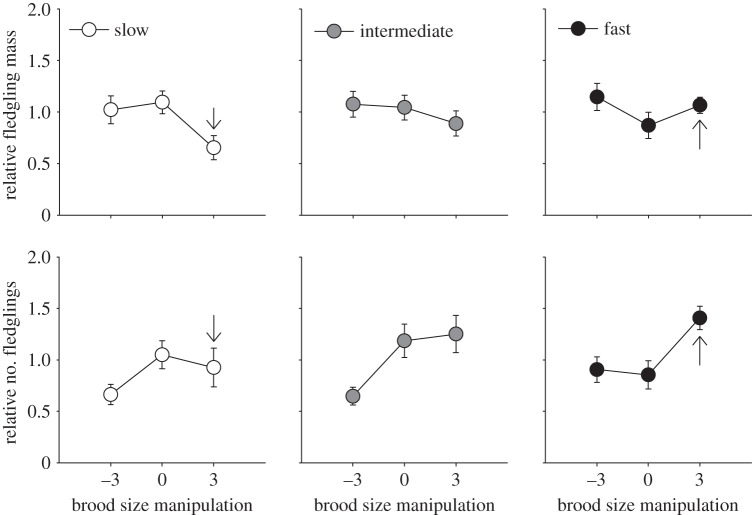
, p = 0.01; overall effect on relative fledgling mass: BSM × exploration: 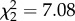 , p = 0.03). For both fitness measures, we found that faster females fledged relatively more and heavier offspring in the enlarged brood size group than the other treatment categories, implying that their natural brood size was below their optimum (table 2 and figure 2). By contrast, slower females performed better at their chosen brood size or when their brood was reduced, implying that their natural brood size was more optimized (table 2 and figure 2). Further analyses revealed that the reduced performance of slower females in the enlarged treatment group was explained by a higher probability of failing to produce any fledglings (electronic supplementary material, S2).
, p = 0.03). For both fitness measures, we found that faster females fledged relatively more and heavier offspring in the enlarged brood size group than the other treatment categories, implying that their natural brood size was below their optimum (table 2 and figure 2). By contrast, slower females performed better at their chosen brood size or when their brood was reduced, implying that their natural brood size was more optimized (table 2 and figure 2). Further analyses revealed that the reduced performance of slower females in the enlarged treatment group was explained by a higher probability of failing to produce any fledglings (electronic supplementary material, S2).
Table 2.
Model summary of phenotypic selection analyses of female exploratory behaviour in a great tit population. The effects of the brood size manipulation (BSM) on relative fitness are studied in relation to female exploratory behaviour standardized within year. Estimated effects sizes (β) are reported with their 95% credible interval (CI; n = 189 females). Significant effects are denoted in bold face.
| relative fledgling mass | relative no. fledglings | |
|---|---|---|
| fixed effects | β (95% CI) | β (95% CI) |
| intercept | 0.90 (0.76, 1.10) | 1.03 (0.82, 1.26) |
| BSMa | ||
| reduced | 0.10 (−0.08, 0.30) | −0.24 (−0.47, −0.05) |
| enlarged | −0.10 (−0.27, 0.10) | 0.15 (0.01, 0.41) |
| exploration | −0.03 (−0.20, 0.06) | −0.04 (−0.25, 0.05) |
| BSMa × exploration | ||
| reduced × exploration | 0.11 (−0.03, 0.35) | 0.11 (−0.11, 0.36) |
| enlarged × exploration | 0.22 (0.07, 0.42) | 0.32 (0.10, 0.50) |
| random effects | σ2 (95% CI) | σ2 (95% CI) |
| female | 0.03 (0.02, 0.04) | 0.04 (0.03, 0.05) |
| nest-box | 0.03 (0.02, 0.04) | 0.00 (0.00, 0.00) |
| plot | 0.03 (0.01, 0.05) | 0.09 (0.04, 0.13) |
| residual | 0.22 (0.19, 0.26) | 0.34 (0.26, 0.40) |
a‘Control’ is used as reference category.
Figure 2.
Selection gradients for female exploratory behaviour across BSM treatments in a great tit population (both years combined). Measures of relative fitness involve relative fledgling mass and relative number of fledglings per nest. For graphical purpose only, exploratory types were divided into three categories of equal sample size (slow explorers: white, intermediate explorers: grey and fast explorers: black). Exploratory behaviour is standardized within year. Means are shown with standard error (raw data). Black arrows indicate where the largest differences among groups exist (inferred from table 2).
(c). Exploratory behaviour and life history
Female exploratory behaviour was not related to clutch size or pre-manipulation brood size (Pearson correlation coefficients (r) ± 95% CI: clutch size: r = 0.07 (−0.07, 0.21), n = 187 females; pre-manipulation brood size: r = −0.05 (−0.10, 0.19), n = 185 females).
4. Discussion
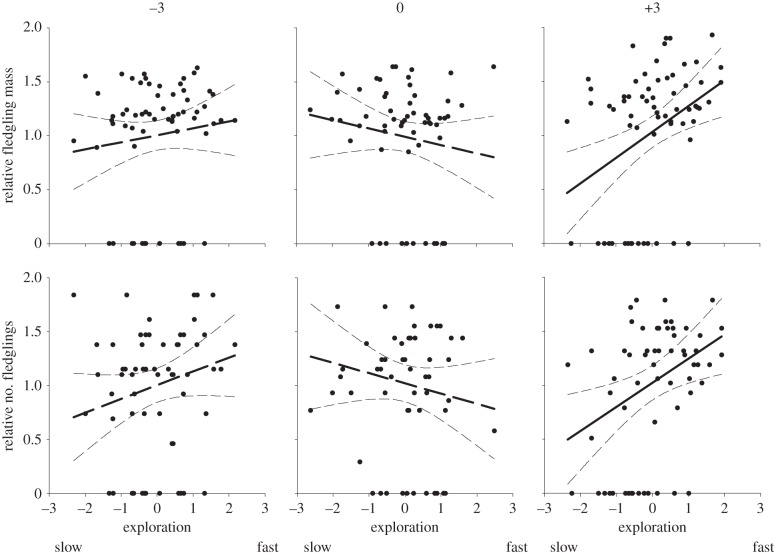
The aim of this study was to test whether females with different coping styles differ in their ability to take optimal reproductive decisions. We predicted that the fitness consequences of BSMs should differ between fast (i.e. proactive individuals) and slow explorers (i.e. reactive individuals), because of associated differences in environmental sensitivity. This should affect their ability to express adaptive phenotypic plasticity, and hence, the production of a brood size that is well matched to the current environment. Our results showed that the fitness consequences of the BSM depended on female coping style or phenotypic traits associated with female exploration tendency. In partial support of the scenario depicted in figure 1b, faster explorers did best when their brood size was enlarged, suggesting that fast females consistently underestimate the clutch size they should produce. By contrast, slower-exploring females performed best at their original brood size (and no different when their brood size was reduced), i.e. their reproductive decisions seemed more optimized. Further phenotypic selection analyses detailed in the electronic supplementary material confirmed that faster females, in fact, performed better than slower females within the enlarged treatment (figure 3; electronic supplementary material, S3). We also expected proactive and reactive females to differ in natural brood sizes, i.e. in general life-history strategy. Female exploratory behaviour was, however, not correlated with these life-history decisions. This non-experimental part of the dataset suggests that proactive, fast explorers did not bias investment towards current reproduction, unless proactive and reactive individuals adjust brood sizes differently with age [22,23]. Whether this is the case can only be addressed with long-term longitudinal data, which we are currently lacking.
Figure 3.
Selection gradients of female exploratory behaviour within BSM treatments in a great tit population (2 years combined). Measures of relative fitness are relative fledgling mass and relative number of fledglings per nest. Exploratory behaviour is standardized within year and within treatment. Selection gradients are shown ±95% CI (raw data). Non-significant trends are shown with dashed lines (see electronic supplementary material, S2).
(a). Population-level optimization of brood size
In agreement with life-history theory [1,2] and findings from other great tit populations [25,36], our experiment revealed the existence of a trade-off between the number and quality of offspring produced. Brood enlargement increased the number of fledged offspring but these were of lower mass. Our manipulation did not, however, reveal a trade-off between investments into current versus future reproduction, because female residual reproductive value (future fecundity and survival) was not affected. We showed previously that reproductive costs are enhanced in more competitive social environments [10]. Therefore, without direct manipulation of social environments (such as the local density of males [10]), survival or fecundity costs of reproduction may not be revealed.
Our two study years differed drastically in ecological conditions. In 2010, the reproductive output of females was lower and they coped less well with brood enlargement than in 2011. These results indicate that in the ‘bad’ year (2010), females had difficulties in meeting increased brood demands and it should have been more advantageous to produce smaller broods. This suggests that brood size is not optimal for the average female in the population, particularly in harsh years, or that early cues used for clutch size decisions were not appropriately predicting later environmental conditions. Yet, because we did not detect clear fitness costs, the conclusion regarding the optimality of brood size depends on whether or not the BSM affected the number of recruits per nest. Unfortunately, recruitment into our population was too low for meaningful analysis because most surviving young probably settled outside our nest-box areas.
(b). Proactive behaviour and individual optimization
This study tested whether females with different coping style differ in their levels of brood size optimization. Our analyses revealed that faster explorers produced a brood size that was below their optimum (i.e. not individually optimized), whereas slower explorers produced a brood size that was a good match to the environment (i.e. individually optimized; figure 1b). These results constitute a special case of the scenario depicted in figure 1b where fast-exploring females consistently underestimate the size of the clutch they should lay.
A prominent explanation for the existence of coping styles involves personality-related differences in information use [37,38]. Sampling information about local environmental features allows individuals to reduce uncertainty around fitness-relevant decisions [39]. Yet, because speed of behavioural decisions is traded-off against precision [38], a central prediction of coping style theory is that reactive individuals (who sample slowly but thoroughly) should be better able to assess the current conditions, or cues for future conditions during brood care, and therefore, should be better able to match their phenotype to the environment than proactive individuals [37,38]. We found that fast-exploring females consistently produced a number of offspring that was lower than the number of offspring they could raise, which is in line with the idea that they produced a brood size based on a biased perception of the environment (i.e. their clutch sizes fall ‘under’ their optimal reaction norm depicted in figure 1b). Studies on great tits have shown that slow-exploring individuals respond more quickly to changes in food distribution [19] and invest more heavily in sampling foraging options compared with fast-exploring individuals [40,41]. If early spring conditions can predict future food availability, such foraging behaviour may be important in determining the optimal number of nestlings that can be raised successfully. This explanation, however, does not clarify why fitness measures of both slow- and fast-exploring females were not significantly lower in the reduced brood size treatment.
The detected patterns may also be caused by physiological constraints acting on fast explorers during egg production. It is known that egg production is costly and that food supply in early spring represents an important determinant of clutch size [42]. If fast-exploring females would, for example, devote more time and energy to defending their resources at the cost of time spent foraging [43], this could reduce the amount of resources available for egg production. As a result, fast-exploring females would consistently produce smaller broods than slow-exploring females. Yet, because fast-exploring parents provision their young at a higher rate ([44], but see [45]), they might be better able to cope with brood enlargement than slow-exploring parents. This explanation warrants further testing and we are currently examining whether personality-related differences in provisioning behaviour could explain our findings [46].
Finally, an adaptive explanation for our results may involve personality-related differences in future fitness expectations. Individuals’ willingness to increase reproductive effort may depend on their expected future survival and reproduction. Increasing work load to meet brood demands may be seen as a ‘risky action’ that increases mortality risk (e.g. through increased predation risk [47] or physiological aging [48]). It has been proposed [23] (and confirmed experimentally [49]) that willingness to take risks is lower for individuals with increased future fitness expectations (or ‘assets'): by behaving more cautiously, individuals with increased survival or reproductive prospects would have higher chances of realizing their assets. There is experimental evidence that slow-exploring individuals are generally more risk averse than fast-exploring individuals [20,50–52]. Because exploration score is expected to be negatively correlated with future fitness expectations [22], it is possible that only fast-exploring females compensate for the manipulation (because they will have fewer future reproductive opportunities) and thus fledge more and heavier offspring in the enlarged treatment. By contrast, slow-exploring females might not be willing to increase their reproductive effort after an experimental increase in brood size, such that their offspring pay the cost.
This paper addressed the question whether coping behaviour predicts level of optimization. We investigated this question by asking whether an individual's exploratory behaviour predicts its level of clutch size optimization within the same year. However, both exploratory behaviour and ability to optimize reproduction represent behaviours that may harbour both among- and within-individual variation (owing to personality and plasticity, respectively; [53]). The demonstrated relationships between coping and level of optimization, similarly, may exist at both levels [54]. We had no a priori reason to predict differences in associations between coping style and level of optimization within versus among individuals, and therefore assume that the reported covariance applies to both levels. Repeated measures for both coping and level of optimization are required to enable partitioning of coping style-related differences in optimization within versus among individuals [55].
5. Conclusion
By applying an ‘individual optimization’ paradigm, we showed that the expression of adaptive phenotypic plasticity depended on a female's (current) coping style. We showed that reproductive decisions of faster explorers (i.e. proactive individuals) were sub-optimal, whereas reproductive decisions of slower explorers (i.e. reactive individuals) were matched with their environment. Those findings lead to the question of which conditions maintain variation in exploratory variation in natural populations [56]. The answer to this question might lie in the notion that reactive behaviour is expected to be favoured only in conditions where the benefits of phenotypic plasticity outweigh its costs [57,58], such as predictably varying environments [59], whereas proactive behaviour should be favoured when the environment is stable [60,61]. In line with this idea, recent theoretical modelling implies that spatio-temporal variation in the availability and predictability of resources can favour the maintenance of proactive and reactive individuals in the same population [62,63]. Similarly, empirical studies have shown that selection on personality traits can vary across sexes or years [64] and can be linked to changes in predator abundance [65], local social environment or habitat quality [66]. A challenge for future studies will be to identify which environmental variables and which spatial scales are relevant to understand the maintenance of variation in proactivity in our population.
Supplementary Material
Acknowledgements
We thank Anne-Lise Olsen, Erica F. Stuber and all the field assistants and students for their help with the collection of field data. We also thank John L. Quinn and an anonymous referee for constructive comments on the previous version of the manuscript. A.M. and Y.G.A.-A. were graduate students of the International Max Planck Research School for Organismal Biology. M.N. and N.J.D. conceived the study; M.N. and all co-authors designed the study; M.N. K.J.M., Y.G.A-A, A.M, J.J.W and N.J.D collected the data; M.N. analysed the data and the current version was written by M.N. with input from all authors.
Funding statement
M.N. and K.J.M. were supported by the Alexander von Humboldt Foundation, K.J.M by an NSERC postdoctoral fellowship and all authors by the Max Planck Society. This work was carried out under licence from the Regierung Oberbayern (permit no. 55.2-1-54-2532-140-11).
References
- 1.Lessells CM. 1991. The evolution of life histories. In Behavioural ecology. An evolutionary approach (eds Krebs JR, Davies NB.), pp. 32–68. Oxford, UK: Blackwell Scientific Publication. [Google Scholar]
- 2.Roff DA. 1992. The evolution of life histories. New York, NY: Chapman & Hall. [Google Scholar]
- 3.Lack D. 1947. The significance of clutch size. Ibis 89, 302–352. ( 10.1111/j.1474-919X.1947.tb04155.x) [DOI] [Google Scholar]
- 4.Lack D. 1966. Population studies of birds. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Charnov EL, Krebs JR. 1974. On clutch size and fitness. Ibis 116, 217–219. ( 10.1111/j.1474-919X.1974.tb00241.x) [DOI] [Google Scholar]
- 6.Williams GC. 1966. Natural selection costs of reproduction and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 7.Perrins CM, Moss D. 1975. Reproductive rates in the Great tit. J. Anim. Ecol. 44, 695–706. ( 10.2307/3712) [DOI] [Google Scholar]
- 8.Pettifor RA, Perrins CM, McCleery RH. 1988. Individual optimization of clutch size in great tits. Nature 336, 160–162. ( 10.1038/336160a0) [DOI] [Google Scholar]
- 9.Parejo D, Danchin E. 2006. Brood size manipulation affects frequency of second clutches in the blue tit. Behav. Ecol. Sociobiol. 60, 184–194. ( 10.1007/s00265-005-0155-z) [DOI] [Google Scholar]
- 10.Nicolaus M, Michler SPM, Ubels R, van der Velde M, Bouwman KM, Both C, Tinbergen JM. 2012. Local sex ratio affects the cost of reproduction. J. Anim. Ecol. 81, 564–572. ( 10.1111/j.1365-2656.2011.01933.x) [DOI] [PubMed] [Google Scholar]
- 11.Rytkönen S, Orell M. 2001. Great tits, Parus major, lay too many eggs: experimental evidence in mid-boreal habitats. Oikos 93, 439–450. ( 10.1034/j.1600-0706.2001.930309.x) [DOI] [Google Scholar]
- 12.Tinbergen JM, Sanz J. 2004. Strong evidence for selection for larger brood size in a great tit population. Behav. Ecol. 15, 525–533. ( 10.1093/beheco/arh045) [DOI] [Google Scholar]
- 13.Both C, Tinbergen JM, van Noordwijk AJ. 1998. Offspring fitness and individual optimization of clutch size. Proc. R. Soc. Lond. B 265, 2303–2307. ( 10.1098/rspb.1998.0575) [DOI] [Google Scholar]
- 14.Tinbergen JM, Both C. 1999. Is clutch size individually optimized? Behav. Ecol. 10, 504–509. ( 10.1093/beheco/10.5.504) [DOI] [Google Scholar]
- 15.Dhondt AA, Adriaensen F, Matthysen E, Kempenaers B. 1990. Nonadaptive clutch sizes in tits. Nature 348, 723–725. ( 10.1038/348723a0) [DOI] [Google Scholar]
- 16.Coppens CM, de Boer SF, Koolhaas JM. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028. ( 10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. ( 10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 18.Groothuis TGG, Carere C. 2005. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150. ( 10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 19.Verbeek MEM, Drent PJ, Wiepkema PR. 1994. Consistent individual differences in early exploratory behavior of male great tits. Anim. Behav. 48, 1113–1121. ( 10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 20.Stuber EF, Araya-Ajoy YG, Mathot KJ, Mutzel A, Nicolaus M, Wijmenga JJ, Mueller JC, Dingemanse NJ. 2013. Slow explorers take less risk: a problem of sampling bias in ecological studies. Behav. Ecol. 24, 1092–1098. ( 10.1093/beheco/art035) [DOI] [Google Scholar]
- 21.Browne WJ, McCleery RH, Sheldon BC, Pettifor RA. 2007. Using cross-classified multivariate mixed response models with application to life history traits in great tits (Parus major). Stat. Model. 7, 217–238. ( 10.1177/1471082X0700700301) [DOI] [Google Scholar]
- 22.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 24.Nicolaus M, Both C, Ubels R, Edelaar P, Tinbergen JM. 2009. No experimental evidence for local competition in the nestling phase as a driving force for density-dependent avian clutch size. J. Anim. Ecol. 78, 828–838. ( 10.1111/j.1365-2656.2009.01535.x) [DOI] [PubMed] [Google Scholar]
- 25.Nicolaus M, Michler SPM, Ubels R, van der Velde M, Komdeur J, Both C, Tinbergen JM. 2009. Sex-specific effects of altered competition on nestling growth and survival: an experimental manipulation of brood size and sex ratio. J. Anim. Ecol. 78, 414–426. ( 10.1111/j.1365-2656.2008.01505.x) [DOI] [PubMed] [Google Scholar]
- 26.Dingemanse NJ, Both C, Drent PJ, Van Oers K, van Noordwijk AJ. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938. ( 10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- 27.Dingemanse NJ, Bouwman KM, van de Pol M, Van Overveld T, Patrick SC, Matthysen E, Quinn JL. 2012. Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J. Anim. Ecol. 81, 116–126. ( 10.1111/j.1365-2656.2011.01877.x) [DOI] [PubMed] [Google Scholar]
- 28.Biro PA, Dingemanse NJ. 2009. Sampling bias resulting from animal personality. Trends Ecol. Evol. 24, 66–67. ( 10.1016/j.tree.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 29.Brodie ED, Moore AJ, Janzen FJ. 1995. Visualizing and quantifying natural selection. Trends Ecol. Evol. 10, 313–318. ( 10.1016/S0169-5347(00)89117-X) [DOI] [PubMed] [Google Scholar]
- 30.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 31.Gosler A, Carruthers T. 1999. Body reserves and social dominance in the great tit Parus major in relation to winter weather in southwest Ireland. J. Avian Biol. 30, 447–459. ( 10.2307/3677017) [DOI] [Google Scholar]
- 32.Enders CK, Tofighi D. 2007. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol. Methods 12, 121–138. ( 10.1037/1082-989X.12.2.121) [DOI] [PubMed] [Google Scholar]
- 33.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 34.Gelman A, Su Y-S, Yajima M, Hill J, Grazia Pittau M, Kerman J, Zheng T. 2012. arm: Data analysis using regression and multilevel/hierarchical models. R package version 1.5–02.
- 35.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 36.Smith HG, Källander H, Nilsson JÅ. 1989. The trade-off between offspring number and quality in the great tit Parus major. J. Anim. Ecol. 58, 383–401. ( 10.2307/4837) [DOI] [Google Scholar]
- 37.Mathot KJ, Wright J, Kempenaers B, Dingemanse NJ. 2012. Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 121, 1009–1020. ( 10.1111/j.1600-0706.2012.20339.x) [DOI] [Google Scholar]
- 38.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dall SRX, Johnstone RA. 2002. Managing uncertainty: information and insurance under the risk of starvation. Phil. Trans. R. Soc. Lond. B 357, 1519–1526. ( 10.1098/rstb.2002.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Overveld T, Matthysen E. 2013. Personality and information gathering in free-ranging great tits. PLoS ONE 8, 1–9. ( 10.1371/journal.pone.0054199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti C, Drent PJ. 2000. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 60, 131–140. ( 10.1006/anbe.2000.1443) [DOI] [PubMed] [Google Scholar]
- 42.Monaghan P, Nager RG. 1997. Why don't birds lay more eggs? Trends Ecol. Evol. 12, 270–274. ( 10.1016/S0169-5347(97)01094-X) [DOI] [PubMed] [Google Scholar]
- 43.Duckworth RA. 2006. Behavioral correlations across breeding contexts provide a mechanism for a cost of aggression. Behav. Ecol. 17, 1011–1019. ( 10.1093/beheco/arl035) [DOI] [Google Scholar]
- 44.Mutzel A, Dingemanse NJ, Araya-Ajoy YG, Kempenaers B. 2013. Parental provisioning behaviour plays a key role in linking personality with reproductive success. Proc. R. Soc. B 280, 20131019 ( 10.1098/rspb.2013.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrick SC, Browning LE. 2011. Exploration behaviour is not associated with chick provisioning in great tits. PLoS ONE 6, e26383 ( 10.1371/journal.pone.0026383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mutzel A. 2013. Behavioural mechanisms underpinning personality-related fitness. PhD thesis, Ludwig Maximilians University of Munich, Germany. [Google Scholar]
- 47.Tilgar V, Moks K, Saag P. 2011. Predator-induced stress changes parental feeding behavior in pied flycatchers. Behav. Ecol. 22, 23–28. ( 10.1093/beheco/arq164) [DOI] [Google Scholar]
- 48.Nilsson J-Å. 2002. Metabolic consequences of hard work. Proc. R. Soc. Lond. B 269, 1735–1739. ( 10.1098/rspb.2002.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolaus M, Tinbergen JM, Bouwman KM, Michler SPM, Ubels R, Both C, Kempenaers B, Dingemanse NJ. 2012. Experimental evidence for adaptive personalities in a wild passerine bird. Proc. R. Soc. B 279, 4885–4892. ( 10.1098/rspb.2012.1936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole EF, Quinn JL. 2014. Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild. Biol. Lett. 10, 20140178 ( 10.1098/rsbl.2014.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones KA, Godin JGJ. 2010. Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc. R. Soc. B 277, 625–632. ( 10.1098/rspb.2009.1607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn JL, Cole EF, Bates J, Payne RW, Cresswell W. 2012. Personality predicts individual responsiveness to the risks of starvation and predation. Proc. R. Soc. B 279, 1919–1926. ( 10.1098/rspb.2011.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 54.Dingemanse NJ, Dochtermann NA, Nakagawa S. 2012. Defining behavioural syndromes and the role of ‘syndrome deviation’ in understanding their evolution. Behav. Ecol. Sociobiol. 66, 1543–1548. ( 10.1007/s00265-012-1416-2) [DOI] [Google Scholar]
- 55.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 56.Dingemanse NJ, Réale D. 2013. What is the evidence for natural selection maintaining animal personality variation? In Animal personalities: behavior, physiology, and evolution (eds Carere C, Maestripieri D.), p. 201 Chicago, IL: Chicago University Press. [Google Scholar]
- 57.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewitt TJ. 1998. Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J. Evol. Biol. 11, 465–480. ( 10.1007/s000360050100) [DOI] [Google Scholar]
- 59.Gabriel W, Luttbeg B, Sih A, Tollrian R. 2005. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 166, 339–353. ( 10.1086/432558) [DOI] [PubMed] [Google Scholar]
- 60.Wilson DS, Yoshimura J. 1994. On the coexistence of specialists and generalists. Am. Nat. 144, 692–707. ( 10.1086/285702) [DOI] [Google Scholar]
- 61.Fischer B, Taborsky B, Kokko H. 2011. How to balance the offspring quality–quantity tradeoff when environmental cues are unreliable. Oikos 120, 258–270. ( 10.1111/j.1600-0706.2010.18642.x) [DOI] [Google Scholar]
- 62.Wolf M, van Doorn GS, Weissing FJ. 2008. Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830. ( 10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf M, Van Doorn GS, Weissing FJ. 2011. On the coevolution of social responsiveness and behavioural consistency. Proc. R. Soc. B 278, 440–448. ( 10.1098/rspb.2010.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852. ( 10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Réale D, Festa-Bianchet M. 2003. Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470. ( 10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- 66.Quinn JL, Patrick SC, Bouwhuis S, Wilkin TA, Sheldon BC. 2009. Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215. ( 10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.